Gastroesophageal Neuroendocrine Tumors: Outcomes and Management
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Gastric NETs
3.2. Esophageal NETs
3.3. Small Intestinal NETs
3.4. Colonic NETs
3.5. Rectal NETs
4. Discussion
4.1. Gastric NETs
4.2. Esophageal NETs
4.3. Small Intestinal NETs
4.4. Colonic NETs
4.5. Rectal NETs
5. Conclusions
6. Take-Home Points
- A higher incidence of GI NETs is observed in the VA hospital system.
- GI NETs are a rare type of GI tumor, and improved endoscopic detection has led to an increase in GI NET diagnoses.
- GI neuroendocrine carcinomas have a median survival of 4 to 15 months depending on the primary site and disease stage.
- Surveillance guidelines following endoscopic or surgical resection depend on the stage of disease at the time of diagnosis.
- Long-term surveillance (up to 10 years) is imperative in patients with metastatic disease at the time of diagnosis as the majority of patients have recurrent disease.
- Immunotherapy, combined with targeted therapy, could represent a new treatment option for E-NECs, especially after surgical resection.
7. Areas of Future Research
- Currently, adjuvant chemotherapy is only reserved for poorly differentiated GI NETs.
- The survival outcomes of patients with locally advanced E-NECs treated with definitive chemoradiation therapy need to be studied further.
- Further studies are needed to establish systemic treatment strategies, especially the role of immunotherapy and targeted therapies such as PRRT in locally advanced or metastatic disease.
- The possible factors (i.e., exposure to chemical carcinogenic substances and occupational exposures) associated with the higher incidence of NETs in veteran populations need to be further explored.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GEP-NEC | Gastroenteropancreatic Neuroendocrine Carcinomas |
| NET | Neuroendocrine tumor |
| NEC | Neuroendocrine carcinoma |
| E-NEC | Esophageal neuroendocrine carcinoma |
| G-NET | Gastric neuroendocrine tumor |
| TACE | Transarterial chemoembolization |
| ICI | Immune checkpoint inhibitor |
| SCCE | Small-cell carcinoma of the esophagus |
| SCLC | Small-cell lung cancer |
| CE-RT | Platinum plus etoposide with radiotherapy |
| CF-RT | Cisplatin plus 5-fluorouracil with radiotherapy |
| PRRT | Peptide receptor radionuclide therapy |
| C-NET | Colonic neuroendocrine tumor |
| R-NET | Rectal neuroendocrine tumor |
| EMR | Endoscopic mucosal resection |
| ESD | Endoscopic submucosal dissection |
| r-EUS | Rectal endoscopic ultrasound |
| LAR | Low anterior resection |
| TME | Total mesorectal excision |
References
- Pape, U.F.; Böhmig, M.; Berndt, U.; Tiling, N.; Wiedenmann, B.; Plöckinger, U. Survival and clinical outcome of patients with neuroendocrine tumors of the gastroenteropancreatic tract in a german referral center. Ann. New York Acad. Sci. 2004, 1014, 222–233. [Google Scholar] [CrossRef]
- Ilett, E.E.; Langer, S.W.; Olsen, I.H.; Federspiel, B.; Kjær, A.; Knigge, U. Neuroendocrine Carcinomas of the Gastroenteropancreatic System: A Comprehensive Review. Diagnostics 2015, 5, 119–176. [Google Scholar] [CrossRef]
- Sok, C.; Ajay, P.S.; Tsagkalidis, V.; Kooby, D.A.; Shah, M.M. Management of Gastric Neuroendocrine Tumors: A Review. Ann. Surg. Oncol. 2024, 31, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wei, L.; Liu, W.; Chen, S.; Guan, M.; Zhang, Y.; Guo, Z.; Liu, R.; Xie, P. Trends in incidence and survival in patients with gastrointestinal neuroendocrine tumors: A SEER database analysis, 1977-2016. Front. Oncol. 2023, 13, 1079575. [Google Scholar] [CrossRef]
- Mastracci, L.; Rindi, G.; Grillo, F.; Solcia, E.; Campora, M.; Fassan, M.; Parente, P.; Vanoli, A.; La Rosa, S. Neuroendocrine neoplasms of the esophagus and stomach. Pathologica 2021, 113, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Egashira, A.; Morita, M.; Kumagai, R.; Taguchi, K.I.; Ueda, M.; Yamaguchi, S.; Yamamoto, M.; Minami, K.; Ikeda, Y.; Toh, Y. Neuroendocrine carcinoma of the esophagus: Clinicopathological and immunohistochemical features of 14 cases. PLoS ONE 2017, 12, e0173501. [Google Scholar] [CrossRef]
- Köseoğlu, H.; Duzenli, T.; Sezikli, M. Gastric neuroendocrine neoplasms: A review. World J. Clin. Cases 2021, 9, 7973–7985. [Google Scholar] [CrossRef]
- Noh, J.H.; Kim, D.H.; Yoon, H.; Hsing, L.C.; Na, H.K.; Ahn, J.Y.; Lee, J.H.; Jung, K.W.; Choi, K.D.; Song, H.J.; et al. Clinical Outcomes of Endoscopic Treatment for Type 1 Gastric Neuroendocrine Tumor. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2021, 25, 2495–2502. [Google Scholar] [CrossRef]
- Vescio, R.A.; Connors, K.M.; Bordin, G.M.; Robb, J.A.; Youngkin, T.; Umbreit, J.N.; Hoffman, R.M. The distinction of small cell and non-small cell lung cancer by growth in native-state histoculture. Cancer Res. 1990, 50, 6095–6099. [Google Scholar]
- Son, C.; Havlichek, D.; Leya, J.; Kalapala, J.; Banerjee, P. An Unusual Cause of Food Impaction: Neuroendocrine Tumor. In Proceedings of the ACG 2024 Annual Scientific Meeting Abstracts, American College of Gastroenterology, North Bethesda, MD, USA, 28 October 2024. [Google Scholar]
- Roberto, G.A.; Rodrigues CM, B.; Peixoto, R.D.; Younes, R.N. Gastric neuroendocrine tumor: A practical literature review. World J. Gastrointest. Oncol. 2020, 12, 850–856. [Google Scholar] [CrossRef]
- Pan, W.X.; Zhang, X.M.; Hao, S.L.; Han, W. Progress in immunotherapy for neuroendocrine neoplasm of the digestive system. World J. Gastroenterol. 2023, 29, 4174–4185. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.L.; O’Connell, J.; Muldoon, C.; Swan, N.; Reynolds, J.V.; Ravi, N.; Geoghegan, J.; Conlon, K.C.; O’Shea, D.; O’Toole, D. Selective Resection of Type 1 Gastric Neuroendocrine Neoplasms and the Risk of Progression in an Endoscopic Surveillance Programme. Dig. Surg. 2021, 38, 38–45. [Google Scholar] [CrossRef]
- Daskalakis, K.; Tsoli, M.; Karapanagioti, A.; Chrysochoou, M.; Thomas, D.; Sougioultzis, S.; Karoumpalis, I.; Kaltsas, G.A.; Alexandraki, K.I. Recurrence and metastatic potential in Type 1 gastric neuroendocrine neoplasms. Clin. Endocrinol. 2019, 91, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Min, B.H.; Hong, M.; Lee, J.H.; Rhee, P.L.; Sohn, T.S.; Kim, S.; Kim, K.M.; Kim, J.J. Clinicopathological features and outcome of type 3 gastric neuroendocrine tumours. Br. J. Surg. 2018, 105, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Lim, Y.J.; Park, S.J.; Jang, B.I.; Choi, S.R.; Kim, J.K.; Kim, Y.T.; Cho, J.Y.; Yang, C.H.; Chun, H.J.; et al. The clinical features and treatment modality of esophageal neuroendocrine tumors: A multicenter study in Korea. BMC Cancer 2014, 14, 569. [Google Scholar] [CrossRef]
- Cai, W.; Ge, W.; Yuan, Y.; Ding, K.; Tan, Y.; Wu, D.; Hu, H. A 10-year Population-based Study of the Differences between NECs and Carcinomas of the Esophagus in Terms of Clinicopathology and Survival. J. Cancer 2019, 10, 1520–1527. [Google Scholar] [CrossRef]
- Giannetta, E.; Guarnotta, V.; Rota, F.; de Cicco, F.; Grillo, F.; Colao, A.; Faggiano, A. NIKE A rare rarity: Neuroendocrine tumor of the esophagus. Crit. Rev. Oncol./Hematol. 2019, 137, 92–107. [Google Scholar] [CrossRef]
- Swied, M.Y.; Turk, Y.A.; Jegadeesan, R. A Poorly Differentiated Esophageal Neuroendocrine Carcinoma With Brain Metastasis. ACG Case Rep. J. 2023, 10, e01156. [Google Scholar] [CrossRef]
- Cho, M.Y.; Kim, J.M.; Sohn, J.H.; Kim, M.J.; Kim, K.M.; Kim, W.H.; Kim, H.; Kook, M.C.; Park, D.Y.; Gastrointestinal Pathology Study Group of Korean Society of Pathologists; et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000–2009: Multicenter Study. Cancer Res. Treat. 2012, 44, 157–165. [Google Scholar] [CrossRef]
- Maru, D.M.; Khurana, H.; Rashid, A.; Correa, A.M.; Anandasabapathy, S.; Krishnan, S.; Komaki, R.; Ajani, J.A.; Swisher, S.G.; Hofstetter, W.L. Retrospective study of clinicopathologic features and prognosis of high-grade neuroendocrine carcinoma of the esophagus. Am. J. Surg. Pathol. 2008, 32, 1404–1411. [Google Scholar] [CrossRef]
- Honma, Y.; Nagashima, K.; Hirano, H.; Shoji, H.; Iwasa, S.; Takashima, A.; Okita, N.; Kato, K.; Boku, N.; Murakami, N.; et al. Clinical outcomes of locally advanced esophageal neuroendocrine carcinoma treated with chemoradiotherapy. Cancer Med. 2020, 9, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Raja, S.; Rice, T.W.; Rajeswaran, J.; Zhong, J.; Blackstone, E.H. Esophageal small-cell cancer: Study of a rare disease. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2013, 26, 690–695. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Liang, H.; Liu, W.; Wang, H.; Li, T. Late-stage esophageal neuroendocrine carcinoma in a patient treated with tislelizumab combined with anlotinib: A case report. J. Int. Med. Res. 2023, 51, 3000605231187942. [Google Scholar] [CrossRef]
- Di Franco, M.; Zanoni, L.; Fortunati, E.; Fanti, S.; Ambrosini, V. Radionuclide Theranostics in Neuroendocrine Neoplasms: An Update. Curr. Oncol. Rep. 2024, 26, 538–550. [Google Scholar] [CrossRef]
- Kaemmerer, D.; Twrznik, M.; Kulkarni, H.R.; Hörsch, D.; Sehner, S.; Baum, R.P.; Hommann, M.; Center for Neuroendocrine Tumors, Bad Berka — ENETS Center of Excellence. Prior Resection of the Primary Tumor Prolongs Survival After Peptide Receptor Radionuclide Therapy of Advanced Neuroendocrine Neoplasms. Ann. Surg. 2021, 274, e45–e53. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.; Abou Azar, S.; Gujarathi, R.; Nordgren, R.; Vaghaiwalla, T.; Millis, J.M.; Feinberg, N.; Liao, C.Y.; Keutgen, X.M. Surgery enhances the effectiveness of peptide receptor radionuclide therapy in metastatic gastroenteropancreatic neuroendocrine tumors. Surgery 2025, 177, 108834. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens LA, A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Lamarca, A.; Bartsch, D.K.; Caplin, M.; Kos-Kudla, B.; Kjaer, A.; Partelli, S.; Rinke, A.; Janson, E.T.; Thirlwell, C.; van Velthuysen, M.F.; et al. European Neuroendocrine Tumor Society (ENETS) 2024 guidance paper for the management of well-differentiated small intestine neuroendocrine tumours. J. Neuroendocrinol. 2024, 36, e13423. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.G.; Sherman, S.K.; Howe, J.R. Small Bowel Neuroendocrine Tumors. Curr. Probl. Surg. 2020, 57, 100823. [Google Scholar] [CrossRef]
- Hellman, P.; Lundström, T.; Ohrvall, U.; Eriksson, B.; Skogseid, B.; Oberg, K.; Tiensuu Janson, E.; Akerström, G. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J. Surg. 2002, 26, 991–997. [Google Scholar] [CrossRef]
- Mayo, S.C.; de Jong, M.C.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Gamblin, T.C.; Celinksi, S.A.; Kooby, D.A.; Staley, C.A.; Stokes, J.B.; et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann. Surg. Oncol. 2010, 17, 3129–3136. [Google Scholar] [CrossRef]
- Fan, S.T.; Le Treut, Y.P.; Mazzaferro, V.; Burroughs, A.K.; Olausson, M.; Breitenstein, S.; Frilling, A. Liver transplantation for neuroendocrine tumour liver metastases. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2015, 17, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.T.; Howe, J.R. Management of Small Bowel Neuroendocrine Tumors. J. Oncol. Pract. 2018, 14, 471–482. [Google Scholar] [CrossRef]
- Volante, M.; Grillo, F.; Massa, F.; Maletta, F.; Mastracci, L.; Campora, M.; Ferro, J.; Vanoli, A.; Papotti, M. Neuroendocrine neoplasms of the appendix, colon and rectum. Pathologica 2021, 113, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Wu, S.; Hamid, M.; Mahipal, A.; Cjakrabarti, S.; Bajor, D.; Selfridge, J.E.; Asa, S.L. Management of Appendix Neuroendocrine Neoplasms: Insights on the Current Guidelines. Cancers 2022, 15, 295. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan Nair Sobha, R.; Jensen, C.T.; Waters, R.; Calimano-Ramirez, L.F.; Virarkar, M.K. Appendiceal Neuroendocrine Neoplasms: A Comprehensive Review. J. Comput. Assist. Tomogr. 2024, 48, 545–562. [Google Scholar] [CrossRef]
- Gallo, C.; Rossi, R.E.; Cavalcoli, F.; Barbaro, F.; Boškoski, I.; Invernizzi, P.; Massironi, S. Rectal neuroendocrine tumors: Current advances in management, treatment, and surveillance. World J. Gastroenterol. 2022, 28, 1123–1138. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, H.; Xie, L.; Li, J.; Cao, W.; Fu, W. Endoscopic resection therapies for rectal neuroendocrine tumors: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2014, 29, 259–268. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Petersen, L.; Hampel, H.; Richardson, P.; Cooper, G. The use of screening colonoscopy for patients cared for by the Department of Veterans Affairs. Arch. Intern. Med. 2006, 166, 2202–2208. [Google Scholar] [CrossRef]
- Williams, S.B.; Janes, J.L.; Howard, L.E.; Yang, R.; De Hoedt, A.M.; Baillargeon, J.G.; Kuo, Y.F.; Tyler, D.S.; Terris, M.K.; Freedland, S.J. Exposure to Agent Orange and Risk of Bladder Cancer Among US Veterans. JAMA Netw. Open 2023, 6, e2320593. [Google Scholar] [CrossRef]
- Balmadrid, B.L.; Thomas, C.M.; Coffman, C.J.; Liddle, R.A.; Fisher, D.A. Factors associated with survival of veterans with gastrointestinal neuroendocrine tumors. J. Cancer Epidemiol. 2012, 2012, 986708. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens JH, M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef] [PubMed]
- Popa Ilie, I.R.; Georgescu, C.E. Immunotherapy in Gastroenteropancreatic Neuroendocrine Neoplasia. Neuroendocrinology 2023, 113, 262–278. [Google Scholar] [CrossRef] [PubMed]
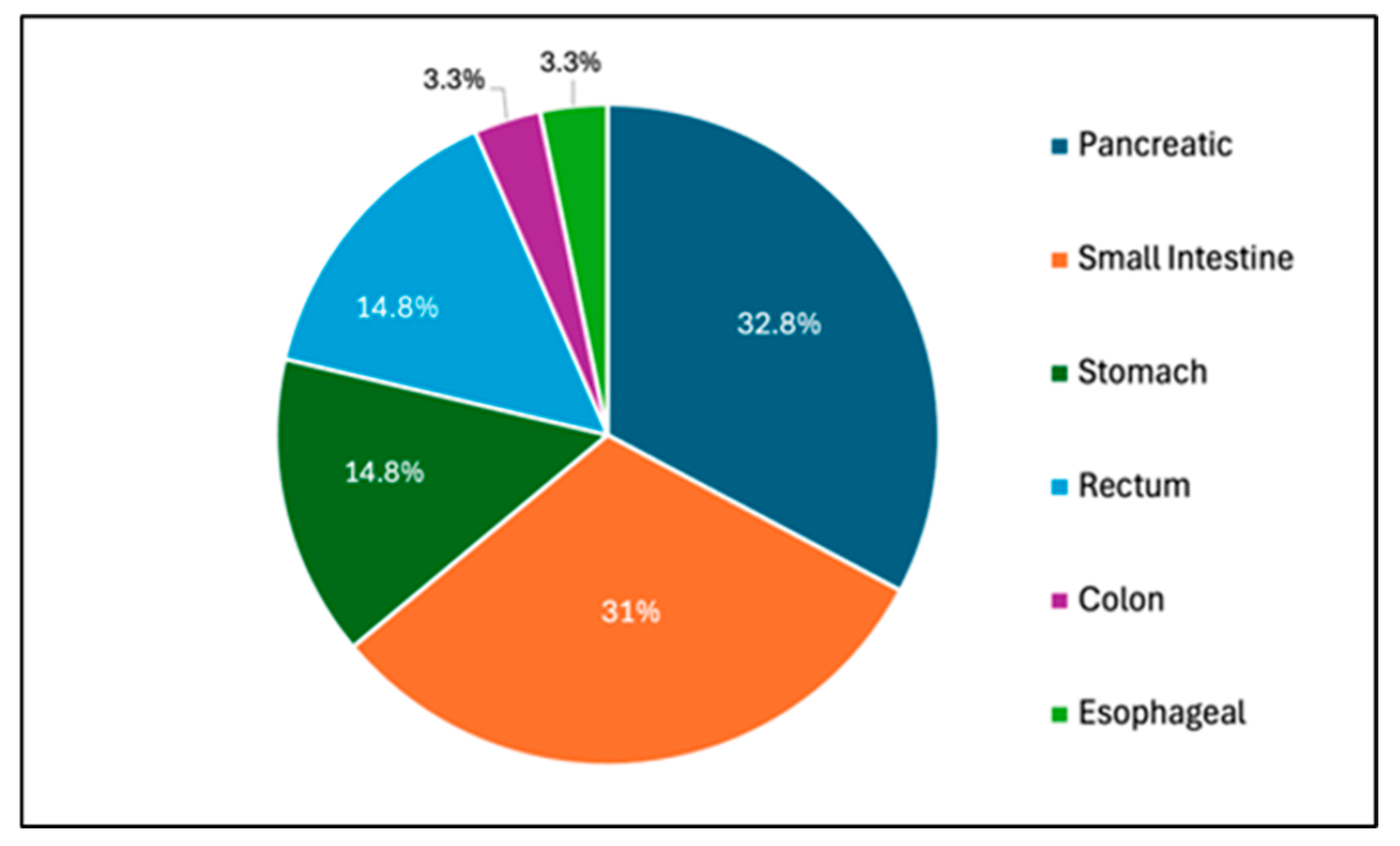
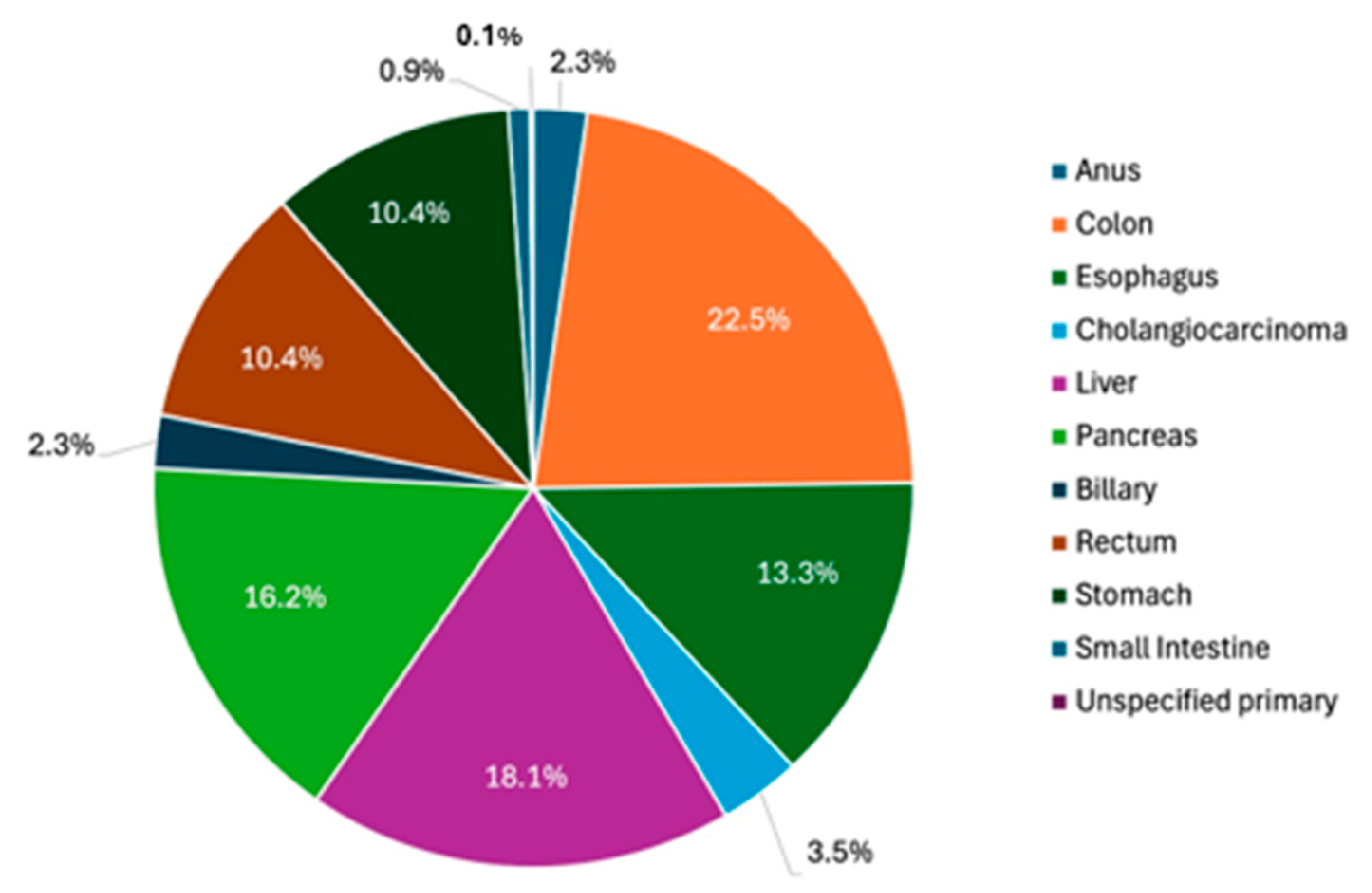

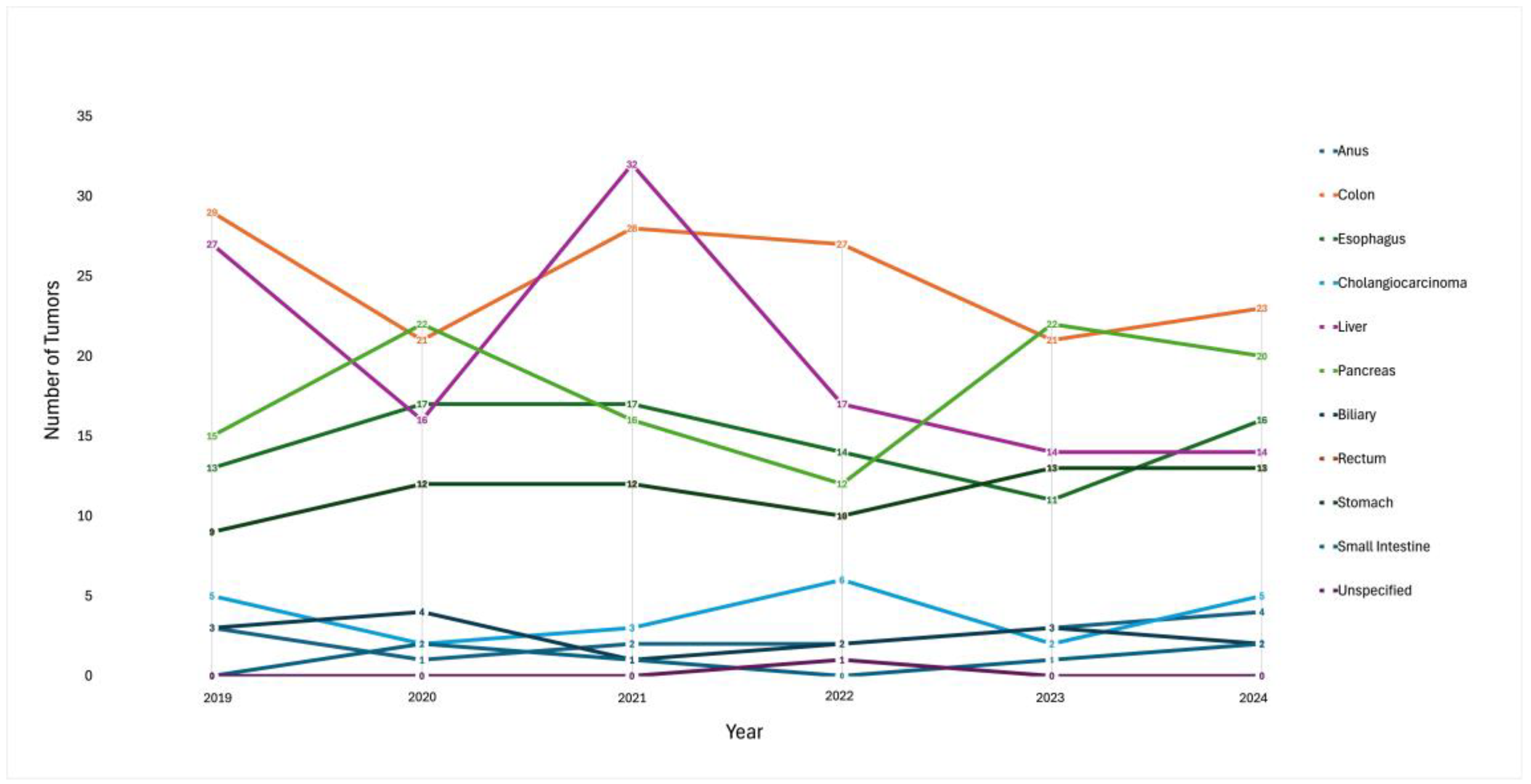
| Gastric NET Subtype | # |
|---|---|
| Type 1 | 8 |
| Type 2 | 0 |
| Type 3 | 0 |
| Unspecified | 1 |
| 9 Total |
| Number | Location | Age | Gender | Race | Path/Grade | Military History | Endoscopic or Surgical Resection (Y/N) | Systemic Therapy (Chemo/RT/Immuno) (Y/N) | Medical Treatment | Surveillance |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Stomach | 77 | M | White | Well-diff, no grade | Vietnam War | Y, polypectomy | N | None | Q4 months then q2 years |
| 2 | Stomach | 69 | M | Black | Well-diff, no grade | Vietnam War | Y, polypectomy | N | None | Annual EGD |
| 3 | Stomach | 52 | M | Black | Well-diff, grade 2 | Persian Gulf War | Y, polypectomy | N | None | Annual EGD |
| 4 | Stomach | 53 | F | Black | Well-diff, grade 1 | Persian Gulf War | Y, polypectomy | N | None | Annual EGD |
| 5 | Stomach | 56 | M | Hispanic | Well-diff, grade 2 | Persian Gulf War | Y, polypectomy | N | None | Annual EGD |
| 6 | Stomach | 82 | M | Black | Well-to-mod diff, no grade concurrent gastric adenocarcinoma | Vietnam War | Y, Robot-assisted distal gastrectomy with roux-en-y | Y, FOLFOX | None | 6-month CT AP |
| 7 | Stomach | 69 | M | Black | Well-diff, grade 2 | Vietnam War | Y, polypectomy | N | None | Annual EGD |
| 8 | Stomach | 63 | M | White | Well-diff, grade 2 | Persian Gulf War | N | N | Octreotide, Lanreotide, Lutathera | PET CT q6 months |
| 9 | Stomach | 55 | M | Hispanic | Well-diff, grade 2 | Persian Gulf War | Y, Subtotal gastrectomy with roux-en-y | N | None | Annual CT/PET |
| Number | Location | Age | Gender | Race | Path/ Grade | Military History | Endoscopic or Surgical Resection (Y/N) | Systemic Therapy (Chemo/RT/Immuno) (Y/N) | Medical Treatment | Surveillance |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Esophagus | 84 | M | White | Poorly diff, grade 3 | Korean War | N | Cisplatin/Etoposide with RT, Ipilimumab and nivolumab, Carboplatin/Etoposide | None | EGD. Patient deceased following recurrence |
| 2 | Esophagus | 58 | M | Hispanic | Poorly diff, grade 3 | Persian Gulf War | N | Cisplatin/etoposide with RT | None | EGD with PET |
| Site of Small Bowel NET | # |
|---|---|
| Duodenal Bulb | 4 |
| Jejunum | 1 |
| Ileum | 1 |
| Ileocecum | 1 |
| Distal Ileum | 3 |
| Terminal Ileum | 4 |
| Multifocal lesions | 5 |
| 19 Total |
| Number | Location | Age | Gender | Race | Path/Grade | Military History | Endoscopic or Surgical Resection (Y/N) | Systemic Therapy (Chemo/RT/Immuno) (Y/N) | Medical Treatment | Surveillance |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Small Intestine | 71 | M | White | Well-diff, no grade | Vietnam War | Y, R hemicolectomy | N | Lanreotide | Hemicolectomy in 2020, did not have repeat cscope until 2025, unclear if intended for earlier. |
| 2 | Small Intestine | 62 | M | White | Well-diff, grade 1 | Vietnam War | N | N | Octreotide, Lanreotide | Unclear; transferred care elsewhere. |
| 3 | Small Intestine | 74 | M | White | poorly diff, grade 3 | Vietnam War | N | Y | None | Initially planned annual c-scope but stopped with poor prognosis. |
| 4 | Small Intestine | 86 | M | White | Well-diff, no grade | Vietnam War | Y, small bowel resection | N | Octreotide | Unclear |
| 5 | Small Intestine | 80 | M | White | Well-diff, grade 1 | Vietnam War | Y, robotic assisted distal ileum resection | N | Octreotide | PET CT q6 months |
| 6 | Small Intestine | 72 | M | White | Well-diff, grade 1 | Vietnam War | N | N | None | Unclear |
| 7 | Small Intestine | 76 | M | White | Well-diff, grade 1 | Vietnam War | Y, partial duodenectomy | N | None | Q6months |
| 8 | Small Intestine | 57 | M | White | Well-diff, grade 2 | Vietnam War | N | N | Octreotide | None. Patient died within 3 months. |
| 9 | Small Intestine | 101 | M | White | Well-diff, grade 1 | World War II | Y, R hemicolectomy | N | None | Unclear |
| 10 | Small Intestine | 73 | M | Black | Well-diff, grade 2 | Vietnam War | N | N | None | Q1year |
| 11 | Small Intestine | 79 | M | White | Well-diff, grade 2 | Vietnam War | N | Y | Everolimus Lantreotide | CT AP q6month |
| 12 | Small Intestine | 65 | F | Black | Well-diff, grade 1 | Vietnam War | N | N | None | Q3years |
| 13 | Small Intestine | 44 | M | White | Well-diff, grade 1 | Persian Gulf War | Y, R hemicolectomy | N | None | Unclear; transferred care elsewhere |
| 14 | Small Intestine | 62 | M | White | Well-diff, grade 1 | Vietnam War | Y, small bowel resection | N | None | Q6months |
| 15 | Small Intestine | 76 | M | Black | Multiple | Vietnam War | N | N | Lanreotide | PET-CT annual |
| 16 | Small Intestine | 55 | M | Unknown | Well-diff, grade 1 | Persian Gulf War | N | N | None | Unclear |
| 17 | Small Intestine | 68 | M | White | Well-diff, grade 1 | Vietnam War | N | N | Octreotide | CT q6month |
| 18 | Small Intestine | 79 | M | White | Well-diff, grade 1 | Vietnam War | N | Y, capecitabine/ temozolomide | None | CT q3 months |
| 19 | Small Intestine | 88 | M | Black | Well-diff, no grade | Vietnam War | N | N | None | CT annual |
| Number | Location | Age | Gender | Race | Stage/Grade | Military History | Endoscopic or Surgical Resection (Y/N) | Systemic Therapy (Chemo/RT/Immuno) (Y/N) | Medical Treatment | Surveillance |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Colon | 86 | M | White | Well-diff, grade 2 | Vietnam War | Y, Appendectomy | N | None | None |
| 2 | Colon | 41 | M | White | Well-diff, Low-grade | Persian Gulf War | Y, Appendectomy | N | None | None |
| Number | Location | Age | Gender | Race | Stage/Grade | Military History | Endoscopic or Surgical Resection (Y/N) | Systemic Therapy (Chemo/RT/Immuno) (Y/N) | Medical Treatment | Surveillance |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rectum | 43 | F | Black | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Rectal EUS in 2 years |
| 2 | Rectum | 56 | M | White | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Colonoscopy in 3 years |
| 3 | Rectum | 56 | M | Black | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Continue surveillance colonoscopy |
| 4 | Rectum | 70 | M | Black | Well-diff, grade 1 | Vietnam War | Y, endoscopic | N | None | Colonoscopy in 2 years |
| 5 | Rectum | 46 | M | White | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Continue surveillance colonoscopy |
| 6 | Rectum | 60 | M | White | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Continue surveillance colonoscopy |
| 7 | Rectum | 53 | M | Black | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Flex sig in 1 year, then sigmoidoscopy in 2 years |
| 8 | Rectum | 55 | M | Black | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Continue surveillance colonoscopy |
| 9 | Rectum | 55 | M | White | Well-diff, grade 1 | Persian Gulf War | Y, endoscopic | N | None | Colonoscopy in 3 years |
| Diagnosis | N (# Dead) | Mortality Rate | Average Age of Death | Treatment Type | Stage at Presentation | Stage at Death | Cause of Death | Time Between Diagnosis and Death (Months) |
|---|---|---|---|---|---|---|---|---|
| Gastric NET | 1 | 11.1% (1/9) | 52 | Polypectomy | Well-diff, Grade 2, No metastases/ No formal staging | Well-diff, Grade 2, No metastases/No formal staging | End-stage renal disease | 47 months |
| Esophageal NET | 1 | 50% (1/2) | 84 | Chemo, RT, and immunotherapy | T4N2M0 Poorly diff, Grade 3 | T4N2M0 Poorly diff, Grade 3 | Recurrent disease | 26 months |
| Small bowel NET | 5 | 26.3% (5/19) | 74 | Lanreotide, Lutathera, Everolimus | Well-diff, Grade 2, No metastases/ No formal staging | Well-diff, Grade 2, No metastases/ No formal staging | Recurrent disease | 4 months |
| Octreotide | T4N2M1, Well-diff, Grade 2 | T4N2M1, Well-diff, grade 2 | Recurrent disease | 5 months | ||||
| Imaging surveillance | Well-diff, Grade 1 No metastases/ No formal staging | Well-diff, Grade 1 No metastases/ No formal staging | Metastatic chondrosarcoma | 20 months | ||||
| Imaging surveillance | Well-diff, Grade 1 | Well-diff, Grade 1 | Multiple Comorbidities | 22 months | ||||
| Chemo- and immunotherapy | T3N1M1, Poorly diff, Grade 3 | T3N2M1, Poorly diff, Grade 3 | Recurrent disease | 24 months | ||||
| Colonic NET | 1 | 50% | 84 | Appendectomy | Well-diff, Grade 1 No metastases/ No formal staging | Well-diff, Grade 1 No metastases/ No formal staging | Old age | 39 months |
| Rectal NET | 0 | 0% | N/A | Polypectomy | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, C.; Kalapala, J.; Leya, J.; Popadiuk, M.M.; Atieh, M.K.; Havlichek, D., III; Feldman, L.; Roach, P.; Banerjee, P. Gastroesophageal Neuroendocrine Tumors: Outcomes and Management. J. Clin. Med. 2025, 14, 2148. https://doi.org/10.3390/jcm14072148
Son C, Kalapala J, Leya J, Popadiuk MM, Atieh MK, Havlichek D III, Feldman L, Roach P, Banerjee P. Gastroesophageal Neuroendocrine Tumors: Outcomes and Management. Journal of Clinical Medicine. 2025; 14(7):2148. https://doi.org/10.3390/jcm14072148
Chicago/Turabian StyleSon, Christine, Joshua Kalapala, Jeff Leya, Michelle Marion Popadiuk, Mohammed K. Atieh, Daniel Havlichek, III, Lawrence Feldman, Paul Roach, and Promila Banerjee. 2025. "Gastroesophageal Neuroendocrine Tumors: Outcomes and Management" Journal of Clinical Medicine 14, no. 7: 2148. https://doi.org/10.3390/jcm14072148
APA StyleSon, C., Kalapala, J., Leya, J., Popadiuk, M. M., Atieh, M. K., Havlichek, D., III, Feldman, L., Roach, P., & Banerjee, P. (2025). Gastroesophageal Neuroendocrine Tumors: Outcomes and Management. Journal of Clinical Medicine, 14(7), 2148. https://doi.org/10.3390/jcm14072148






