Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Shows Reduced Graft Failure Rates and Superior Residual Rotational Stability Regardless of Anterolateral Ligament Reconstruction Graft: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
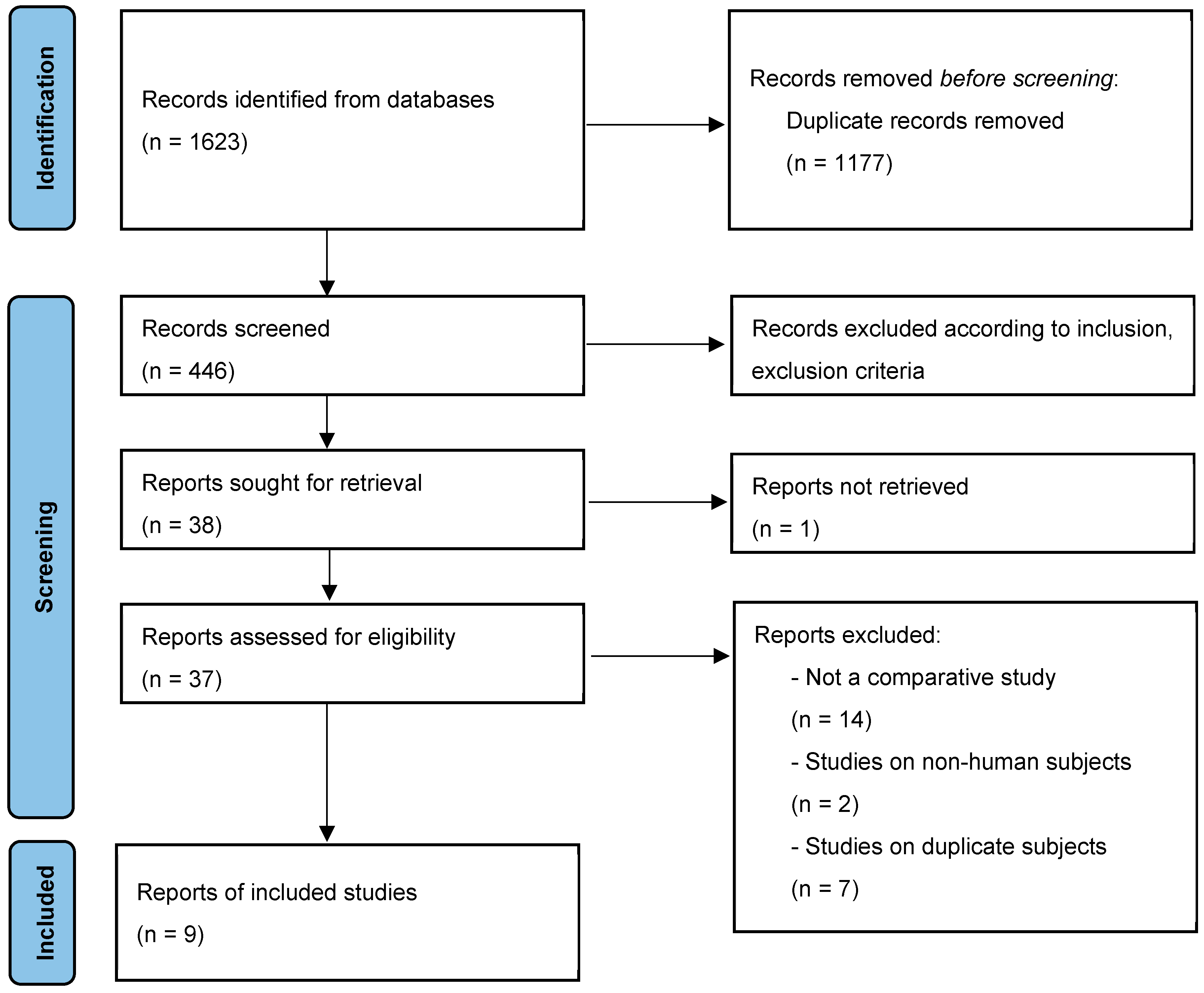
2.2. Identification of Eligibility
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Methodological Quality Assessment of Included Studies
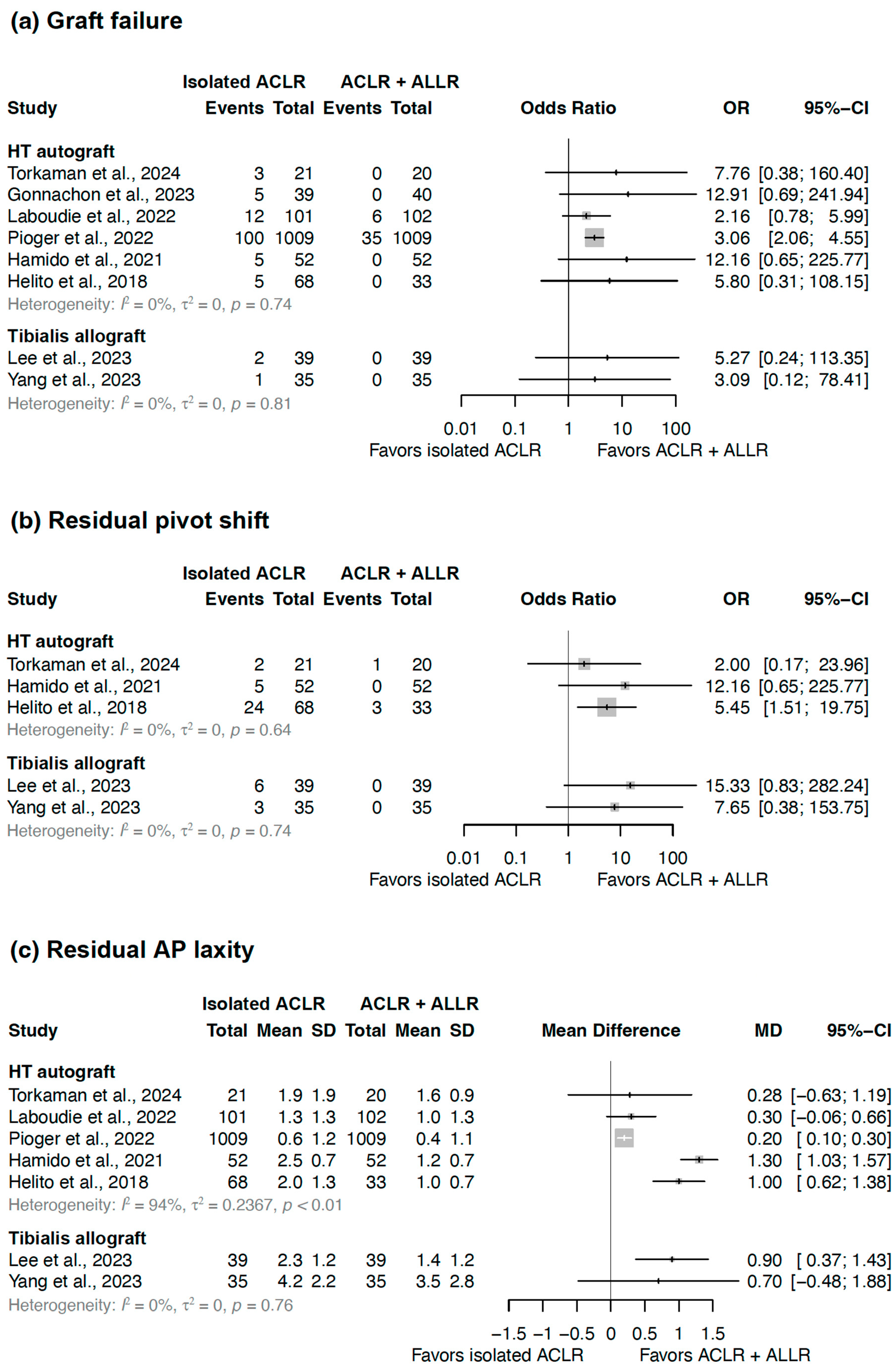
3.3. Graft Failure
3.4. Residual Pivot Shift
3.5. Residual Anterior–Posterior Laxity
3.6. Patient-Reported Outcome Measures
3.7. Complications
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACLR | Anterior cruciate ligament reconstruction |
| ALLR | Anterolateral ligament reconstruction |
| AP | Anterior–posterior |
| HT | Hamstring tendon |
| OR | Odds ratio |
| ACL | Anterior cruciate ligament |
| ALL | Anterolateral ligament |
| BPTB | Bone–patellar tendon–bone |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| IKDC | International Knee Documentation Committee |
| RCT | Randomized controlled trial |
| MINORS | Methodological Index for Non-Randomized Studies |
| MD | Mean difference |
| CI | Confidence interval |
Appendix A

| Study | Torkaman et al. [33] | Gonnachon et al. [27] | Lee et al. [26] | Yang et al. [25] | Laboudie et al. [29] | Pioger et al. [28] | Goncharov et al. [31] | Helito et al. [32] |
|---|---|---|---|---|---|---|---|---|
| Year | 2024 | 2023 | 2023 | 2023 | 2022 | 2022 | 2019 | 2018 |
| Level of evidence | 2 | 3 | 3 | 3 | 3 | 3 | 2 | 3 |
| 1. A stated aim of the study | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 2. Inclusion of consecutive patients | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 3. Prospective collection of data | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 4. Endpoint appropriate to the study aim | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 5. Unbiased evaluation of endpoints | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 6. Follow-up period appropriate to the major endpoint | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 7. Loss to follow up not exceeding 5% | 2 | 2 | 0 | 0 | 1 | 2 | 2 | 0 |
| 8. Prospective calculation of the sample size | 2 | 0 | 0 | 2 | 1 | 0 | 0 | 1 |
| 9. A control group having the gold standard intervention | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| 10. Contemporary groups | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 1 |
| 11. Baseline equivalence of groups | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 |
| 12. Statistical analyses adapted to the study design | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total scores | 24 | 21 | 19 | 22 | 22 | 22 | 21 | 20 |
| Study | Year | Graft Used for ALLR |
|---|---|---|
| Torkaman et al. [33] | 2024 | Patients had unilateral ACL injury, underwent ACLR using a quadruple-bundle semitendinosus tendon autograft, had an intact meniscus, exhibited a pivot shift test grade of 2+ or 3+, and had a minimum follow-up of two years. |
| Gonnachon et al. [27] | 2023 | Patients had ACL rupture and participated in pivot contact sports such as team sports or martial arts, regardless of competition level. |
| Lee et al. [26] | 2023 | Female patients had ACL rupture and a high-grade pivot shift (grade 2) and underwent combined ACLR and ALLR. |
| Yang et al. [25] | 2023 | Patients undergoing primary ACLR + ALLR met one or more of the following criteria: participation in high-level sporting activities (training at least twice a week, competing to win, professional or elite players), involvement in pivoting sports, presence of ligamentous hyperlaxity/genu recurvatum based on the modified Beighton scale, or a grade 3 pivot shift. |
| Laboudie et al. [29] | 2022 | Patients had a pivot shift grade ≥2, hyperlaxity (knee recurvatum >10°), were high-level athletes, or had a Segond fracture. |
| Pioger et al. [28] | 2022 | Patients underwent combined ACLR + ALLR due to high-grade pivot shift, chronic injuries, hyperlaxity, or participation in pivoting sports at a young age. |
| Hamido et al. [30] | 2021 | Patients had a high-grade pivot shift (grade III), a Segond fracture, participated in high-level sports, or engaged in sports involving frequent pivoting. |
| Goncharov et al. [31] | 2019 | Patients trained at least three times a week, participated in competitions, were involved in professional sports, and were aged between 16 and 40 years. |
| Helito et al. [32] | 2018 | Patients had an ACL injury for more than 12 months, confirmed by clinical and imaging examinations, and had no associated peripheral ligament injuries apart from the anterolateral corner. |
References
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. Incidence of anterior cruciate ligament tears and reconstruction: A 21-year population-based study. Am. J. Sports Med. 2016, 44, 1502–1507. [Google Scholar] [PubMed]
- Diermeier, T.; Rothrauff, B.B.; Engebretsen, L.; Lynch, A.D.; Ayeni, O.R.; Paterno, M.V.; Xerogeanes, J.W.; Fu, F.H.; Karlsson, J.; Musahl, V.; et al. Treatment after anterior cruciate ligament injury: Panther symposium acl treatment consensus group. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2390–2402. [Google Scholar] [PubMed]
- Lind, M.; Menhert, F.; Pedersen, A.B. Incidence and outcome after revision anterior cruciate ligament reconstruction: Results from the danish registry for knee ligament reconstructions. Am. J. Sports Med. 2012, 40, 1551–1557. [Google Scholar]
- Kamath, G.V.; Redfern, J.C.; Greis, P.E.; Burks, R.T. Revision anterior cruciate ligament reconstruction. Am. J. Sports Med. 2011, 39, 199–217. [Google Scholar]
- Koga, H.; Engebretsen, L.; Fu, F.H.; Muneta, T. Revision anterior cruciate ligament surgery: State of the art. J. ISAKOS 2017, 2, 36–46. [Google Scholar]
- Bae, B.S.; Yoo, S.; Lee, S.H. Ramp lesion in anterior cruciate ligament injury: A review of the anatomy, biomechanics, epidemiology, and diagnosis. Knee Surg. Relat. Res. 2023, 35, 23. [Google Scholar]
- Yoon, K.H.; Lee, S.M.; Park, J.Y.; Lee, H.S.; Hwang, S.H. A comparison of results in older, middle-aged, and younger patients after primary anterior cruciate ligament reconstruction: Minimum 10-year follow-up. Clin. Orthop. Surg. 2024, 16, 57–65. [Google Scholar]
- Ayeni, O.R.; Chahal, M.; Tran, M.N.; Sprague, S. Pivot shift as an outcome measure for acl reconstruction: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 767–777. [Google Scholar]
- Littlefield, C.P.; Belk, J.W.; Houck, D.A.; Kraeutler, M.J.; LaPrade, R.F.; Chahla, J.; McCarty, E.C. The anterolateral ligament of the knee: An updated systematic review of anatomy, biomechanics, and clinical outcomes. Arthroscopy 2021, 37, 1654–1666. [Google Scholar]
- Morgan, A.M.; Bi, A.S.; Kaplan, D.J.; Alaia, M.J.; Strauss, E.J.; Jazrawi, L.M. An eponymous history of the anterolateral ligament complex of the knee. Knee Surg. Relat. Res. 2022, 34, 45. [Google Scholar]
- Na, B.R.; Kwak, W.K.; Seo, H.Y.; Seon, J.K. Clinical outcomes of anterolateral ligament reconstruction or lateral extra-articular tenodesis combined with primary acl reconstruction: A systematic review with meta-analysis. Orthop. J. Sports Med. 2021, 9, 23259671211023099. [Google Scholar] [PubMed]
- Musahl, V.; Engler, I.D.; Nazzal, E.M.; Dalton, J.F.; Lucidi, G.A.; Hughes, J.D.; Zaffagnini, S.; Della Villa, F.; Irrgang, J.J.; Fu, F.H.; et al. Current trends in the anterior cruciate ligament part ii: Evaluation, surgical technique, prevention, and rehabilitation. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 34–51. [Google Scholar] [PubMed]
- Mouarbes, D.; Menetrey, J.; Marot, V.; Courtot, L.; Berard, E.; Cavaignac, E. Anterior cruciate ligament reconstruction: A systematic review and meta-analysis of outcomes for quadriceps tendon autograft versus bone-patellar tendon-bone and hamstring-tendon autografts. Am. J. Sports Med. 2019, 47, 3531–3540. [Google Scholar]
- Lee, D.W.; Ro, D.H.; Lee, M.C.; Han, H.S. Rectangular-tunnel anterior cruciate ligament reconstruction using quadriceps tendon-patellar bone autograft can reduce early donor site morbidity while maintaining comparable short-term clinical outcomes. Clin. Orthop. Surg. 2024, 16, 49–56. [Google Scholar]
- Bottoni, C.R.; Smith, E.L.; Shaha, J.; Shaha, S.S.; Raybin, S.G.; Tokish, J.M.; Rowles, D.J. Autograft versus allograft anterior cruciate ligament reconstruction: A prospective, randomized clinical study with a minimum 10-year follow-up. Am. J. Sports Med. 2015, 43, 2501–2509. [Google Scholar]
- Cruz, A.I., Jr.; Beck, J.J.; Ellington, M.D.; Mayer, S.W.; Pennock, A.T.; Stinson, Z.S.; VandenBerg, C.D.; Barrow, B.; Gao, B.; Ellis, H.B., Jr. Failure rates of autograft and allograft acl reconstruction in patients 19 years of age and younger: A systematic review and meta-analysis. JBJS Open Access 2020, 5, e20.00106. [Google Scholar]
- Runer, A.; Keeling, L.; Wagala, N.; Nugraha, H.; Özbek, E.A.; Hughes, J.D.; Musahl, V. Current trends in graft choice for primary anterior cruciate ligament reconstruction—Part II: In-vivo kinematics, patient reported outcomes, re-rupture rates, strength recovery, return to sports and complications. J. Exp. Orthop. 2023, 10, 40. [Google Scholar]
- Liau, Z.Q.G.; Ng, M.S.P.; Low, S.S.E.; Chin, B.Z.; Hui, J.H.P.; Kagda, F.H.Y. A novel practical method to predict anterior cruciate ligament hamstring graft size using preoperative mri. Knee Surg. Relat. Res. 2024, 36, 17. [Google Scholar]
- Boksh, K.; Sheikh, N.; Chong, H.H.; Ghosh, A.; Aujla, R. The role of anterolateral ligament reconstruction or lateral extra-articular tenodesis for revision anterior cruciate ligament reconstruction: A systematic review and meta-analysis of comparative clinical studies. Am. J. Sports Med. 2024, 52, 269–285. [Google Scholar]
- Bosco, F.; Giustra, F.; Masoni, V.; Capella, M.; Sciannameo, V.; Camarda, L.; Massè, A.; LaPrade, R.F. Combining an anterolateral complex procedure with anterior cruciate ligament reconstruction reduces the graft reinjury rate and improves clinical outcomes: A systematic review and meta-analysis of randomized controlled trials. Am. J. Sports Med. 2024, 52, 2129–2147. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar]
- Furukawa, T.A.; Barbui, C.; Cipriani, A.; Brambilla, P.; Watanabe, N. Imputing missing standard deviations in meta-analyses can provide accurate results. J. Clin. Epidemiol. 2006, 59, 7–10. [Google Scholar]
- Gheibi, S.; Mahmoodzadeh, A.; Kashfi, K.; Jeddi, S.; Ghasemi, A. Data extraction from graphs using adobe photoshop: Applications for meta-analyses. Int. J. Endocrinol. Metab. 2019, 17, e95216. [Google Scholar]
- Yang, H.Y.; Cheon, J.H.; Choi, J.H.; Song, E.K.; Seon, J.K. Combined anterior cruciate ligament and anterolateral ligament reconstruction decreases passive anterior tibial subluxation compared with isolated anterior cruciate ligament reconstruction despite similar rotational stability and clinical outcomes. Arthroscopy 2023, 39, 2513–2524.e2. [Google Scholar] [CrossRef]
- Lee, D.W.; Lee, D.H.; Cho, S.I.; Yang, S.J.; Kim, W.J.; Lee, J.K.; Kim, J.G. Comparison of acl and anterolateral ligament reconstruction with isolated acl reconstruction using hamstring autograft: Outcomes in young female patients with high-grade pivot shift. Orthop. J. Sports Med. 2023, 11, 23259671231178048. [Google Scholar]
- Gonnachon, A.; Labattut, L.; Abdoul Carime, N.; Orta, C.; Baulot, E.; Martz, P. Does combined anterior cruciate ligament and anterolateral ligament reconstruction improve return to sport? Eur. J. Orthop. Surg. Traumatol. 2023, 34, 981–987. [Google Scholar] [CrossRef]
- Pioger, C.; Gousopoulos, L.; Hopper, G.P.; Vieira, T.D.; Campos, J.P.; El Helou, A.; Philippe, C.; Saithna, A.; Sonnery-Cottet, B. Clinical outcomes after combined acl and anterolateral ligament reconstruction versus isolated acl reconstruction with bone-patellar tendon-bone grafts: A matched-pair analysis of 2018 patients from the santi study group. Am. J. Sports Med. 2022, 50, 3493–3501. [Google Scholar]
- Laboudie, P.; Douiri, A.; Bouguennec, N.; Biset, A.; Graveleau, N. Combined acl and all reconstruction reduces the rate of reoperation for graft failure or secondary meniscal lesions in young athletes. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3488–3498. [Google Scholar]
- Hamido, F.; Habiba, A.A.; Marwan, Y.; Soliman, A.S.I.; Elkhadrawe, T.A.; Morsi, M.G.; Shoaeb, W.; Nagi, A. Anterolateral ligament reconstruction improves the clinical and functional outcomes of anterior cruciate ligament reconstruction in athletes. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 1173–1180. [Google Scholar]
- Goncharov, E.N.; Koval, O.A.; Dubrov, V.E.; Bezuglov, E.N.; Filimonova, A.M.; Goncharov, N.G. Clinical experience with combined reconstruction of the anterior cruciate and anterolateral ligaments of the knee in sportsmen. Int. Orthop. 2019, 43, 2781–2788. [Google Scholar] [PubMed]
- Helito, C.P.; Camargo, D.B.; Sobrado, M.F.; Bonadio, M.B.; Giglio, P.N.; Pécora, J.R.; Camanho, G.L.; Demange, M.K. Combined reconstruction of the anterolateral ligament in chronic acl injuries leads to better clinical outcomes than isolated acl reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3652–3659. [Google Scholar]
- Torkaman, A.; Hosseinzadeh, M.; Mohammadyahya, E.; Torkaman, P.; Bahaeddini, M.R.; Aminian, A.; Tayyebi, H. All-inside anterior cruciate ligament reconstruction with and without anterolateral ligament reconstruction: A prospective study. BMC Musculoskelet. Disord. 2024, 25, 16. [Google Scholar]
- Tavlo, M.; Eljaja, S.; Jensen, J.T.; Siersma, V.D.; Krogsgaard, M.R. The role of the anterolateral ligament in acl insufficient and reconstructed knees on rotatory stability: A biomechanical study on human cadavers. Scand. J. Med. Sci. Sports 2016, 26, 960–966. [Google Scholar]
- Maletis, G.B.; Chen, J.; Inacio, M.C.S.; Love, R.M.; Funahashi, T.T. Increased risk of revision after anterior cruciate ligament reconstruction with bone-patellar tendon-bone allografts compared with autografts. Am. J. Sports Med. 2017, 45, 1333–1340. [Google Scholar]
- Zeng, C.; Gao, S.G.; Li, H.; Yang, T.; Luo, W.; Li, Y.S.; Lei, G.H. Autograft versus allograft in anterior cruciate ligament reconstruction: A meta-analysis of randomized controlled trials and systematic review of overlapping systematic reviews. Arthroscopy 2016, 32, 153–163.e18. [Google Scholar]
- Dai, W.; Leng, X.; Wang, J.; Cheng, J.; Hu, X.; Ao, Y. Quadriceps tendon autograft versus bone-patellar tendon-bone and hamstring tendon autografts for anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Am. J. Sports Med. 2022, 50, 3425–3439. [Google Scholar]
- He, X.; Yang, X.G.; Feng, J.T.; Wang, F.; Huang, H.C.; He, J.Q.; Hu, Y.C. Clinical outcomes of the central third patellar tendon versus four-strand hamstring tendon autograft used for anterior cruciate ligament reconstruction: A systematic review and subgroup meta-analysis of randomized controlled trials. Injury 2020, 51, 1714–1725. [Google Scholar]
- Mohtadi, N.G.; Chan, D.S.; Dainty, K.N.; Whelan, D.B. Patellar tendon versus hamstring tendon autograft for anterior cruciate ligament rupture in adults. Cochrane Database Syst. Rev. 2011, 2011, Cd005960. [Google Scholar]
- Chalidis, B.; Pitsilos, C.; Kitridis, D.; Givissis, P. Graft choices for anterolateral ligament knee reconstruction surgery: Current concepts. World J. Clin. Cases 2022, 10, 8463–8473. [Google Scholar]
- Wytrykowski, K.; Swider, P.; Reina, N.; Murgier, J.; Laffosse, J.M.; Chiron, P.; Cavaignac, E. Cadaveric study comparing the biomechanical properties of grafts used for knee anterolateral ligament reconstruction. Arthroscopy 2016, 32, 2288–2294. [Google Scholar] [PubMed]
- Pailhé, R.; Cavaignac, E.; Murgier, J.; Laffosse, J.M.; Swider, P. Biomechanical study of acl reconstruction grafts. J. Orthop. Res. 2015, 33, 1188–1196. [Google Scholar] [PubMed]
- Almqvist, K.F.; Jan, H.; Vercruysse, C.; Verbeeck, R.; Verdonk, R. The tibialis tendon as a valuable anterior cruciate ligament allograft substitute: Biomechanical properties. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 1326–1330. [Google Scholar] [PubMed]
- Nazari, G.; Barton, K.I.; Bryant, D.; Getgood, A.; Brown, C.H., Jr. Five- and six-strand hamstring grafts consistently produce appropriate graft diameters for anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 2940–2947. [Google Scholar]
- Itoh, M.; Itou, J.; Okazaki, K.; Iwasaki, K. Estimation failure risk by 0.5-mm differences in autologous hamstring graft diameter in anterior cruciate ligament reconstruction: A meta-analysis. Am. J. Sports Med. 2024, 52, 535–543. [Google Scholar]
- Zhao, D.; Pan, J.K.; Lin, F.Z.; Luo, M.H.; Liang, G.H.; Zeng, L.F.; Huang, H.T.; Han, Y.H.; Xu, N.J.; Yang, W.Y.; et al. Risk factors for revision or rerupture after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Am. J. Sports Med. 2023, 51, 3053–3075. [Google Scholar]
- Guglielmetti, L.G.B.; Shimba, L.G.; do Santos, L.C.; Severino, F.R.; Severino, N.R.; de Moraes Barros Fucs, P.M.; de Paula Leite Cury, R. The influence of femoral tunnel length on graft rupture after anterior cruciate ligament reconstruction. J. Orthop. Traumatol. 2017, 18, 243–250. [Google Scholar] [CrossRef][Green Version]
- Moon, H.S.; Choi, C.H.; Yoo, J.H.; Jung, M.; Lee, T.H.; Choi, K.H.; Kim, S.H. The graft insertion length in the femoral tunnel during anterior cruciate ligament reconstruction with suspensory fixation and tibialis anterior allograft does not affect surgical outcomes but is negatively correlated with tunnel widening. Arthroscopy 2021, 37, 2903–2914.e1. [Google Scholar]
- Mistry, H.; Metcalfe, A.; Colquitt, J.; Loveman, E.; Smith, N.A.; Royle, P.; Waugh, N. Autograft or allograft for reconstruction of anterior cruciate ligament: A health economics perspective. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 1782–1790. [Google Scholar]
- Lee, J.K.; Seo, Y.J.; Jeong, S.Y.; Yang, J.H. Biomechanical function of the anterolateral ligament of the knee: A systematic review. Knee Surg. Relat. Res. 2020, 32, 6. [Google Scholar]

| Study | Year | Journal | Country | Study Design | Level of Evidence | Number of Patients | Graft Used for ACLR | Graft Used for ALLR |
|---|---|---|---|---|---|---|---|---|
| Torkaman et al. [33] | 2024 | BMC Musculoskeletal. Disord. | Iran | Prospective comparative study | 2 | 41 | 4 strand ST | 1 strand GT |
| Gonnachon et al. [27] | 2023 | Eur. J. Orthop. Surg. Traumatol. | France | Cohort study | 3 | 79 | 3 or 4 strand ST + GT | 1 strand ST |
| Lee et al. [26] | 2023 | Orthop. J. Sports Med. | Republic of Korea | Cohort study | 3 | 78 | 4 strand ST | TA allograft |
| Yang et al. [25] | 2023 | Arthroscopy | Republic of Korea | Cohort study | 3 | 70 | 4 strand ST + GT | Tibialis allograft |
| Laboudie et al. [29] | 2022 | Knee Surg. Sports Traumatol. Arthrosc. | France | Retrospective comparative study | 3 | 203 | 4 strand ST | 1 strand GT |
| Pioger et al. [28] | 2022 | Am. J. Sports Med. | France | Cohort study | 3 | 2018 | 4 strand ST + GT | 1 strand GT |
| Hamido et al. [30] | 2021 | Knee Surg. Sports Traumatol. Arthrosc. | Kuwait | Randomized controlled trial | 1 | 102 | 4 strand ST | 2 strand GT |
| Goncharov et al. [31] | 2019 | Int. Orthop. | Russia | Prospective comparative study | 2 | 48 | BPTB Autograft | ST + GT |
| Helito et al. [32] | 2018 | Knee Surg. Sports Traumatol. Arthrosc. | Brazil | Cohort study | 3 | 101 | 4 strand ST + GT | 1 strand GT |
| Graft Used for ALLR | Study | Follow-Up Duration (Mean ± SD) | Sex (n, Male/Female) | Age (Years, Mean ± SD) | BMI (kg/m2, Mean ± SD) |
|---|---|---|---|---|---|
| HT Autograft | Torkaman et al., 2024 [33] | 39.8 ± 14.1 (isolated ACLR), 41.3 ± 15 (ACLR + ALLR) months | 17/4 (isolated ACLR), 18/2 (ACLR + ALLR) | 26.7 ± 8.9 (isolated ACLR), 25.9 ± 6.9 (ACLR + ALLR) | 24.5 ± 2.2 (isolated ACLR), 24.2 ± 2.1 (ACLR + ALLR) |
| Gonnachon et al., 2023 [27] | 54 months | 67/16 | 24 ± 7.2 | NR | |
| Laboudie et al., 2022 [29] | 4.8 ± 0.9 years | 57/44 (isolated ACLR), 62/40 (ACLR + ALLR) | 16.5 ± 2.2 (isolated ACLR), 16.8 ± 1.9 (ACLR + ALLR) | 21.8 ± 3 (isolated ACLR), 22.1 ± 3 (ACLR + ALLR) | |
| Pioger et al., 2022 [28] | 101.3 ± 59.9 months | 840/169 (isolated ACLR), 845/163 (ACLR + ALLR) | 25.8 ± 7.5 (isolated ACLR), 25.8 ± 7.9 (ACLR + ALLR) | 24.4 ± 3.4 (isolated ACLR), 24.3 ± 3.4 (ACLR + ALLR) | |
| Hamido et al., 2021 [30] | median 60 months (range 55–65) | 52/0 (isolated ACLR), 50/0 (ACLR + ALLR) | 26 (isolated ACLR), 24 (ACLR + ALLR) | NR | |
| Goncharov et al., 2019 [31] | 24 months | NR | NR | NR | |
| Helito et al., 2018 [32] | 26 (isolated ACLR), 25 (ACLR + ALLR) months | 59/9 (isolated ACLR), 30/3 (ACLR + ALLR) | 33.9 ± 6.1 (isolated ACLR), 33.1 ± 8.8 (ACLR + ALLR) | NR | |
| Tibialis Allograft | Lee et al., 2023 [26] | 30.4 ± 3.9 (isolated ACLR), 29.3 ± 3.5 (ACLR + ALLR) months | 0/39 (isolated ACLR), 0/39 (ACLR + ALLR) | 31.1 ± 5.7 (isolated ACLR), 30.4 ± 6.1 (ACLR + ALLR) | 19.4 ± 2.5 (isolated ACLR), 19.7 ± 2.7 (ACLR + ALLR) |
| Yang et al., 2023 [25] | 46.6 months, 42.5 months | 31/4 (isolated ACLR), 29/6 (ACLR + ALLR) | 26.8 ± 9.9 (isolated ACLR), 26.1 ± 10.8 (ACLR + ALLR) | 26.2 ± 4.6 (isolated ACLR), 26.6 ± 4.5 (ACLR + ALLR) |
| Graft Used for ALLR | Study | Graft Failure | Residual Pivot Shift | Residual AP Laxity (mm, Mean ± SD) | ||||
|---|---|---|---|---|---|---|---|---|
| Isolated ACLR | ACLR + ALLR | Isolated ACLR | ACLR + ALLR | Isolated ACLR | ACLR + ALLR | Method | ||
| HT Autograft | Torkaman et al., 2024 [33] | 3/21 (14.3%) | 0/20 (0%) | 2/21 (9.5%) | 1/20 (5.0%) | 1.9 ± 1.9 | 1.7 ± 0.9 | KT-1000 |
| Gonnachon et al., 2023 [27] | 5/39 (12.8%) | 0/40 (0%) | NR | NR | NR | NR | NR | |
| Laboudie et al., 2022 [29] | 12/101 (11.9%) | 6/102 (5.9%) | NR | NR | 1.3 ± 1.3 | 1 ± 1.3 | GNRB | |
| Pioger et al., 2022 [28] | 100/1009 (9.9%) | 35/1009 (3.5%) | NR | NR | 0.6 ± 1.2 | 0.4 ± 1.1 | Rolimeter | |
| Hamido et al., 2021 [30] | 5/52 (9.6%) | 0/50 (0%) | 5/52 (9.6%) | 0/50 (0%) | 2.5 ± 0.7 | 1.2 ± 0.7 | KT-1000 | |
| Goncharov et al., 2019 [31] | NR | NR | NR | NR | NR | NR | NR | |
| Helito et al., 2018 [32] | 5/68 (7.4%) | 0/33 (0%) | 24/68 (35.3%) | 3/33 (9.1%) | 2 (95% CI 1.5–2.1) | 1 (95% CI 1.14–1.6) | KT-1000 | |
| Tibialis Allograft | Lee et al., 2023 [26] | 2/39 (5.1%) | 0/39 (0%) | 6/39 (15.4%) | 0/39 (0%) | 2.3 ± 1.2 | 1.4 ± 1.2 | KT-2000 |
| Yang et al., 2023 [25] | 1/35 (2.9%) | 0/35 (0%) | 3/35 (8.6%) | 0/35 (0%) | 4.2 ± 2.2 | 3.5 ± 2.8 | Telos stress | |
| Graft Used for ALLR | Study | Lysholm Score (Mean ± SD) | IKDC Score (Mean ± SD) | Tegner Activity Scale (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Isolated ACLR | ACLR + ALLR | Isolated ACLR | ACLR + ALLR | Isolated ACLR | ACLR + ALLR | ||
| HT Autograft | Torkaman et al., 2024 [33] | 89.1 ± 8.7 | 90.2 ± 9.3 | 79 ± 9.2 | 81.5 ± 8.8 | NR | NR |
| Gonnachon et al., 2023 [27] | 80.5 ± 11.7 | 82.05 ± 14.6 | 85.6 ± 12.3 | 85.8 ± 13.8 | 6.2 ± 1.3 | 6.6 ± 1.6 | |
| Laboudie et al., 2022 [29] | 83.3 ± 14.3 | 82 ± 14.4 | 86.4 ± 15.2 | 86 ± 16.8 | 7 | 7 | |
| Hamido et al., 2021 [30] | NR | NR | 94 ± 4.5 | 96 ± 5 | 7.8 ± 1.4 | 7.9 ± 0.8 | |
| Goncharov et al., 2019 [31] | 90.3 ± 10 | 96.3 ± 3.6 | 92.1 ± 10.5 | 97.4 ± 2.3 | NR | NR | |
| Helito et al., 2018 [32] | 87.1 ± 9 | 92.7 ± 5.9 | 90 ± 7.1 | 95.4 ± 5.3 | NR | NR | |
| Tibialis Allograft | Lee et al., 2023 [26] | 82.9 ± 8.8 | 87.1 ± 9.8 | 87.3 ± 7.9 | 90.7 ± 8.1 | 5.6 ± 1.3 | 6.1 ± 1.4 |
| Yang et al., 2023 [25] | 84.3 ± 15.9 | 84.7 ± 12.2 | 89.6 ± 17.6 | 89.7 ± 13.3 | 5.5 ± 2.1 | 6 ± 1.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.H.; Kim, S.-H.; Jung, M.; Moon, H.-S.; Chung, K. Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Shows Reduced Graft Failure Rates and Superior Residual Rotational Stability Regardless of Anterolateral Ligament Reconstruction Graft: A Systematic Review. J. Clin. Med. 2025, 14, 2237. https://doi.org/10.3390/jcm14072237
Han JH, Kim S-H, Jung M, Moon H-S, Chung K. Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Shows Reduced Graft Failure Rates and Superior Residual Rotational Stability Regardless of Anterolateral Ligament Reconstruction Graft: A Systematic Review. Journal of Clinical Medicine. 2025; 14(7):2237. https://doi.org/10.3390/jcm14072237
Chicago/Turabian StyleHan, Joo Hyung, Sung-Hwan Kim, Min Jung, Hyun-Soo Moon, and Kwangho Chung. 2025. "Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Shows Reduced Graft Failure Rates and Superior Residual Rotational Stability Regardless of Anterolateral Ligament Reconstruction Graft: A Systematic Review" Journal of Clinical Medicine 14, no. 7: 2237. https://doi.org/10.3390/jcm14072237
APA StyleHan, J. H., Kim, S.-H., Jung, M., Moon, H.-S., & Chung, K. (2025). Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Shows Reduced Graft Failure Rates and Superior Residual Rotational Stability Regardless of Anterolateral Ligament Reconstruction Graft: A Systematic Review. Journal of Clinical Medicine, 14(7), 2237. https://doi.org/10.3390/jcm14072237






