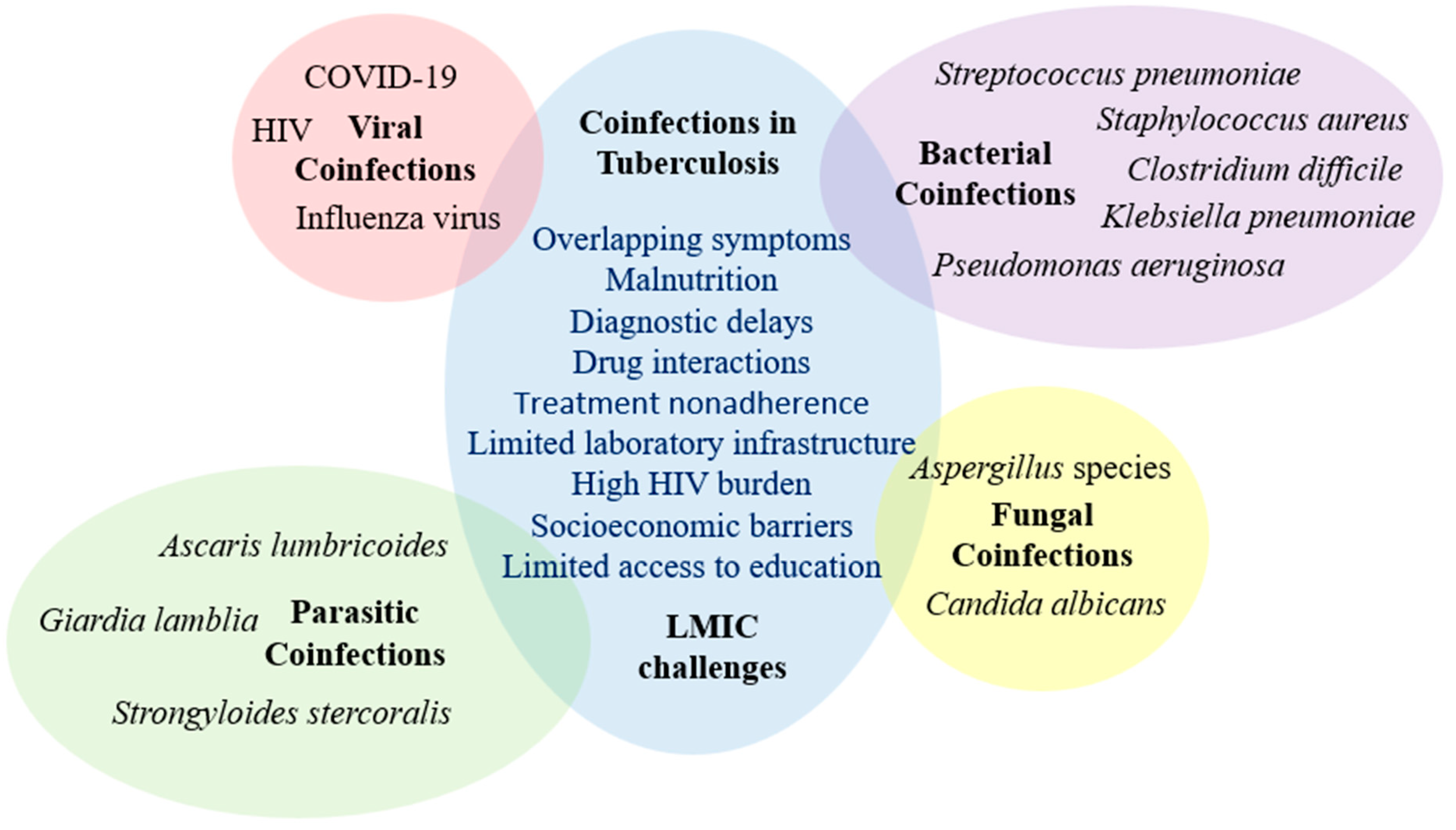
Coinfections in Tuberculosis in Low- and Middle-Income Countries: Epidemiology, Clinical Implications, Diagnostic Challenges, and Management Strategies—A Narrative Review
Abstract
1. Introduction
2. Viral Coinfections
2.1. Influenza Coinfection
2.2. HIV Coinfection
2.3. SARS-CoV-2 Coinfection
3. Bacterial Coinfections
4. Fungal Coinfections
5. Parasitic Coinfections
6. The Impact of Socioeconomic Factors on Coinfection Rates in LMICs
7. TB Coinfection Management Models
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Alemu, A.; Bitew, Z.W.; Seid, G.; Diriba, G.; Gashu, E.; Berhe, N.; Mariam, S.H.; Gumi, B. Tuberculosis in Individuals Who Recovered from COVID-19: A Systematic Review of Case Reports. PLoS ONE 2022, 17, e0277807. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report 2024; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Goletti, D.; Meintjes, G.; Andrade, B.B.; Zumla, A.; Shan Lee, S. Insights from the 2024 WHO Global Tuberculosis Report—More Comprehensive Action, Innovation, and Investments Required for Achieving WHO End TB Goals. Int. J. Infect. Dis. 2025, 150, 107325. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H. Tuberculosis Infection and Latent Tuberculosis. Tuberc. Respir. Dis. 2016, 79, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Jarde, A.; Romano, E.; Afaq, S.; Elsony, A.; Lin, Y.; Huque, R.; Elsey, H.; Siddiqi, K.; Stubbs, B.; Siddiqi, N. Prevalence and Risks of Tuberculosis Multimorbidity in Low-Income and Middle-Income Countries: A Meta-Review. BMJ Open 2022, 12, e060906. [Google Scholar] [CrossRef]
- Cioboata, R.; Nicolosu, D.; Balasoiu, A.T.; Balteanu, M.A.; Zlatian, O.M.; Osman, A.; Biciusca, V.; Tieranu, E.N.; Mogos, G.F.R.; Ghenea, A.E. Vitamin C and Tuberculosis: Examining the Relationship Between Antioxidant Defense and Disease Severity—Preliminary Findings from a Southwestern Romanian Study. J. Clin. Med. 2024, 13, 6715. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Che, Y.; Xiao, Y.; Jiang, F.; Chen, Y.; Zhou, J.; Yang, T. Impact of Multimorbidity Subgroups on the Health Care Use and Clinical Outcomes of Patients with Tuberculosis: A Population-Based Cohort Analysis. Front. Public Health 2021, 9, 756717. [Google Scholar] [CrossRef]
- Oh, J.; Lee, M.; Kim, M.; Jin Kim, H.; Won Lee, S.; Youl Rhee, S.; Koyanagi, A.; Smith, L.; Seo Kim, M.; Lee, H.; et al. Incident Allergic Diseases in Post-COVID-19 Condition: Multinational Cohort Studies from South Korea, Japan and the UK. Nat. Commun. 2024, 15, 2830. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P.R. The Immune Response in Tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- de Martino, M.; Lodi, L.; Galli, L.; Chiappini, E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front. Pediatr. 2019, 7, 350. [Google Scholar] [CrossRef]
- Joslyn, L.R.; Flynn, J.A.L.; Kirschner, D.E.; Linderman, J.J. Concomitant Immunity to M. tuberculosis Infection. Sci. Rep. 2022, 12, 20731. [Google Scholar] [CrossRef]
- Kumar Nathella, P.; Babu, S. Influence of Diabetes Mellitus on Immunity to Human Tuberculosis. Immunology 2017, 152, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tarashi, S.; Fateh, A.; Mirsaeidi, M.; Siadat, S.D.; Vaziri, F. Mixed Infections in Tuberculosis: The Missing Part in a Puzzle. Tuberculosis 2017, 107, 168–174. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Cai, J.; Shao, L.; Jiang, X.; Yin, X.; Zhao, X.; Wang, S. Clinical and Microbiological Characteristics of Klebsiella pneumoniae Co-Infections in Pulmonary Tuberculosis: A Retrospective Study. Infect. Drug Resist. 2023, 16, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Attia, E.F.; Pho, Y.; Nhem, S.; Sok, C.; By, B.; Phann, D.; Nob, H.; Thann, S.; Yin, S.; Noce, R.; et al. Tuberculosis and Other Bacterial Co-Infection in Cambodia: A Single Center Retrospective Cross-Sectional Study. BMC Pulm. Med. 2019, 19, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Bhunu, C.P.; Garira, W.; Mukandavire, Z. Modeling HIV/AIDS and Tuberculosis Coinfection. Bull. Math. Biol. 2009, 71, 1745–1780. [Google Scholar] [CrossRef]
- McIvor, A.; Koornhof, H.; Kana, B.D. Relapse, Re-Infection and Mixed Infections in Tuberculosis Disease. Pathog. Dis. 2017, 75, ftx020. [Google Scholar] [CrossRef]
- Walaza, S.; Tempia, S.; Dawood, H.; Variava, E.; Wolter, N.; Dreyer, A.; Moyes, J.; Von Mollendorf, C.; McMorrow, M.; Von Gottberg, A.; et al. The Impact of Influenza and Tuberculosis Interaction on Mortality Among Individuals Aged ≥15 Years Hospitalized with Severe Respiratory Illness in South Africa, 2010–2016. Open Forum Infect. Dis. 2019, 6, ofz020. [Google Scholar] [CrossRef]
- Ring, S.; Eggers, L.; Behrends, J.; Wutkowski, A.; Schwudke, D.; Kröger, A.; Hierweger, A.M.; Hölscher, C.; Gabriel, G.; Schneider, B.E. Blocking IL-10 Receptor Signaling Ameliorates Mycobacterium tuberculosis Infection during Influenza-Induced Exacerbation. JCI Insight 2019, 4, e126533. [Google Scholar] [CrossRef]
- Redford, P.S.; Mayer-Barber, K.D.; McNab, F.W.; Stavropoulos, E.; Wack, A.; Sher, A.; O’Garra, A. Influenza A Virus Impairs Control of Mycobacterium tuberculosis Coinfection through a Type i Interferon Receptor-Dependent Pathway. J. Infect. Dis. 2014, 209, 270–274. [Google Scholar] [CrossRef]
- Mendy, J.; Jarju, S.; Heslop, R.; Bojang, A.L.; Kampmann, B.; Sutherland, J.S. Changes in Mycobacterium tuberculosis-Specific Immunity with Influenza Co-Infection at Time of TB Diagnosis. Front. Immunol. 2019, 10, 3093. [Google Scholar] [CrossRef]
- Noh, J.Y.; Lee, J.; Choi, W.S.; Song, J.Y.; Seo, Y.B.; Kim, I.S.; Cheong, H.J.; Kim, W.J. Concurrent Tuberculosis and Influenza, South Korea. Emerg. Infect. Dis. 2013, 19, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Fry, A.; Shay, D.; Gubareva, L.; Bresee, J.; Uyeki, T. Antiviral Agents for the Treatment and Chemoprophylaxis of Influenza—Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011, 60, 1–24. [Google Scholar] [PubMed]
- Getahun, H.; Gunneberg, C.; Granich, R.; Nunn, P. HIV Infection-Associated Tuberculosis: The Epidemiology and the Response. Clin. Infect. Dis. 2010, 50 (Suppl. S3), 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.M.; Cohn, D.L. Tuberculosis and HIV Coinfection. Semin. Respir. Crit. Care Med. 2013, 34, 032–043. [Google Scholar] [CrossRef]
- Martino, R.J.; Chirenda, J.; Mujuru, H.A.; Ye, W.; Yang, Z. Characteristics Indicative of Tuberculosis/HIV Coinfection in a High-Burden Setting: Lessons from 13,802 Incident Tuberculosis Cases in Harare, Zimbabwe. Am. J. Trop. Med. Hyg. 2020, 103, 214–220. [Google Scholar] [CrossRef]
- Moore-Pardo, S.M.; Addisu, A.; Reljic, T.; Aslam, S.; Casanas, B.C. 370. Comparing Trends and Outcomes among HIV-Infected vs. HIV Uninfected Patients with Tuberculosis: A 5-Year Experience Within the Florida Department of Health in Hillsborough County. Open Forum Infect. Dis. 2019, 6, 194. [Google Scholar] [CrossRef]
- Addisu, A.; Aslam, S.; Holt, D.; Alrabaa, S.; Casanas, B. 1482. Changing Patterns of HIV-TB Coinfection Among Patients in a Public Health Department Ambulatory Care Setting: A 5-Year Experience from a US Metropolitan Area. Open Forum Infect. Dis 2018, 5, 458. [Google Scholar] [CrossRef]
- Frolova, O.P.; Butylchenko, O.V.; Gadzhieva, P.G.; Timofeeva, M.Y.; Basangova, V.A.; Petrova, V.O.; Fadeeva, I.A.; Kashutina, M.I.; Zabroda, N.N.; Basov, A.A.; et al. Medical Care for Tuberculosis-HIV-Coinfected Patients in Russia with Respect to a Changeable Patients’ Structure. Trop. Med. Infect. Dis. 2022, 7, 86. [Google Scholar] [CrossRef]
- Khan, M.S.; Rego, S.; Rajal, J.B.; Bond, V.; Fatima, R.K.; Isani, A.K.; Sutherland, J.; Kranzer, K. Mitigating the Impact of COVID-19 on Tuberculosis and HIV Services: A Cross-Sectional Survey of 669 Health Professionals in 64 Low and Middle-Income Countries. PLoS ONE 2020, 16, e0244936. [Google Scholar] [CrossRef]
- Hogan, A.B.; Jewell, B.L.; Sherrard-Smith, E.; Vesga, J.F.; Watson, O.J.; Whittaker, C.; Hamlet, A.; Smith, J.A.; Winskill, P.; Verity, R.; et al. Potential Impact of the COVID-19 Pandemic on HIV, Tuberculosis, and Malaria in Low-Income and Middle-Income Countries: A Modelling Study. Lancet Glob. Health 2020, 8, 1132–1141. [Google Scholar] [CrossRef]
- Cioboata, R.; Biciusca, V.; Olteanu, M.; Vasile, C.M. COVID-19 and Tuberculosis: Unveiling the Dual Threat and Shared Solutions Perspective. J. Clin. Med. 2023, 12, 4784. [Google Scholar] [CrossRef] [PubMed]
- Bruchfeld, J.; Correia-Neves, M.; Kä Llenius, G. Tuberculosis and HIV Coinfection. Perspect. Med. 2015, 15, 017871. [Google Scholar] [CrossRef]
- Muñoz-Lombo, J.P.; Llanos-Torres, J.; Rebellon-Sanchez, D.E.; Montes-Tello, S.A.; García-Goez, J.F.; Rosso, F. Clinical Characterization of TB/HIV Naive Coinfection Patients Enrolled in a TB Program in Cali—Colombia. Open Forum Infect. Dis. 2023, 10, ofad500.1340. [Google Scholar] [CrossRef]
- Putra Gofur, N.R.; Putri Gofur, A.R. Clinical Manifestation of Oral Tuberculosis in HIV Patient: A Review Article. J. Dent. Sci. Res. Rev. Rep. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Mehta, S.; Peters, R.P.H.; Smit, D.P.; Gupta, V. Ocular Tuberculosis in HIV-Infected Individuals. Ocul. Immunol. Inflamm. 2020, 28, 1251–1258. [Google Scholar] [CrossRef]
- Wasserman, S.; Barr, D.; Meintjes, G. Clinical Manifestations of HIV-Associated Tuberculosis in Adults. In HIV and Tuberculosis; Springer: Cham, Switzerland, 2019; pp. 73–97. [Google Scholar] [CrossRef]
- Vázquez García, J.C.; Sada Díaz, E.; Rivera Martínez, E.; Narváez Porras, O.; Salazar Lezama, M.A. Tuberculosis Associated with HIV Infection. Rev. Investig. Clin. 1994, 46, 473–477. [Google Scholar]
- The Impact of COVID-19 on HIV, TB and Malaria Services and Systems for Health—Updates—The Global Fund to Fight AIDS, Tuberculosis and Malaria. Available online: https://resources.theglobalfund.org/en/updates/2021-04-13-the-impact-of-covid-19-on-hiv-tb-and-malaria-services-and-systems-for-health/ (accessed on 2 February 2025).
- Sharma, S.; Mohan, A.; Kadhiravan, T. HIV-TB Co-Infection: Epidemiology, Diagnosis & Management. Indian. J. Med. Res. 2005, 121, 550–567. [Google Scholar]
- Swaminathan, S.; Narendran, G.; Venkatesan, P.; Iliayas, S.; Santhanakrishnan, R.; Menon, P.A.; Padmapriyadarsini, C.; Ramachandran, R.; Chinnaiyan, P.; Suhadev, M.; et al. Efficacy of a 6-Month versus 9-Month Intermittent Treatment Regimen in HIV-Infected Patients with Tuberculosis. Am. J. Respir. Crit. Care Med. 2012, 181, 743–751. [Google Scholar] [CrossRef]
- Li, Q. Treatment of TB and HIV Coinfection. In Handbook of Global Tuberculosis Control; Springer: Boston, MA, USA, 2017; pp. 173–190. [Google Scholar] [CrossRef]
- Toossi, Z.; Mayanja-Kizza, H.; Hirsch, C.S.; Edmonds, K.L.; Spahlinger, T.; Hom, D.L.; Aung, H.; Mugyenyi, P.; Ellner, J.J.; Whalen, C.W. Impact of Tuberculosis (TB) on HIV-1 Activity in Dually Infected Patients. Clin. Exp. Immunol. 2001, 123, 233–238. [Google Scholar] [CrossRef]
- Pandie, M.; Wiesner, L.; McIlleron, H.; Hughes, J.; Siwendu, S.; Conradie, F.; Variava, E.; Maartens, G. Drug-Drug Interactions between Bedaquiline and the Antiretrovirals Lopinavir/Ritonavir and Nevirapine in HIV-Infected Patients with Drug-Resistant TB. J. Antimicrob. Chemother. 2016, 71, 1037–1040. [Google Scholar] [CrossRef]
- Nabisere, R.; Musaazi, J.; Denti, P.; Aber, F.; Lamorde, M.; Dooley, K.E.; Aarnoutse, R.; Sloan, D.J.; Sekaggya-Wiltshire, C. Pharmacokinetics, SAfety/Tolerability, and EFficacy of High-Dose RIFampicin in Tuberculosis-HIV Co-Infected Patients on Efavirenz- or Dolutegravir-Based Antiretroviral Therapy: Study Protocol for an Open-Label, Phase II Clinical Trial (SAEFRIF). Trials 2020, 21, 181. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Brima, N.; Almajid, F.; Pozniak, A.L.; Janmohamed, A.; Mandalia, S.; Basnayake, S.; Kellgren, L.; Copas, A.J.; Miller, R.F. Outcomes from Treating Tuberculosis with Rifampicin or Rifabutin in HIV-Infected Persons Also Receiving Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2015, 68, e84–e87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Sadr, W.M.; Perlman, D.C.; Denning, E.; Matts, J.P.; Cohn, D.L. A Review of Efficacy Studies of 6-Month Short-Course Therapy for Tuberculosis among Patients Infected with Human Immunodeficiency Virus: Differences in Study Outcomes. Clin. Infect. Dis. 2001, 32, 623–632. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, S.; Wei, X.; Dong, Q.; Xu, N.; Li, H.; Zhao, J.; Sun, Q. Global Prevalence, Treatment and Outcome of Tuberculosis and COVID-19 Coinfection: A Systematic Review and Meta-Analysis (from November 2019 to March 2021). BMJ Open 2022, 12, e059396. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, P.; Ratho, R.K.; Sethi, S. Immunopathogenesis in SARS-CoV-2 and Mycobacterium Tuberculosis: The Danger of Overlapping Crises. Front. Pharmacol. 2022, 13, 1065124. [Google Scholar] [CrossRef]
- Zaini, J.; Fadhillah, M.R.; Reisa, T.; Isbaniah, F.; Handayani, R.R.D. The Tuberculosis and COVID-19 Coinfection: A Report of Two Cases at a Tertiary Referral in Indonesia. J. Infect. Dev. Ctries. 2022, 16, 478–483. [Google Scholar] [CrossRef]
- Koupaei, M.; Naimi, A.; Moafi, N.; Mohammadi, P.; Tabatabaei, F.S.; Ghazizadeh, S.; Heidary, M.; Khoshnood, S. Clinical Characteristics, Diagnosis, Treatment, and Mortality Rate of TB/COVID-19 Coinfectetd Patients: A Systematic Review. Front. Med. 2021, 8, 740593. [Google Scholar] [CrossRef]
- Cioboata, R.; Vasile, C.M.; Balteanu, M.A.; Georgescu, D.E.; Toma, C.; Dracea, A.S.; Nicolosu, D. Evaluating Serum Calcium and Magnesium Levels as Predictive Biomarkers for Tuberculosis and COVID-19 Severity: A Romanian Prospective Study. Int. J. Mol. Sci. 2024, 25, 418. [Google Scholar] [CrossRef]
- Song, W.M.; Zhao, J.Y.; Zhang, Q.Y.; Liu, S.Q.; Zhu, X.H.; An, Q.Q.; Xu, T.T.; Li, S.J.; Liu, J.Y.; Tao, N.N.; et al. COVID-19 and Tuberculosis Coinfection: An Overview of Case Reports/Case Series and Meta-Analysis. Front. Med. 2021, 8, 657006. [Google Scholar] [CrossRef]
- Huangfu, P.; Laurence, Y.V.; Alisjahbana, B.; Ugarte-Gil, C.; Riza, A.-L.; Walzl, G.; Ruslami, R.; Moore, D.A.J.; Ioana, M.; McAllister, S.; et al. Point of Care HbA1c Level for Diabetes Mellitus Management and Its Accuracy among Tuberculosis Patients: A Study in Four Countries. Int. J. Tuberc. Lung Dis. 2019, 23, 283–292. [Google Scholar] [CrossRef]
- Meca, A.-D.; Ștefănescu, S.; Bogdan, M.; Turcu-stiolică, A.; Nițu, F.; Matei, M.; Cioboată, R.; Bugă, A.; Pisoschi, C.-G. Crosstalk between Vitamin D Axis, Inflammation and Host Immunity Mechanisms: A Prospective Study. Exp. Ther. Med. 2021, 21, 608. [Google Scholar] [CrossRef]
- Hazra, D.; Siddalingaiah, N.; Gupta, N.; Chawla, K.; Prabhu, A.R.; Datta, D.; Khader, N.; Swaminathan, S.M. COVID-19 and Tuberculosis Coinfection: A Case–Control Study from a Tertiary Care Center in South India. J. Fam. Med. Prim. Care 2023, 12, 3200–3203. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Naqvi, R.A.; Alizadehsani, R.; Hussain, S.; Moqurrab, S.A.; Lee, S.W. An Adaptive Ensemble Deep Learning Framework for Reliable Detection of Pandemic Patients. Comput. Biol. Med. 2024, 168, 107836. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.R.; Abbas, Z.; Zahir, A.; Lee, S.W. Federated Learning in Smart Healthcare: A Comprehensive Review on Privacy, Security, and Predictive Analytics with IoT Integration. Healthcare 2024, 12, 2587. [Google Scholar] [CrossRef] [PubMed]
- Udristoiu, A.L.; Ghenea, A.E.; Udristoiu, S.; Neaga, M.; Zlatian, O.M.; Vasile, C.M.; Popescu, M.; Tieranu, E.N.; Salan, A.I.; Turcu, A.A.; et al. COVID-19 and Artificial Intelligence: An Approach to Forecast the Severity of Diagnosis. Life 2021, 11, 1281. [Google Scholar] [CrossRef]
- Flores-Lovon, K.; Ortiz-Saavedra, B.; Cueva-Chicaña, L.A.; Aperrigue-Lira, S.; Montes-Madariaga, E.S.; Soriano-Moreno, D.R.; Bell, B.; Macedo, R. Immune Responses in COVID-19 and Tuberculosis Coinfection: A Scoping Review. Front. Immunol. 2022, 13, 992743. [Google Scholar] [CrossRef] [PubMed]
- Nuwagira, E.; Mpagama, S.G.; Katusiime, A.; Natamba, B.; Baluku, J.B.; Lai, P.S. Coinfection of COVID-19 and Tuberculosis in Uganda. Am. J. Trop. Med. Hyg. 2023, 108, 1240–1243. [Google Scholar] [CrossRef]
- Murdaca, G.; Paladin, F.; Mangini, G.; Tiso, D.; Gangemi, S. TBC and COVID: An Interplay between Two Infections. Expert. Opin. Drug Saf. 2023, 22, 303–311. [Google Scholar] [CrossRef]
- Parvez, A.; Ahmed, S.; Sattar, A.; Gomes, D.J. Incidence of Bacterial and Fungal Infection Associated with Clinically Diagnosed Pulmonary Tuberculosis Patients. Indian. J. Microbiol. 2002, 42, 323–326. [Google Scholar]
- Garcia, R. Community-Acquired Pneumonia Due to Streptococcus Pneumoniae: When to Consider Coinfection with Active Pulmonary Tuberculosis. Case Rep. Infect. Dis. 2019, 2019, 4618413. [Google Scholar] [CrossRef]
- Meli, H.; Cissoko, Y.; Konaté, I.; Soumaré, M.; Fofana, A.; Dembélé, J.P.; Kaboré, M.; Cissé, M.A.; Zaré, A.; Dao, S. Co-Infection Tuberculose-VIH Compliquée d’une Sur Infection Nosocomiale à Klebsiella pneumoniae: À Propos de 4 Observations Dans Un Service de Maladies Infectieuses Au Mali. Pan Afr. Med. J. 2020, 37, 141. [Google Scholar] [CrossRef]
- Cojocaru, I.; Luculescu, L.; Negoescu, D.; Strâmbu, I. Profile of Patients Admitted in a Pulmonology Ward and Developing Clostridium difficile Enterocolitis. Pneumologia 2019, 68, 31–36. [Google Scholar] [CrossRef]
- Halala, B.K.; Ali, M.M.; Ormago, M.D. Prevalence and Multi-Drug Resistance of Streptococcus Pneumoniae Infection Among Presumptive Tuberculosis Adult Cases at Dilla University Referral Hospital, Dilla, Ethiopia. Infect. Drug Resist. 2022, 15, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Droz, N.; Hsia, Y.; Ellis, S.; Dramowski, A.; Sharland, M.; Basmaci, R. Bacterial Pathogens and Resistance Causing Community Acquired Paediatric Bloodstream Infections in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control 2019, 8, 207. [Google Scholar] [CrossRef]
- Schleicher, G.; Feldman, C. Dual Infection with Streptococcus pneumoniae and Mycobacterium tuberculosis in HIV-Seropositive Patients with Community Acquired Pneumonia. Int. J. Tuberc. Lung Dis. 2003, 7, 1207–1208. [Google Scholar] [PubMed]
- Moore, D.P.; Klugman, K.P.; Madhi, S.A. Role of Streptococcus pneumoniae in Hospitalization for Acute Community-Acquired Pneumonia Associated with Culture-Confirmed Mycobacterium tuberculosis in Children: A Pneumococcal Conjugate Vaccine Probe Study. Pediatr. Infect. Dis. J. 2010, 29, 1099–1104. [Google Scholar] [CrossRef]
- Shin, J.H.; Sung, S.I.; Kim, J.K.; Jung, J.M.; Kim, E.S.; Choi, S.H.; Kim, Y.J.; Ahn, K.M.; Chang, Y.S.; Park, W.S. Retropharyngeal Abscess Coinfected with Staphylococcus aureus and Mycobacterium tuberculosis after Rhinoviral Infection in a 1-Month-Old Infant. Korean J. Pediatr. 2013, 56, 86–89. [Google Scholar] [CrossRef]
- Yadav, S.; Madan Lal Khurana, S. Tuberculosis of the Cervical Vertebrae with Retropharyngeal and Parapharyngeal Abscesses Due to Staphylococcus aureus and Mycobacterium tuberculosis in an Adult: A Report of a Rare Case. Cureus 2024, 16, e61412. [Google Scholar] [CrossRef]
- Hossain, H.T.; Noor, N.; Akhter, S.; Ahmed, M.; Islam, Q.T. Co-Infection by Mycobacterium tuberculosis And Klebsiella pneumoniae in an Elderly Male with Multiple Co-Morbidities: A Rare Entity With High Mortality. Bangladesh J. Med. 2022, 34, 52–57. [Google Scholar] [CrossRef]
- Li, L.; Shao, J.; Tong, C.; Gao, W.; Pan, P.; Qi, C.; Gao, C.; Zhang, Y.; Zhu, Y.; Chen, C. Non-Tuberculous Mycobacteria Enhance the Tryptophan-Kynurenine Pathway to Induce Immunosuppression and Facilitate Pulmonary Colonization. Front. Cell Infect. Microbiol. 2024, 14, 1455605. [Google Scholar] [CrossRef]
- Bir, R.; Ranjan, R.; Gunasekaran, J.; Chatterjee, K.; Karteeka, D.; Rai, A.; Gupta, S.; Karlapudi, P.; Joshi, I.; Gupta, R.M. Prevalence of Co-Infection of Culture-Proven Bacterial Pathogens in Microbiologically Confirmed Pulmonary Tuberculosis Patients From a Tertiary Care Center. Cureus 2024, 16, e66482. [Google Scholar] [CrossRef] [PubMed]
- Regmi, R.S.; Khadka, S.; Sapkota, S.; Adhikari, S.; Dhakal, K.K.; Dhakal, B.; Lamsal, B.; Kafle, S.C. Bacterial Etiology of Sputum from Tuberculosis Suspected Patients and Antibiogram of the Isolates. BMC Res. Notes 2020, 13, 520. [Google Scholar] [CrossRef]
- Kebede, W.; Abebe, G.; De Boeck, I.; Gudina, E.K.; Cauwenberghs, E.; Lebeer, S.; Van Rie, A. Bacterial Pathogens in Xpert MTB/RIF Ultra-Negative Sputum Samples of Patients with Presumptive Tuberculosis in a High TB Burden Setting: A 16S RRNA Analysis. Microbiol. Spectr. 2024, 12, e0293123. [Google Scholar] [CrossRef] [PubMed]
- Obuch-Woszczatyński, P.; Dubiel, G.; Harmanus, C.; Kuijper, E.; Duda, U.; Wultańska, D.; Van Belkum, A.; Pituch, H. Emergence of Clostridium difficile Infection in Tuberculosis Patients Due to a Highly Rifampicin-Resistant PCR Ribotype 046 Clone in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1027–1030. [Google Scholar] [CrossRef]
- Chirtsova, M.V.; Khvorostov, A.A.; Tursunova, N.V. Diagnosis of Hemostatic Disorders in Patient with Sepsis Caused by a Triple Infection of M. Tuberculosis, P. Aeruginosa, Kl. Pneumoniae. Tuberc. Lung Dis. 2023, 101, 71–79. [Google Scholar] [CrossRef]
- von Baum, H.; Welte, T.; Marre, R.; Suttorp, N.; Ewig, S.; CAPNETZ Study Group. Community-acquired pneumonia through Enterobacteriaceae and Pseudomonas aeruginosa: Diagnosis, incidence and predictors. Eur. Respir. J. 2010, 35, 598–615. [Google Scholar] [CrossRef]
- Fend, R.; Kolk, A.H.J.; Bessant, C.; Buijtels, P.; Klatser, P.R.; Woodman, A.C. Prospects for Clinical Application of Electronic-Nose Technology to Early Detection of Mycobacterium tuberculosis in Culture and Sputum. J. Clin. Microbiol. 2006, 44, 2039–2045. [Google Scholar] [CrossRef]
- Landeta, C.; McPartland, L.; Tran, N.Q.; Meehan, B.M.; Zhang, Y.; Tanweer, Z.; Wakabayashi, S.; Rock, J.; Kim, T.; Balasubramanian, D.; et al. Inhibition of Pseudomonas aeruginosa and Mycobacterium tuberculosis Disulfide Bond Forming Enzymes. Mol. Microbiol. 2019, 111, 918–937. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Maccari, G.; Esin, S.; Batoni, G. Analogs of the Frog-Skin Antimicrobial Peptide Temporin 1Tb Exhibit a Wider Spectrum of Activity and a Stronger Antibiofilm Potential as Compared to the Parental Peptide. Front. Chem. 2017, 5, 24. [Google Scholar] [CrossRef]
- Lee, Y.M.; Huh, K.C.; Yoon, S.M.; Jang, B.I.; Shin, J.E.; Koo, H.S.; Jung, Y.; Kim, S.H.; Moon, H.S.; Lee, S.W. Incidence and Clinical Outcomes of Clostridium difficile Infection after Treatment with Tuberculosis Medication. Gut Liver 2016, 10, 250–254. [Google Scholar] [CrossRef]
- Crobach, M.J.T.; Planche, T.; Eckert, C.; Barbut, F.; Terveer, E.M.; Dekkers, O.M.; Wilcox, M.H.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Diagnostic Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2016, 22, S63–S81. [Google Scholar] [CrossRef]
- Kullin, B.R.; Reid, S.; Abratt, V. Clostridium difficile in Patients Attending Tuberculosis Hospitals in Cape Town, South Africa, 2014–2015. Afr. J. Lab. Med. 2018, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Rahmat Ullah, S.; Majid, M.; Rashid, M.I.; Mehmood, K.; Andleeb, S. Immunoinformatics Driven Prediction of Multiepitopic Vaccine Against Klebsiella pneumoniae and Mycobacterium tuberculosis Coinfection and Its Validation via In Silico Expression. Int. J. Pept. Res. Ther. 2021, 27, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Foreman, T.W.; Mehra, S.; LoBato, D.N.; Malek, A.; Alvarez, X.; Golden, N.A.; Bucşan, A.N.; Didier, P.J.; Doyle-Meyers, L.A.; Russell-Lodrigue, K.E.; et al. CD4+ T-Cell–Independent Mechanisms Suppress Reactivation of Latent Tuberculosis in a Macaque Model of HIV Coinfection. Proc. Natl. Acad. Sci. USA 2016, 113, E5636–E5644. [Google Scholar] [CrossRef]
- Soeroso, N.N.; Siahaan, L.; Khairunnisa, S.; Anggriani, R.A.H.; Aida, A.; Eyanoer, P.C.; Daulay, E.R.; Burhan, E.; Rozaliyani, A.; Ronny, R.; et al. The Association of Chronic Pulmonary Aspergillosis and Chronic Pulmonary Histoplasmosis with MDR-TB Patients in Indonesia. J. Fungi 2024, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Bohra, G.K.; Bohra, G.K.; Shankar, D.; Kumar, D.; Midha, N.K.; Jain, V.; Sharma, S.; N, S.; Garg, M.K.; Singh, K.; et al. P144 The Uncommon Meets the Common: Invasive Aspergillosis and Tuberculosis Co-Infection in Non-Neutropenic Patients—Arare Association. Med. Mycol. 2022, 60, 94. [Google Scholar] [CrossRef]
- Hosseini, M.; Shakerimoghaddam, A.; Ghazalibina, M.; Khaledi, A. Aspergillus Coinfection among Patients with Pulmonary Tuberculosis in Asia and Africa Countries; A Systematic Review and Meta-Analysis of Cross-Sectional Studies. Microb. Pathog. 2020, 141, 104018. [Google Scholar] [CrossRef]
- Hadadi-Fishani, M.; Shakerimoghaddam, A.; Khaledi, A. Candida Coinfection among Patients with Pulmonary Tuberculosis in Asia and Africa; A Systematic Review and Meta-Analysis of Cross-Sectional Studies. Microb. Pathog. 2019, 139, 103898. [Google Scholar] [CrossRef]
- Ghazalibina, M.; Shakerimoghaddam, A.; Khaledi, A. Candida Coinfection among Patients with Pulmonary Tuberculosis in the World; A Systematic Review and Meta-Analysis of Cross-Sectional Studies. Microb. Pathog. 2019, 139, 103898. [Google Scholar] [CrossRef]
- Iamwat, W.; Cheawcharnprapan, K.; Yenjabog, P.; Lertkovit, O.; Isaranimitkul, D.; Kanjanaphan, T. Aspergillosis and Pulmonary Tuberculosis Co-Infection in a 9-Year-Old with B-Cell Acute Lymphoblastic Leukemia. Oxf. Med. Case Rep. 2023, 2023, 295–297. [Google Scholar] [CrossRef]
- Murugavel, M.; Howlader, A.; Nagarajan, P.; Kuthalaramalingam, S. Prevalence and Antifungal Susceptibility of Candida Infections in Patients with Pulmonary Tuberculosis: A Cross-Sectional Study from the Union Territory of Puducherry, India. J. Clin. Diagn. Res. 2024, 18, DC10–DC15. [Google Scholar] [CrossRef]
- Parsons, L.M.; Somoskövi, Á.; Gutierrez, C.; Lee, E.; Paramasivan, C.N.; Abimiku, A.; Spector, S.; Roscigno, G.; Nkengasong, J. Laboratory Diagnosis of Tuberculosis in Resource-Poor Countries: Challenges and Opportunities. Clin. Microbiol. Rev. 2011, 24, 314–350. [Google Scholar] [CrossRef] [PubMed]
- Dube, P.; Saroa, R.; Palta, S. Coinfections in Intensive Care Unit with Pulmonary Tuberculosis and Mucormycosis: A Clinical Dilemma. Indian J. Crit. Care Med. 2016, 20, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Gao, Y.C.; Zhang, Y.; Tang, Z.H.; Yu, Y.S.; Zang, G.Q. Tuberculosis Infection Might Increase the Risk of Invasive Candidiasis in an Immunocompetent Patient. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 273–275. [Google Scholar] [CrossRef][Green Version]
- Craciun, O.M.; Torres, M.D.R.; Llanes, A.B.; Romay-Barja, M. Tuberculosis Knowledge, Attitudes, and Practice in Middle- and Low-Income Countries: A Systematic Review. J. Trop. Med. 2023, 2023, 1014666. [Google Scholar] [CrossRef]
- Shibabaw, A.; Tilahun, M.; Gedefie, A.; Sahle, Z.; Belete, M.A.; Ebrahim, H.; Debash, H.; Sharew, B. Magnitude and Predisposing Factors of Intestinal Parasitosis and Tuberculosis Coinfection at Five Health Institutions in Southern Ethiopia: A Cross-sectional Study. Health Sci. Rep. 2023, 6, e1569. [Google Scholar] [CrossRef]
- Alemu, A.; Bitew, Z.W.; Worku, T. Intestinal Parasites Co-Infection among Tuberculosis Patients in Ethiopia: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2020, 20, 510. [Google Scholar] [CrossRef]
- Baluku, J.B.; Nakazibwe, B.; Wasswa, A.; Naloka, J.; Ntambi, S.; Waiswa, D.; Okwir, M.; Nabwana, M.; Bongomin, F.; Katuramu, R.; et al. Prevalence of Intestinal Helminth Coinfection in Drug-Resistant Tuberculosis in Uganda. Open Forum Infect. Dis. 2022, 9, ofac541. [Google Scholar] [CrossRef]
- Wong, W.K.; Mohd-Nor, N.; Noordin, R.; Foo, P.C.; Mohamed, Z.; Haq, J.A.; Acosta, A.; Sarmiento, M.E.; Subramaniam, P.; Dony, J.F.; et al. Parasitic Infections in Malaysian Aborigines with Pulmonary Tuberculosis: A Comparative Cross-Sectional Study. Parasitol. Res. 2019, 118, 2635–2642. [Google Scholar] [CrossRef]
- Djibougou, D.A.; Mensah, G.I.; Cissé, M.; Inoussa, T.; Sawadogo, L.T.; Combary, A.; Sanou, A.; Bonfoh, B.; Addo, K.K.; Belem, A.M.G.; et al. Intestinal Protozoa, Helminth Infection, and Associated Factors among Tuberculosis Patients and Nontuberculosis Persons in Bobo-Dioulasso City, Burkina Faso. Am. J. Trop. Med. Hyg. 2024, 111, 1265–1272. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Helminth-Tuberculosis Co-Infection: An Immunologic Perspective. Trends Immunol. 2016, 37, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Monin, L.; Griffiths, K.L.; Lam, W.Y.; Gopal, R.; Kang, D.D.; Ahmed, M.; Rajamanickam, A.; Cruz-Lagunas, A.; Zúñiga, J.; Babu, S.; et al. Helminth-Induced Arginase-1 Exacerbates Lung Inflammation and Disease Severity in Tuberculosis. J. Clin. Investig. 2015, 125, 4699–4713. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Zhou, X.N. Co-Infection of Tuberculosis and Parasitic Diseases in Humans: A Systematic Review. Parasit. Vectors 2013, 6, 79. [Google Scholar] [CrossRef]
- Lello, J.; Gassó, D.; Gonçalves, P.; Risco, D.; García, W.L.; Segalés, J.; Garrido-Amaro, C.; Mentaberre, G.; Torres-Blas, I.; Velarde, R.; et al. Annual Short-Burst Mass Anthelmintic Administration Reduces Tuberculosis Severity but Not Prevalence in a Wildlife Reservoir. Front. Ecol. Evol. 2023, 11, 1186295. [Google Scholar] [CrossRef]
- Lambura, A.G.; Mwanga, G.G.; Luboobi, L.; Kuznetsov, D. Modeling the Effects of Helminth Infection on the Transmission Dynamics of Mycobacterium Tuberculosis under Optimal Control Strategies. Comput. Math. Methods Med. 2020, 2020, 8869377. [Google Scholar] [CrossRef]
- Djibougou, D.A.; Mensah, G.I.; Kaboré, A.; Toé, I.; Sawadogo, L.T.; Lompo, P.F.; Kone, A.M.M.; Hien, H.; Meda, C.Z.; Combary, A.; et al. Immunological and Haematological Relevance of Helminths and Mycobacterium Tuberculosis Complex Coinfection among Newly Diagnosed Pulmonary Tuberculosis Patients from Bobo-Dioulasso, Burkina Faso. Biomedicines 2024, 12, 1472. [Google Scholar] [CrossRef]
- Steel, L.B.; Narasimhan, P.B.; Chaudhari, M.; Dauphinais, M.R.; Huang, S.; Beall, K.; Carwile, M.E.; Cintron, C.; Du, X.; Heysell, S.K.; et al. Intestinal Parasitic Infections May Be Overlooked Drivers of the Tuberculosis Pandemic. Am. J. Trop. Med. Hyg. 2024, 111, 719–723. [Google Scholar] [CrossRef]
- Tosepu, R.; Sani, A.; Effendy, D.S.; Ahmad, L.O.A.I. The Association between Climate Variables and Tuberculosis in Kolaka District, Southeast Sulawesi Province, Indonesia, 2013–2020: A Bayesian Autoregressive Model. F1000Research 2023, 12, 1507. [Google Scholar] [CrossRef]
- Maharjan, B.; Gopali, R.S.; Zhang, Y. A Scoping Review on Climate Change and Tuberculosis. Int. J. Biometeorol. 2021, 65, 1579–1595. [Google Scholar] [CrossRef]
- Jacobson, K.B.; Moll, A.P.; Friedland, G.H.; Shenoi, S.V. Successful Tuberculosis Treatment Outcomes among HIV/TB Coinfected Patients Down-Referred from a District Hospital to Primary Health Clinics in Rural South Africa. PLoS ONE 2015, 10, e0127024. [Google Scholar] [CrossRef]
- Silva, C.J.; Torres, D.F.M. A TB-HIV/AIDS Coinfection Model and Optimal Control Treatment. Discret. Contin. Dyn. Syst. 2015, 35, 4639–4663. [Google Scholar] [CrossRef]
- Denysiuk, R.; Silva, C.J.; Torres, D.F.M. Multiobjective Optimization to a TB-HIV/AIDS Coinfection Optimal Control Problem. Comput. Appl. Math. 2017, 37, 2112–2128. [Google Scholar] [CrossRef]
- White, A.D.; Sibley, L.; Gullick, J.; Sarfas, C.; Clark, S.; Fagrouch, Z.; Verschoor, E.; Salguero, F.J.; Dennis, M.; Sharpe, S. TB and SIV Coinfection; a Model for Evaluating Vaccine Strategies against TB Reactivation in Asian Origin Cynomolgus Macaques: A Pilot Study Using BCG Vaccination. Vaccines 2021, 9, 945. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioboata, R.; Balteanu, M.A.; Osman, A.; Vlasceanu, S.G.; Zlatian, O.M.; Mitroi, D.M.; Catana, O.M.; Socaci, A.; Tieranu, E.-N. Coinfections in Tuberculosis in Low- and Middle-Income Countries: Epidemiology, Clinical Implications, Diagnostic Challenges, and Management Strategies—A Narrative Review. J. Clin. Med. 2025, 14, 2154. https://doi.org/10.3390/jcm14072154
Cioboata R, Balteanu MA, Osman A, Vlasceanu SG, Zlatian OM, Mitroi DM, Catana OM, Socaci A, Tieranu E-N. Coinfections in Tuberculosis in Low- and Middle-Income Countries: Epidemiology, Clinical Implications, Diagnostic Challenges, and Management Strategies—A Narrative Review. Journal of Clinical Medicine. 2025; 14(7):2154. https://doi.org/10.3390/jcm14072154
Chicago/Turabian StyleCioboata, Ramona, Mara Amalia Balteanu, Andrei Osman, Silviu Gabriel Vlasceanu, Ovidiu Mircea Zlatian, Denisa Maria Mitroi, Oana Maria Catana, Adriana Socaci, and Eugen-Nicolae Tieranu. 2025. "Coinfections in Tuberculosis in Low- and Middle-Income Countries: Epidemiology, Clinical Implications, Diagnostic Challenges, and Management Strategies—A Narrative Review" Journal of Clinical Medicine 14, no. 7: 2154. https://doi.org/10.3390/jcm14072154
APA StyleCioboata, R., Balteanu, M. A., Osman, A., Vlasceanu, S. G., Zlatian, O. M., Mitroi, D. M., Catana, O. M., Socaci, A., & Tieranu, E.-N. (2025). Coinfections in Tuberculosis in Low- and Middle-Income Countries: Epidemiology, Clinical Implications, Diagnostic Challenges, and Management Strategies—A Narrative Review. Journal of Clinical Medicine, 14(7), 2154. https://doi.org/10.3390/jcm14072154







