The Efficacy and Safety of Direct Oral Anticoagulants Compared to Warfarin for Left Ventricular Thrombus Resolution
Abstract
1. Introduction
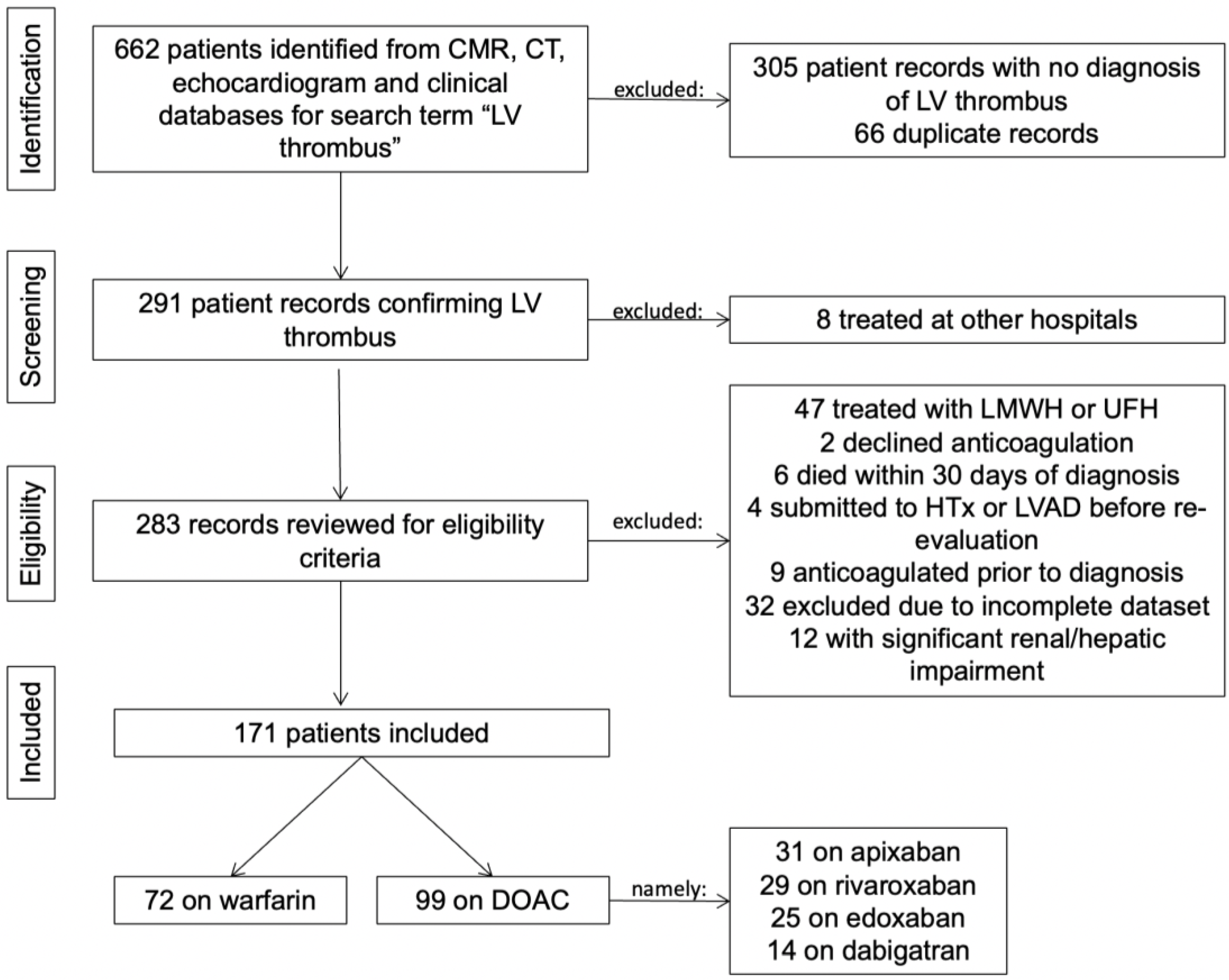
2. Methods
2.1. Study Design
2.2. Participants
2.3. Imaging Assessment
2.4. Endpoints
2.5. Statistical Analysis
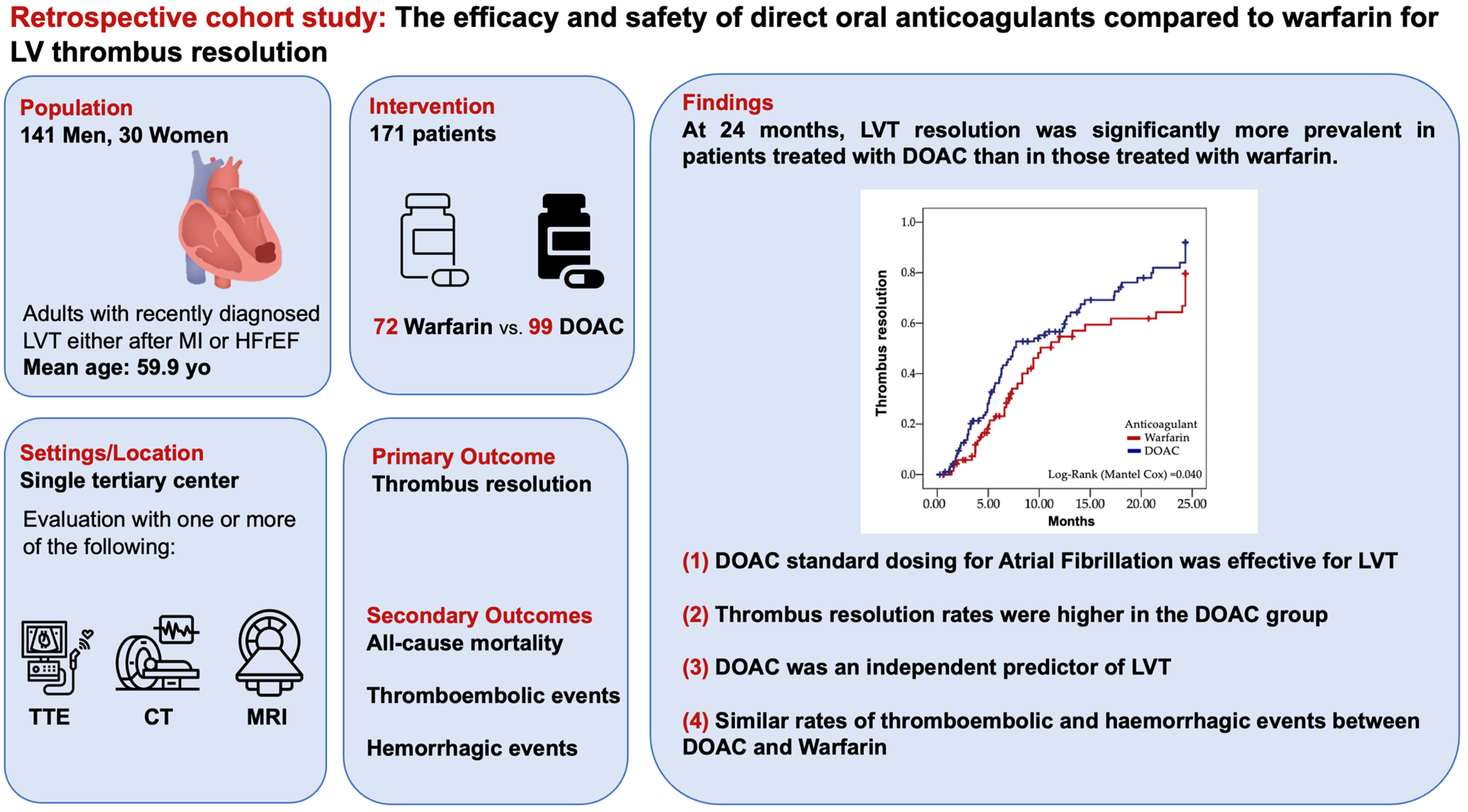
3. Results
3.1. Primary Endpoint—LVT Resolution
3.2. Secondary Endpoints
4. Discussion
4.1. Thrombus Regression
4.2. Safety Endpoints
4.3. Study Limitations
5. Conclusions
Key Messages
- LVT is a serious complication often following myocardial infarction or in patients with reduced left ventricular ejection fraction, increasing embolic risk.
- While warfarin has been the standard treatment, DOACs offer potential benefits, including fixed dosing and fewer drug interactions, though their efficacy and safety in LVT resolution remain unclear.
- This study compares DOACs and warfarin for LVT resolution, assessing both efficacy (thrombus resolution) and safety (bleeding risks, embolic events), suggesting DOACs may be equally effective with potentially lower bleeding risks.
- These findings provide real-world evidence to guide clinicians and may support greater use of DOACs.
- However, randomized controlled trials are needed to confirm the benefits and risks of DOACs in this specific patient population.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| APT | Antiplatelet therapy |
| BARC | Bleeding Academic Research Consortium |
| BSA | Body surface area |
| CMR | Cardiac magnetic resonance |
| CT | Computed tomography |
| DAPT | Double antiplatelet therapy |
| DOAC | Direct oral anticoagulants |
| HfrEF | Heart failure with reduced ejection fraction |
| HTx | Heart transplantation |
| INR | International normalized ratio |
| IQR | Interquartile range |
| LMWH | Low-molecular-weight heparin |
| LV | Left ventricle |
| LVAD | Left ventricle assist device |
| LVT | Left ventricular thrombus |
| MACE | Major adverse cardiovascular event |
| MI | Myocardial infarction |
| PCI | Percutaneous coronary intervention |
| PT | Patient |
| SAPT | Single antiplatelet therapy |
| SD | Standard deviation |
| STEMI | ST-elevation myocardial infarction |
| TDVi | Telediastolic indexed volume |
| TTE | Transthoracic echocardiography |
| UFH | Unfractionated heparin |
| VKA | Vitamin K antagonist |
| WMSI | Wall motion severity index |
References
- Johannessen, K.A.; Nordrehaug, J.E.; von der Lippe, G. Left ventricular thrombosis and cerebrovascular accident in acute myocardial infarction. Heart 1984, 51, 553–556. [Google Scholar] [CrossRef]
- Solheim, S.; Seljeflot, I.; Lunde, K.; Bjørnerheim, R.; Aakhus, S.; Forfang, K.; Arnesen, H. Frequency of Left Ventricular Thrombus in Patients with Anterior Wall Acute Myocardial Infarction Treated with Percutaneous Coronary Intervention and Dual Antiplatelet Therapy. Am. J. Cardiol. 2010, 106, 1197–1200. [Google Scholar] [CrossRef]
- Adams, P.C.; Cohen, M.; Chesebro, J.H.; Fuster, V. Thrombosis and embolism from cardiac chambers and infected valves. J. Am. Coll. Cardiol. 1986, 8, 76B–87B. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Makam, R.C.P.; Hoaglin, D.C.; McManus, D.D.; Wang, V.; Gore, J.M.; Spencer, F.A.; Pradhan, R.; Tran, H.; Yu, H.; Goldberg, R.J. Efficacy and safety of direct oral anticoagulants approved for cardiovascular indications: Systematic review and meta-analysis. PLoS ONE 2018, 13, e0197583. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, M.; Saleh, Y.; Fareed, A.; Nossikof, A.; Wang, L.; Morsi, M.; Eshak, N.; Abdelkarim, O.; Badran, H.; Almaghraby, A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial). J. Am. Coll. Cardiol. 2021, 77, 1590–1592. [Google Scholar] [CrossRef]
- Alcalai, R.; Butnaru, A.; Moravsky, G.; Yagel, O.; Rashad, R.; Ibrahimli, M.; Planer, D.; Amir, O.; Elbaz-Greener, G.; Leibowitz, D. Apixaban vs. warfarin in patients with left ventricular thrombus: A prospective multicentre randomized clinical trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 660–667. [Google Scholar] [CrossRef]
- Isa, W.Y.H.W.; Hwong, N.; Yusof, A.K.M.; Yusof, Z.; Loong, N.S.; Wan-Arfah, N.; Naing, N.N. Apixaban versus Warfarin in Patients with Left Ventricular Thrombus: A Pilot Prospective Randomized Outcome Blinded Study Investigating Size Reduction or Resolution of Left Ventricular Thrombus. J. Clin. Prev. Cardiol. 2020, 9, 150. [Google Scholar] [CrossRef]
- Youssef, A.A.; Alrefae, M.A.; Khalil, H.H.; Abdullah, H.I.; Khalifa, Z.S.; Al Shaban, A.A.; Wali, H.A.; AlRajab, M.R.; Saleh, O.M.; Nashy, B.N. Apixaban in Patients with Post-Myocardial Infarction Left Ventricular Thrombus: A Randomized Clinical Trial. CJC Open 2023, 5, 191–199. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A consensus report from the bleeding academic research consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Z.; Zheng, H.; Qu, M.; Li, S.; Yang, P.; Si, D.; Zhang, W. Rivaroxaban in heart failure patients with left ventricular thrombus: A retrospective study. Front. Pharmacol. 2022, 13, 1008031. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Wright, P.; Alizadeh, M.A.; Fhadil, S.; Rathod, K.S.; Guttmann, O.; Knight, C.; Timmis, A.; Baumbach, A.; Wragg, A.; et al. The use of novel oral anticoagulants compared to vitamin K antagonists (warfarin) in patients with left ventricular thrombus after acute myocardial infarction. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 398–404. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Lin, Y.; Peng, W. Direct oral anticoagulants compared with warfarin in patients with left ventricular thrombus: A cohort study from China. J. Thorac. Dis. 2024, 16, 884–892. [Google Scholar] [CrossRef]
- Al-Abcha, A.; Clay, S.; Wang, L.; Prasad, R.M.; Salam, M.F.; Srivastava, S.; Boumegouas, M.; Abela, G.S.; Saleh, Y.; Essa, E.M. Direct Oral Anticoagulants Versus Warfarin for the Treatment of Left Ventricular Thrombus: A Multicenter Retrospective Observational Study. Am. J. Cardiol. 2025, 238, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, B.S.; Dixon, D.L.; Neyens, R.R.; Page, R.L.; Gluckman, T.J. Select Drug-Drug Interactions with Direct Oral Anticoagulants: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 1341–1350. [Google Scholar] [CrossRef]
- Lattuca, B.; Bouziri, N.; Kerneis, M.; Portal, J.-J.; Zhou, J.; Hauguel-Moreau, M.; Mameri, A.; Zeitouni, M.; Guedeney, P.; Hammoudi, N.; et al. Antithrombotic Therapy for Patients with Left Ventricular Mural Thrombus. J. Am. Coll. Cardiol. 2020, 75, 1676–1685. [Google Scholar] [CrossRef]
- Lip, G.Y.; Gibbs, C.R. Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J. Am.Coll. Cardiol. 1999, 33, 1424–1426. [Google Scholar]
- Guddeti, R.R.; Anwar, M.; Walters, R.W.; Apala, D.; Pajjuru, V.; Kousa, O.; Gujjula, N.R.; Alla, V.M. Treatment of Left Ventricular Thrombus with Direct Oral Anticoagulants: A Retrospective Observational Study. Am. J. Med. 2020, 133, 1488–1491. [Google Scholar] [CrossRef]
- McCarthy, C.P.; Murphy, S.; Venkateswaran, R.V.; Singh, A.; Chang, L.L.; Joice, M.G.; Rivero, J.M.; Vaduganathan, M.; Januzzi, J.L.; Bhatt, D.L. Left Ventricular Thrombus: Contemporary Etiologies, Treatment Strategies, and Outcomes. J. Am. Coll. Cardiol. 2019, 73, 2007–2009. [Google Scholar] [CrossRef] [PubMed]
- Fleddermann, A.M.; Hayes, C.H.; Magalski, A.; Main, M.L. Efficacy of Direct Acting Oral Anticoagulants in Treatment of Left Ventricular Thrombus. Am. J. Cardiol. 2019, 124, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.A.; Trankle, C.R.; Eubanks, G.; Schumann, C.; Thompson, P.; Wallace, R.L.; Gottiparthi, S.; Ruth, B.; Kramer, C.M.; Salerno, M.; et al. Off-label Use of Direct Oral Anticoagulants Compared with Warfarin for Left Ventricular Thrombi. JAMA Cardiol. 2020, 5, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Isom, N.; Dalia, T.; Sami, F.; Mahmood, U.; Shah, Z.; Gupta, K. Direct oral anticoagulant use in left ventricular thrombus. Thromb. J. 2020, 18, 29. [Google Scholar] [CrossRef]
- Iqbal, H.; Straw, S.; Craven, T.P.; Stirling, K.; Wheatcroft, S.B.; Witte, K.K. Direct oral anticoagulants compared to vitamin K antagonist for the management of left ventricular thrombus. ESC Heart Fail. 2020, 7, 2032–2041. [Google Scholar] [CrossRef]
- Daher, J.; Da Costa, A.; Hilaire, C.; Ferreira, T.; Pierrard, R.; Guichard, J.B.; Romeyer, C.; Isaaz, K. Management of Left Ventricular Thrombi with Direct Oral Anticoagulants: Retrospective Comparative Study with Vitamin K Antagonists. Clin. Drug Investig. 2020, 40, 343–353. [Google Scholar] [CrossRef]
- Cochran, J.M.; Jia, X.; Kaczmarek, J.; Staggers, K.A.; Al Rifai, M.; Hamzeh, I.R.; Birnbaum, Y. Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus: A Retrospective, Multicenter Study and Meta-Analysis of Existing Data. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 173–178. [Google Scholar] [CrossRef]
- Camaj, A.; Fuster, V.; Giustino, G.; Bienstock, S.W.; Sternheim, D.; Mehran, R.; Dangas, G.D.; Kini, A.; Sharma, S.K.; Halperin, J.; et al. Left Ventricular Thrombus Following Acute Myocardial Infarction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1010–1022. [Google Scholar] [CrossRef]
- Weinsaft, J.W.; Kim, J.; Medicherla, C.B.; Ma, C.L.; Codella, N.C.F.; Kukar, N.; Alaref, S.; Kim, R.J.; Devereux, R.B. Echocardiographic Algorithm for Post–Myocardial Infarction LV Thrombus: A Gatekeeper for Thrombus Evaluation by Delayed Enhancement CMR. JACC Cardiovasc. Imaging 2016, 9, 505–515. [Google Scholar] [CrossRef]

| Overall (N = 171) | Warfarin (N = 72) | DOAC (N = 99) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Male, n (%) | 141 (82.5) | 55 (76.4) | 86 (86.9) | 0.08 |
| Age, years (mean ± SD) | 59.8 ± 14.7 | 65.9 ± 14.0 | 55.4 ± 13.7 | <0.0001 |
| BSA, m2 (median (IQR)) | 1.88(1.77–2.03) | 1.86 (1.73–2.02) | 1.89 (1.80–2.04) | 0.20 |
| Diabetes, n (%) | 30 (17.5) | 10 (13.9) | 20 (20.2) | 0.28 |
| Hypertension, n (%) | 114 (66.7) | 47 (65.3) | 67 (67.7) | 0.74 |
| Dyslipidemia, n (%) | 97 (56.7) | 40 (55.6) | 57 (57.6) | 0.79 |
| Current smoker, n (%) | 45 (26.3) | 21 (29.2) | 24 (24.2) | 0.49 |
| Laboratory findings | ||||
| NTproBNP, mg/dL (median (IQR)) | 2272 (747–4898) | 1320 (488–4087) | 2490 (954–6602) | 0.04 |
| Clearance, mL/min/1.73 m2 (mean ± SD) | 106.3 ± 57.4 | 89.7 ± 35.6 | 119 ± 67.1 | 0.001 |
| Atrial fibrillation, n (%) | 31 (18.1) | 7 (9.7) | 24 (24.2) | 0.02 |
| Etiology | ||||
| Myocardial infarction, n (%) | 121 (70.8) | 55 (76.4) | 66 (66.7) | 0.17 |
| STEMI, | 71 (41.5) | 26 (36.2) | 45 (45.5%) | 0.22 |
| HFrEF, n (%) | 102 (59.6) | 31 (43.1) | 71 (71.7) | <0.0001 |
| Baseline echo findings | ||||
| Ejection fraction, % (mean ± SD) | 36 ± 11 | 38 ± 11 | 34 ± 12 | 0.04 |
| TDVi, mL/m2 (median(IQR)) | 73.0 (57.4–96.8) | 66.0 (52.0–83.0) | 79.3 (62.3–107.3) | 0.007 |
| WMSI, n (mean ± SD) | 2.1 ± 0.4 | 2.0 ± 0.4 | 2.1 ± 0.4 | 0.10 |
| Follow-up period, days (median (IQR)) | 934 (436–1536) | 1480 (719–2675) | 757 (400–1146) | <0.001 |
| Overall (N = 171) | Thrombus Maintenance (N = 60) | Thrombus Resolution (N = 111) | p-Value | |
|---|---|---|---|---|
| VKA, n (%) | 72 (42.1) | 31 (51.7) | 41 (37.0) | 0.06 |
| DOAC, n (%) | 99 (57.9) | 29 (48.3) | 70 (63.0) | 0.06 |
| Edoxaban, n (%) | 25 (14.6) | 5 (8.3) | 20 (18.0) | 0.64 |
| Apixaban, n (%) | 31 (18.1) | 9 (15.0) | 22 (19.8) | |
| Dabigatran, n (%) | 14 (8.2) | 5 (8.3) | 9 (8.1) | |
| Rivaroxaban, n (%) | 29 (17.0) | 10 (16.7) | 19 (17.1) | |
| Concomitant antiplatelet therapy | ||||
| SAPT + OAC, n (%) | 29 (17.0) | 11 (18.3) | 18 (10.5) | 0.73 |
| Duration of SAPT + OAC, months | 6 (2.5–12) | 11 (3–12) | 5 (1.75–8.3) | 0.19 |
| DAPT + OAC, n (%) | 25 (14.6) | 1 (1.7) | 24 (21.6) | <0.0001 |
| Duration of DAPT + OAC, months | 3 (1.5–3) | 0 | 3 (2–3) | 0.16 |
| HFrEF, N (%) | 102 (59.6) | 30 (50.0) | 72 (64.9) | 0.06 |
| Echocardiographic findings | ||||
| Initial ejection fraction, % | 34 ± 11 | 34 ± 12 | 34 ± 11 | 0.98 |
| Initial WMSI, n | 2.0 ± 0.3 | 2.1 ± 0.3 | 2.0 ± 0.4 | 0.02 |
| Follow up ejection fraction, % | 37 ± 12 | 37 ± 11 | 37 ± 13 | 0.24 |
| Follow up WMSI, n | 2.0 ± 0.4 | 2.1 ± 0.4 | 1.9 ± 0.4 | 0.10 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| DOAC | 1.69 (1.13–2.53) | 0.01 | 1.87 (1.01–3.45) | 0.047 |
| BSA | 1.00 (0.92–1.10) | 0.95 | NA | NA |
| Age | 0.99 (0.98–1.01) | 0.34 | 0.99 (0.97–1.01) | 0.28 |
| Ischemic cardiomyopathy | 0.76 (0.52–1.12) | 0.16 | NA | NA |
| STEMI at diagnosis | 1.21 (0.83–1.76) | 0.32 | 0.89 (0.49–1.59) | 0.686 |
| Atrial fibrillation | 0.97 (0.59–1.57) | 0.89 | NA | NA |
| Concomitant antiplatelet therapy | 1.86 (1.27–2.73) | 0.002 | 2.37 (1.33–4.23) | 0.003 |
| Echocardiographic findings | ||||
| Initial ejection fraction | 0.99 (0.97–1.00) | 0.15 | NA | NA |
| Initial WMSI | 0.59 (0.31–1.10) | 0.10 | NA | NA |
| Final ejection fraction | 1.00 (0.99–1.02) | 0.74 | NA | NA |
| Final WMSI | 0.51 (0.26–0.99) | 0.05 | 0.49 (0.25–0.97) | 0.04 |
| DOAC (N = 99) | Warfarin (N = 72) | p-Value (Chi-Square Test) | |||||
|---|---|---|---|---|---|---|---|
| Endpoints | N (%) | Patient/Years | New Cases/100 Pt Years | N (%) | Patient/Years | New Cases/100 Pt Years | |
| Bleeding | |||||||
| Any | 1 (1.0) | 0.01 | 0.8 | 4 (5.6) | 0.04 | 3.8 | 0.082 |
| BARC ≥ 3 | 1 (1.0) | 0.01 | 0.8 | 3 (4.2) | 0.03 | 2.9 | 0.233 |
| Blood transfusion | 1 (1.0) | 0.01 | 0.8 | 2 (2.8) | 0.02 | 1.9 | 0.385 |
| Thromboembolic events, N (%) | |||||||
| Any, N (%) | 3 (3.0) | 0.02 | 2.4 | 6 (8.3) | 0.06 | 5.7 | 0.125 |
| Peripheral, N (%) | 1 (1.0) | 0.01 | 0.8 | 2 (2.8) | 0.02 | 1.9 | 0.385 |
| Stroke or TIA | 2 (2.0) | 0.02 | 1.6 | 4 (5.6) | 0.04 | 3.8 | 0.109 |
| Death | 5 (5.1) | 0.04 | 4.0 | 4 (5.6) | 0.04 | 3.8 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, M.S.; Gama, F.; Azevedo, S.; Lopes, P.M.; Albuquerque, F.; Reis, C.; Freitas, P.; Guerreiro, S.; Abecasis, J.; Trabulo, M.; et al. The Efficacy and Safety of Direct Oral Anticoagulants Compared to Warfarin for Left Ventricular Thrombus Resolution. J. Clin. Med. 2025, 14, 2129. https://doi.org/10.3390/jcm14062129
Paiva MS, Gama F, Azevedo S, Lopes PM, Albuquerque F, Reis C, Freitas P, Guerreiro S, Abecasis J, Trabulo M, et al. The Efficacy and Safety of Direct Oral Anticoagulants Compared to Warfarin for Left Ventricular Thrombus Resolution. Journal of Clinical Medicine. 2025; 14(6):2129. https://doi.org/10.3390/jcm14062129
Chicago/Turabian StylePaiva, Mariana Sousa, Francisco Gama, Samuel Azevedo, Pedro M. Lopes, Francisco Albuquerque, Carla Reis, Pedro Freitas, Sara Guerreiro, João Abecasis, Marisa Trabulo, and et al. 2025. "The Efficacy and Safety of Direct Oral Anticoagulants Compared to Warfarin for Left Ventricular Thrombus Resolution" Journal of Clinical Medicine 14, no. 6: 2129. https://doi.org/10.3390/jcm14062129
APA StylePaiva, M. S., Gama, F., Azevedo, S., Lopes, P. M., Albuquerque, F., Reis, C., Freitas, P., Guerreiro, S., Abecasis, J., Trabulo, M., Ferreira, A. M., Ribeiras, R., Ferreira, J., & Pulido Adragão, P. (2025). The Efficacy and Safety of Direct Oral Anticoagulants Compared to Warfarin for Left Ventricular Thrombus Resolution. Journal of Clinical Medicine, 14(6), 2129. https://doi.org/10.3390/jcm14062129





