Serum Desmosine Levels Might Be Associated with the Size of Ruptured Cerebral Aneurysms in Patients with Aneurysmal Subarachnoid Hemorrhage—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Definition of Aneurysm Morphological Parameters
2.3. Biomarker Measurements
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
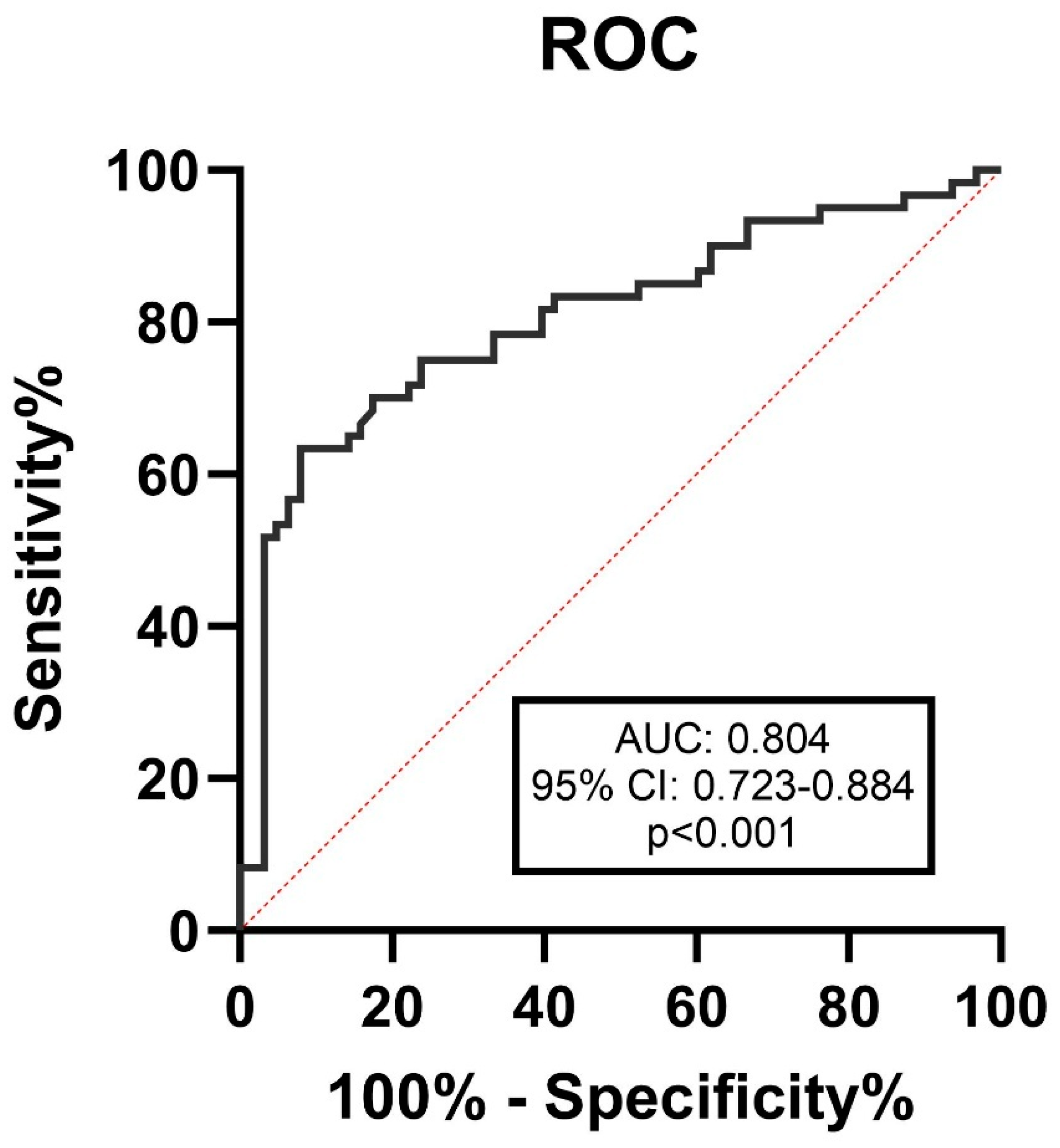
3.2. Relationship Between Serum Desmosine Levels and Aneurysm Morphology
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aSAH | Aneurysmal Subarachnoid Hemorrhage |
| AAA | Abdominal Aortic Aneurysm |
| ACA | Anterior Cerebral Artery |
| Acom | Anterior Communicating Artery |
| AMI | Acute Myocardial Infarction |
| AR | Aspect Ratio |
| AUC | Area Under the Curve |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| CT | Computed Tomography |
| DSA | Digital Subtraction Angiography |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EVD | Extraventricular Drainage |
| HRP | Horseradish Peroxidase |
| ICA | Internal Carotid Artery |
| IQR | Interquartile Range |
| IVH | Intraventricular Hemorrhage |
| MCA | Middle Cerebral Artery |
| mFisher | Modified Fisher Scale |
| MRI | Magnetic Resonance Imaging |
| mRS | Modified Rankin Scale |
| Pcom | Posterior Communicating Artery |
| PHASES | Population, Hypertension, Age, Size, Earlier Subarachnoid Hemorrhage, Site |
| ROC | Receiver Operating Characteristic |
| SAH | Subarachnoid Hemorrhage |
| SR | Size Ratio |
| SD | Standard Deviation |
| SPSS | Statistical Package for the Social Sciences |
| TMB | Tetramethylbenzidine |
| UIA | Unruptured Intracranial Aneurysm |
| UIATS | Unruptured Intracranial Aneurysm Treatment Score |
| WFNS | World Federation of Neurological Societies |
| WBC | White Blood Count |
References
- Bugazia, S.; Boshnaf, M.; Sreenivasan, A. Subarachnoid hemorrhage mortality trends in U.S. patients with circulatory disease (1999–2020). Crit. Care Med. 2024, 52, S114. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Korja, M.; Lehto, H.; Juvela, S. Lifelong rupture risk of intracranial aneurysms depends on risk factors: A prospective Finnish cohort study. Stroke 2014, 45, 1958–1963. [Google Scholar] [CrossRef]
- Bijlenga, P.; Gondar, R.; Schilling, S.; Morel, S.; Hirsch, S.; Cuony, J.; Corniola, M.-V.; Perren, F.; Rüfenacht, D.; Schaller, K. PHASES Score for the Management of Intracranial Aneurysm: A Cross-Sectional Population-Based Retrospective Study. Stroke 2017, 48, 2105–2112. [Google Scholar] [CrossRef]
- Molenberg, R.; Aalbers, M.W.; Mazuri, A.; Luijckx, G.J.; Metzemaekers, J.D.M.; Groen, R.J.M.; Uyttenboogaart, M.; van Dijk, J.M.C. The Unruptured Intracranial Aneurysm Treatment Score as a predictor of aneurysm growth or rupture. Eur. J. Neurol. 2021, 28, 837–843. [Google Scholar] [CrossRef]
- Sanchez, S.; Hickerson, M.; Patel, R.R. Morphological characteristics of ruptured brain aneurysms: A systematic literature review and meta-analysis. Stroke Vasc. Interv. Neurol. 2023, 3, e000707. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, L.; Wen, L.; Wu, Q.; Leng, X.; Xiang, J.; Zhang, X. Morphological and hemodynamic characteristics associated with the rupture of multiple intracranial aneurysms. Front. Neurol. 2022, 12, 811281. [Google Scholar] [CrossRef]
- Duan, Z.; Li, Y.; Guan, S.; Ma, C.; Han, Y.; Ren, X.; Wei, L.; Li, W.; Lou, J.; Yang, Z. Morphological parameters and anatomical locations associated with rupture status of small intracranial aneurysms. Sci. Rep. 2018, 8, 6440. [Google Scholar] [CrossRef]
- Ma, S.; Lieberman, S.; Turino, G.M.; Lin, Y.Y. The detection and quantitation of free desmosine and isodesmosine in human urine and their peptide-bound forms in sputum. Proc. Natl. Acad. Sci. USA 2003, 100, 12941–12943. [Google Scholar] [CrossRef]
- Cantor, J. The role of the extracellular matrix in the pathogenesis and treatment of pulmonary emphysema. Int. J. Mol. Sci. 2024, 25, 10613. [Google Scholar] [CrossRef]
- Luisetti, M.; Ma, S.; Iadarola, P.; Stone, P.J.; Viglio, S.; Casado, B.; Lin, Y.Y.; Snider, G.L.; Turino, G.M. Desmosine as a biomarker of elastin degradation in COPD: Current status and future directions. Eur. Respir. J. 2008, 32, 1146–1157. [Google Scholar] [CrossRef]
- Mordi, I.R.; Forsythe, R.O.; Gellatly, C.; Iskandar, Z.; McBride, O.M.; Saratzis, A.; Chalmers, R.; Chin, C.; Bown, M.J.; Newby, D.E.; et al. Plasma desmosine and abdominal aortic aneurysm disease. J. Am. Heart Assoc. 2019, 8, e013743. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, Z.; Dodd, M.; Huang, J.; Chin, C.W.L.; Stuart, G.; Caputo, M.; Clayton, T.; Child, A.; Jin, X.Y.; Aragon-Martin, J.A.; et al. Exaggerated elastin turnover in young individuals with Marfan syndrome: New insights from the AIMS trial. Eur. Heart J. Open 2023, 3, oead095. [Google Scholar] [CrossRef] [PubMed]
- Mikagi, A.; Tashiro, R.; Inoue, T.; Anzawa, R.; Imura, A.; Tanigawa, T.; Ishida, T.; Inoue, T.; Niizuma, K.; Tominaga, T.; et al. Isotope-dilution LC-MS/MS analysis of the elastin crosslinkers desmosine and isodesmosine in acute cerebral stroke patients. RSC Adv. 2022, 12, 31769–31777. [Google Scholar] [CrossRef]
- Nakagawa, D.; Zanaty, M.; Hudson, J.; Teferi, N.; Ishii, D.; Allan, L.; Jabbour, P.; Ortega-Gutierrez, S.; Samaniego, E.A.; Hasan, D.M. Plasma soluble human elastin fragments as an intra-aneurysmal localized biomarker for ruptured intracranial aneurysm. J. Am. Heart Assoc. 2018, 7, e010051. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H. New pathophysiological considerations on cerebral aneurysms. Neurointervention 2018, 13, 73–83. [Google Scholar] [CrossRef]
- Diringer, M.N.; Bleck, T.P.; Hemphill, J.C.; Menon, D.; Shutter, L.; Vespa, P.; Bruder, N.; Connolly, E.S.; Citerio, G.; Gress, D.; et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: Recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit. Care 2011, 15, 211–240. [Google Scholar] [CrossRef]
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef]
- Claassen, J.; Bernardini, G.L.; Kreiter, K.; Bates, J.; Du, Y.E.; Copeland, D.; Connolly, E.S.; Mayer, S.A. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: The Fisher scale revisited. Stroke 2001, 32, 2012–2020. [Google Scholar] [CrossRef]
- Drake, C.G. Report of World Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage scale. J. Neurosurg. 1988, 68, 985–986. [Google Scholar]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Mocco, J.; Brown, R.D., Jr.; Torner, J.C.; Capuano, A.W.; Fargen, K.M.; Raghavan, M.L.; Piepgras, D.G.; Meissner, I.; John, H., III; on behalf of the International Study of Unruptured Intracranial Aneurysms Investigators. Aneurysm morphology and prediction of rupture: An international study of unruptured intracranial aneurysms analysis. Neurosurgery 2018, 82, 491–496. [Google Scholar] [CrossRef]
- Göttsche, J.; Piffko, A.; Pantel, T.F.; Westphal, M.; Dührsen, L.; Czorlich, P.; Sauvigny, T. Aneurysm location affects clinical course and mortality in patients with subarachnoid hemorrhage. Front. Neurol. 2022, 13, 846066. [Google Scholar] [CrossRef] [PubMed]
- Shiue, I.; Arima, H.; Hankey, G.J.; Anderson, C.S.; for the ACROSS Group. Location and size of ruptured intracranial aneurysm and serious clinical outcomes early after subarachnoid hemorrhage: A population-based study in Australasia. Cerebrovasc. Dis. 2011, 31, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Moffitt, K.L.; Suarez-Cuartin, G.; Sibila, O.; Finch, S.; Furrie, E.; Dicker, A.; Wrobel, K.; Elborn, J.S.; Walker, B.; et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am. J. Respir. Crit. Care Med. 2017, 195, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Israr, M.Z.; Ng, L.L.; Mordi, I.; Lang, C.C.; Kuzmanova, E.; Huang, J.T.-J.; Choy, A.-M. Plasma desmosine for prediction of outcomes after acute myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 992388. [Google Scholar] [CrossRef]
- Rabinovich, R.A.; Miller, B.E.; Wrobel, K.; Ranjit, K.; Williams, M.C.; Drost, E.; Edwards, L.D.; Lomas, D.A.; Rennard, S.I.; Agustí, A.; et al. Circulating desmosine levels do not predict emphysema progression but are associated with cardiovascular risk and mortality in COPD. Eur. Respir. J. 2016, 47, 1365–1373. [Google Scholar] [CrossRef]
- Huang, J.T.-J.; Kuzmanova, E.; Dicker, A.J.; Keir, H.R.; Finch, S.; Aliberti, S.; Fardon, T.C.; Chalmers, J.D. Serum desmosine is associated with long-term all-cause and cardiovascular mortality in bronchiectasis. Am. J. Respir. Crit. Care Med. 2020, 202, 897–899. [Google Scholar] [CrossRef]
- Iskandar, Z.; Mordi, I.; Huang, J.T.J.; Newby, D.; Chalmers, J.; Bown, M.; Lang, C.; Choy, A.M. Plasma desmosine, an elastin degradation product, predicts outcomes in at-risk populations. J. Am. Coll. Cardiol. 2019, 73 (Suppl. S1), 1805. [Google Scholar] [CrossRef]
- Kashiwazaki, D.; Kuroda, S. Size ratio can highly predict rupture risk in intracranial small (<5 mm) aneurysms. Stroke 2013, 44, 2169–2173. [Google Scholar]
- Chien, A.; Sayre, J.; Viñuela, F. Comparative morphological analysis of the geometry of ruptured and unruptured aneurysms. Neurosurgery 2011, 69, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.P.J.; Dofferhoff, A.S.M.; van den Ouweland, J.M.W.; van Daal, H.; Kramers, C.; Schurgers, L.J.; Janssen, R.; Walk, J. Effects of Vitamin D and K on Interleukin-6 in COVID-19. Front. Nutr. 2022, 8, 761191. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, F.; Pop, R.; Ganau, M.; Cebula, H.; Scibilia, A.; Gallinaro, P.; Zaed, I.; Todeschi, J.; Lefevre, E.; Nannavecchia, B.; et al. Endovascular versus surgical treatment for improvement of oculomotor nerve palsy caused by unruptured posterior communicating artery aneurysms. J. Neurointerv. Surg. 2020, 10, 964–967. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total | <7 mm | >7 mm | p-Value |
|---|---|---|---|---|
| Number of patients, n | 135 | 69 | 66 | N/A |
| Age, years, mean ± SD | 58.4 ± 12.4 | 57 ± 13 | 59 ± 12 | 0.360 |
| Female, n (%) | 99 (73) | 52 (75) | 47 (73) | 0.518 |
| Hypertension, n (%) | 71 (53) | 37 (54) | 34 (53) | 0.544 |
| Smoking, n (%) | 46 (34) | 23 (37) | 23 (38) | 0.491 |
| Diabetes, n (%) | 14 (9) | 7 (9.5) | 7 (8.5) | 0.584 |
| WFNS, median (IQR) | 2 (1–4) | 2 (1–4) | 2.5 (1–5) | 0.364 |
| mFisher score, median (IQR) | 3 (2–4) | 3 (2–3) | 3 (2–4) | 0.344 |
| 3-month mRS score, median (IQR) | 3 (2–5) | 2 (1–4) | 4 (2–5) | 0.003 |
| 3-month mRS (0–3), n (%) | 75 (56) | 48 (70) | 27 (41) | 0.004 |
| Delayed cerebral ischemia, n (%) | 25 (19) | 13 (19) | 12 (18) | 0.482 |
| Extraventricular drainage, n (%) | 60 (44) | 26 (37) | 34 (52) | 0.092 |
| Mechanical ventilation, n (%) | 64 (47) | 24 (35) | 40 (61) | 0.008 |
| creatinine a, µmol/L, median (IQR) | 60 (48–71) | 59 (47–77) | 61 (49–70) | 0.677 |
| CRP a, mg/L, median (IQR) | 17 (5–59) | 16 (5–45) | 14 (3–77) | 0.745 |
| WBC a, G/L, median (IQR) | 10 (8–13) | 10.6 (8–13) | 10 (9–12) | |
| Size of aneurysm, mm, median (IQR) | 7 (5–10.7) | 5 (4–6) | 10.8 (9–13) | <0.001 |
| Neck width, mm, median (IQR) | 3.3 (2.4–4) | 2.6 (2.2–3.3) | 3.7 (3.3–4.8) | <0.001 |
| Aspect ratio, median (IQR) | 1.7 (1.4–2.2) | 1.52 (1.2–2.1) | 1.8 (1.5–2.3) | 0.022 |
| Size ratio, median (IQR) | 3.8 (2.6–5) | 2.72 (1.9–3.9) | 4.42 (3.7–5.5) | <0.001 |
| Daughter sac, n (%) | 54 (44) | 22 (32) | 32 (55) | 0.018 |
| Location of ruptured aneurysm, n (%) | 0.003 | |||
| ICA | 6 (4.8) | 2 (3.3) | 4 (6.8) | |
| MCA | 33 (24) | 9 (13.1) | 25 (37.3) | |
| Acom | 42 (31.2) | 31 (44.3) | 9 (13.6) | |
| Pcom | 20 (14.4) | 10 (14.8) | 10 (15.3) | |
| ACA | 3 (2.4) | 2 (3.3) | 1 (1.7) | |
| Vertebrobasilar | 31 (23.2) | 15 (21.3) | 17 (25.4) | |
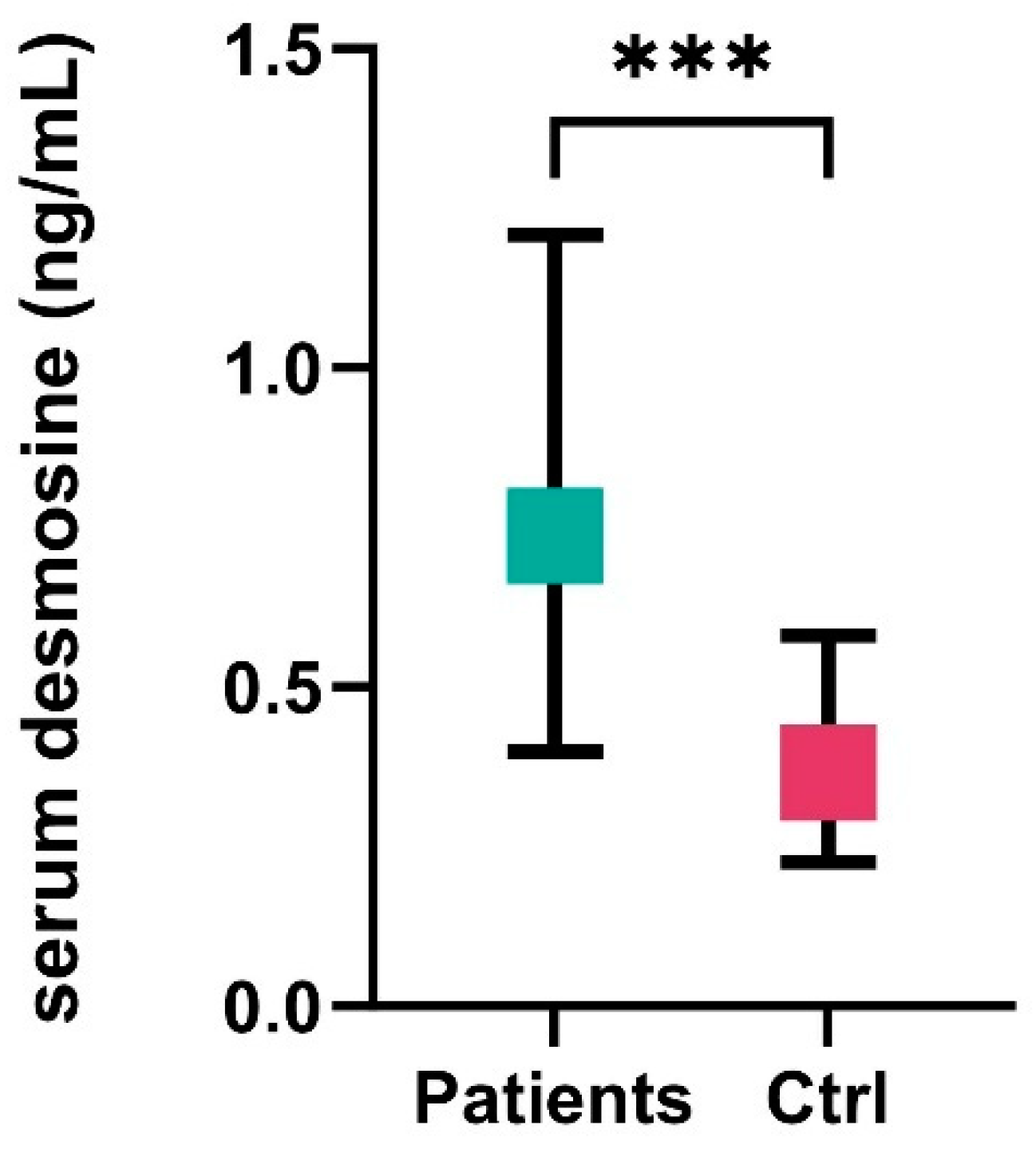
| serum desmosine, ng/mL, median (IQR) | 0.64 (0.37–1.12) | 0.503 (0.285–0.740) | 1.196 (0.714–1.665) | <0.001 |
| serum desmosine, ng/mL, mean ± SD | 0.932 | 0.588 ± 0.46 | 1.281 ± 0.78 | <0.001 |
| <7 mm (n = 69) | >7 mm (n = 66) | |||
|---|---|---|---|---|
| Variable | ρ | p-Value | ρ | p-Value |
| Size of aneurysm | 0.327 | 0.009 | 0.287 | 0.026 |
| Size ratio | −0.336 | 0.008 | −0.006 | 0.966 |
| Aspect ratio | 0.145 | 0.266 | 0.046 | 0.731 |
| Neck width | −0.326 | 0.01 | −0.202 | 0.132 |
| CRP | −0.003 | 0.450 | −0.111 | 0.450 |
| Creatinine | 0.024 | 0.856 | −0.029 | 0.838 |
| Age | 0.229 | 0.071 | −0.116 | 0.380 |
| WFNS score | 0.037 | 0.775 | −0.047 | 0.721 |
| mRS score (3 month) | −0.078 | 0.544 | 0.009 | 0.948 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csecsei, P.; Bogdan, A.; Molnar, T.; Zavori, L.; Schwarcz, A.; Lenzser, G. Serum Desmosine Levels Might Be Associated with the Size of Ruptured Cerebral Aneurysms in Patients with Aneurysmal Subarachnoid Hemorrhage—A Preliminary Study. J. Clin. Med. 2025, 14, 2056. https://doi.org/10.3390/jcm14062056
Csecsei P, Bogdan A, Molnar T, Zavori L, Schwarcz A, Lenzser G. Serum Desmosine Levels Might Be Associated with the Size of Ruptured Cerebral Aneurysms in Patients with Aneurysmal Subarachnoid Hemorrhage—A Preliminary Study. Journal of Clinical Medicine. 2025; 14(6):2056. https://doi.org/10.3390/jcm14062056
Chicago/Turabian StyleCsecsei, Peter, Agnes Bogdan, Tihamer Molnar, Laszlo Zavori, Attila Schwarcz, and Gabor Lenzser. 2025. "Serum Desmosine Levels Might Be Associated with the Size of Ruptured Cerebral Aneurysms in Patients with Aneurysmal Subarachnoid Hemorrhage—A Preliminary Study" Journal of Clinical Medicine 14, no. 6: 2056. https://doi.org/10.3390/jcm14062056
APA StyleCsecsei, P., Bogdan, A., Molnar, T., Zavori, L., Schwarcz, A., & Lenzser, G. (2025). Serum Desmosine Levels Might Be Associated with the Size of Ruptured Cerebral Aneurysms in Patients with Aneurysmal Subarachnoid Hemorrhage—A Preliminary Study. Journal of Clinical Medicine, 14(6), 2056. https://doi.org/10.3390/jcm14062056







