Pharmacokinetic–Pharmacodynamic Simulation of Muscle Relaxation Antagonistic Conditions for Post-Operative Recurarization Prevention
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
- (1)
- Males and females aged 20 years at the time of obtaining consent.
- (2)
- Patients with the American Society of Anesthesiologists–physical status classification system (ASA-PS) of 1–3 who will receive general anesthesia using propofol, remifentanil, and rocuronium.
- (1)
- Patients with a history of hypersensitivity to propofol, remifentanil, rocuronium, or sugammadex.
- (2)
- Patients who could not have the Bispectal Index (BIS) sensor attached during surgery.
- (3)
- Patients who could not receive additional rocuronium after the initial single dose.
- (4)
- Patients who cannot undergo noninvasive blood pressure measurements during surgery.
- (5)
- Patients who underwent hypothermic surgery.
- (6)
- Patients undergoing cardiovascular surgery.
- (7)
- Pregnant, breastfeeding, or planning to become pregnant.
- (8)
- Participation in another study within 12 weeks prior to obtaining consent or planning to participate in another study during the study period.
- (9)
- Patients whom the principal investigator or sub-investigator deems unsuitable for inclusion in the study.
2.2. Anesthesia Procedures
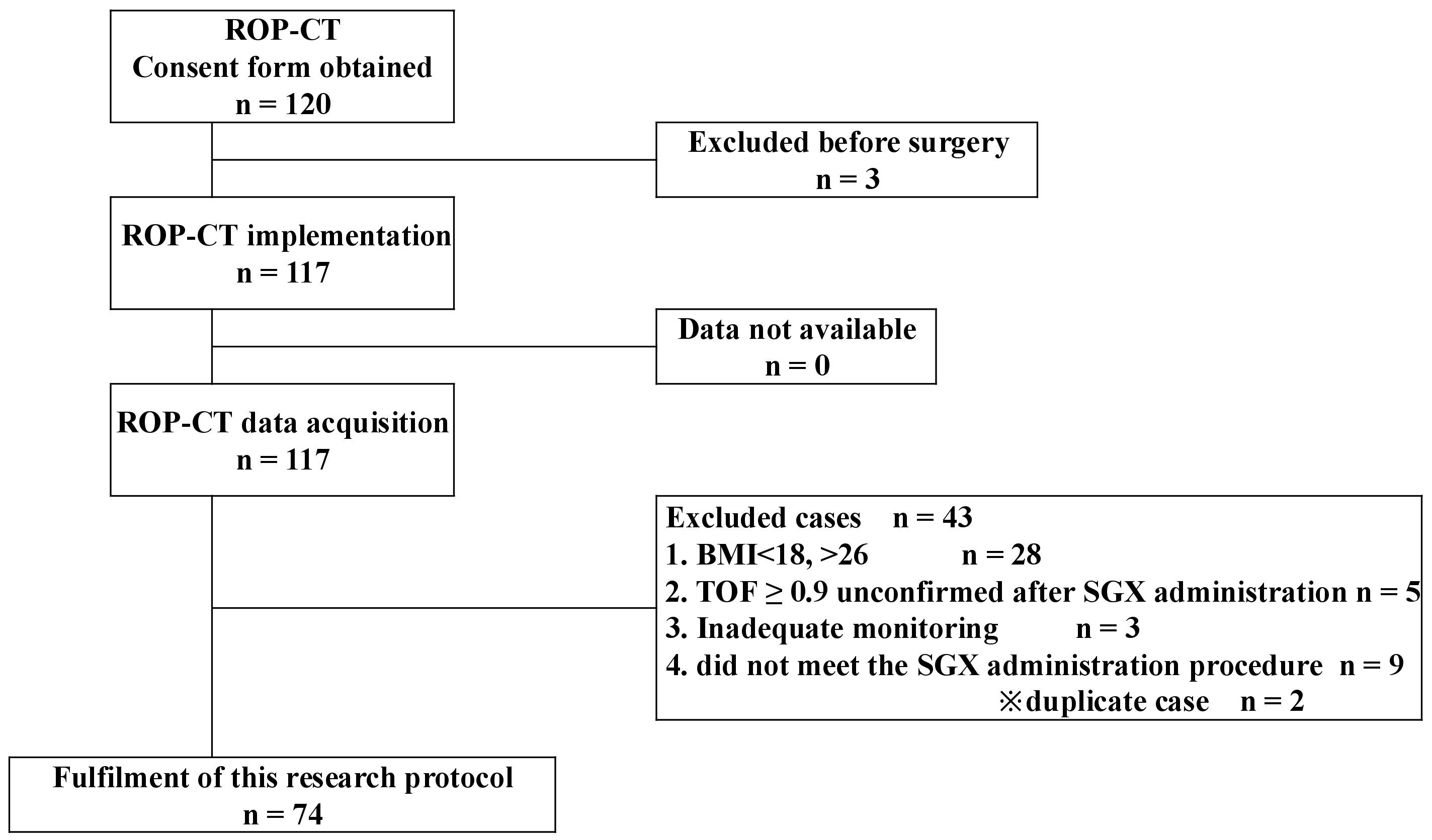
2.3. Analysis of Patient Demographics
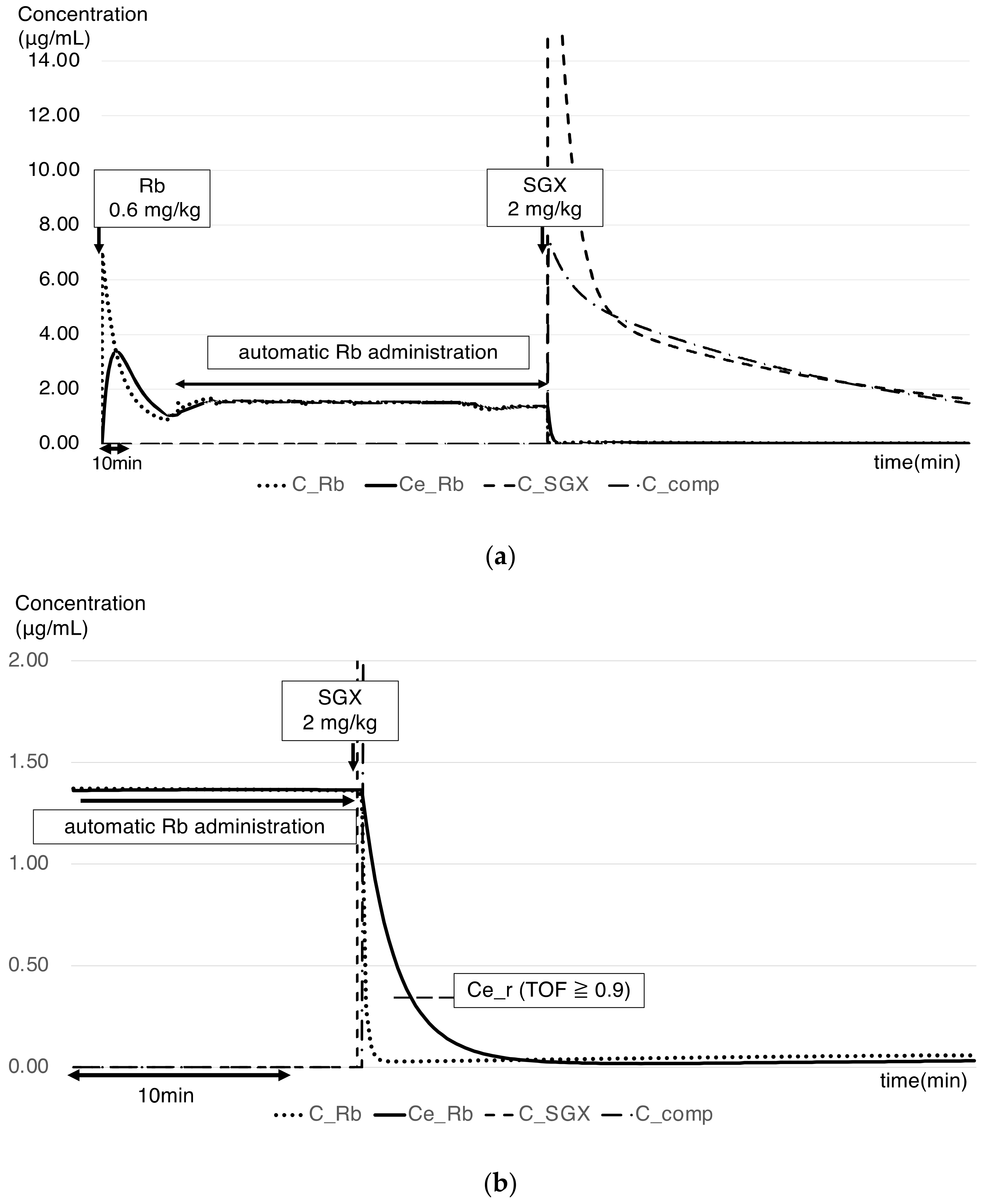
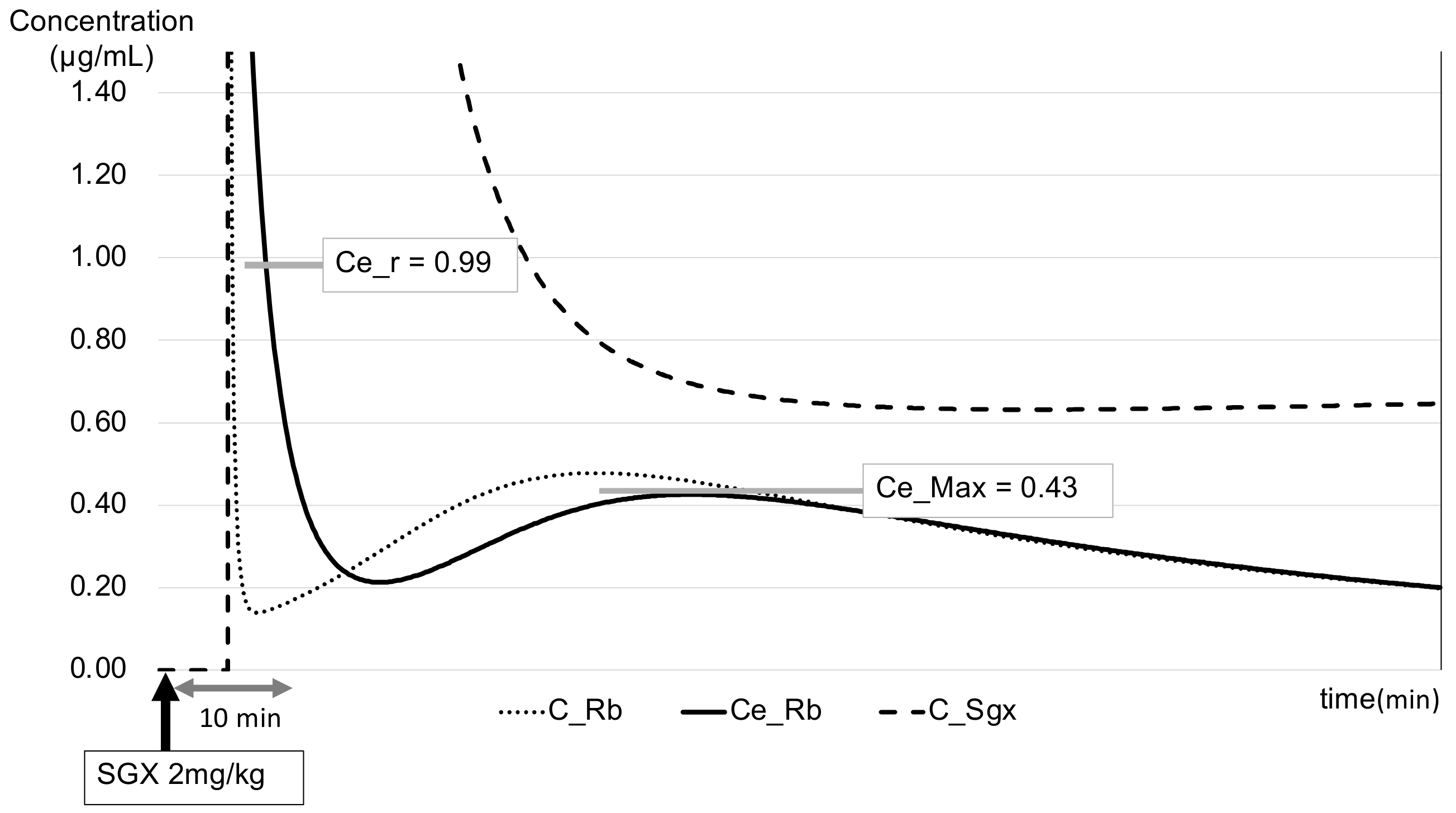
2.4. PK–PD Model of the Rb–SGX Complex
2.5. Statistical Analysis
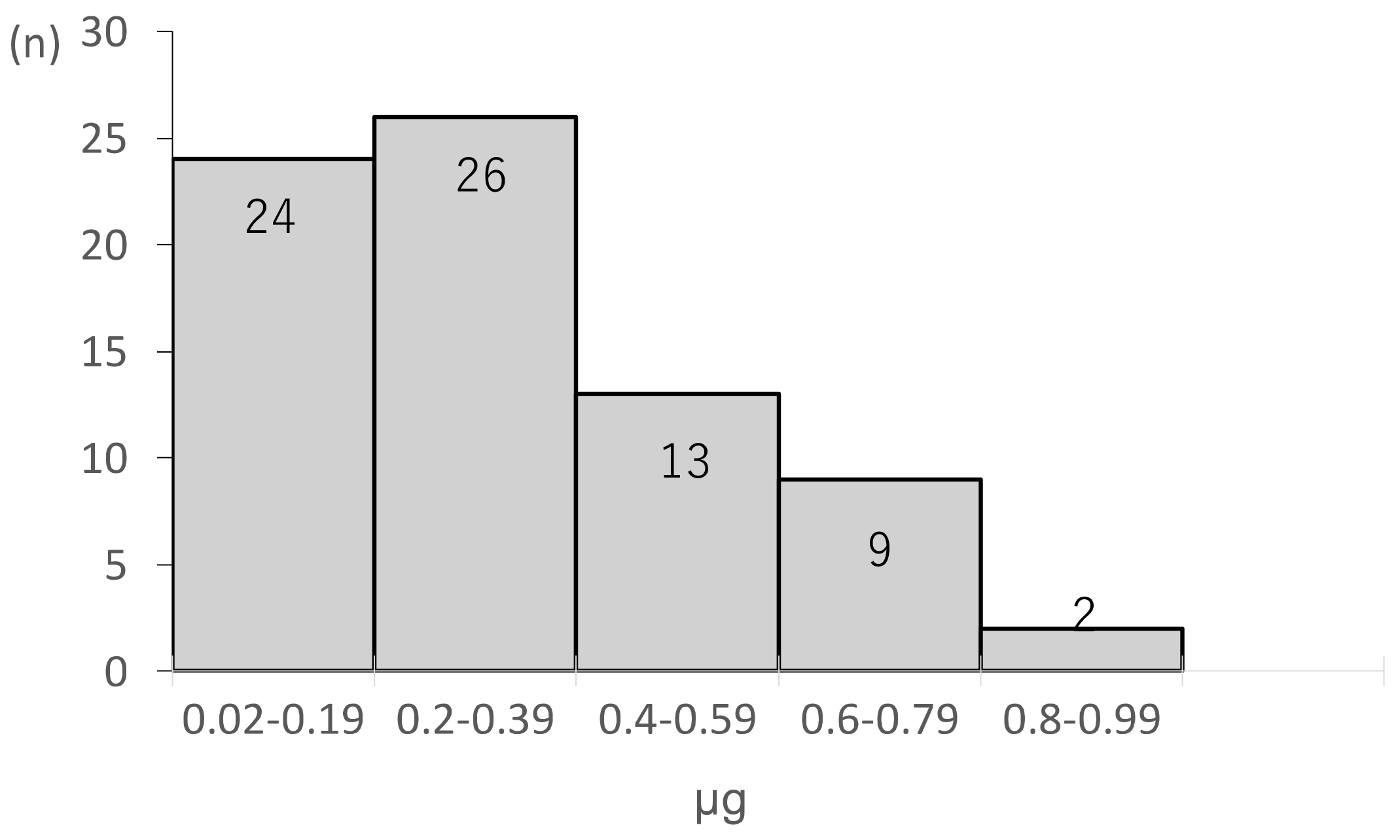
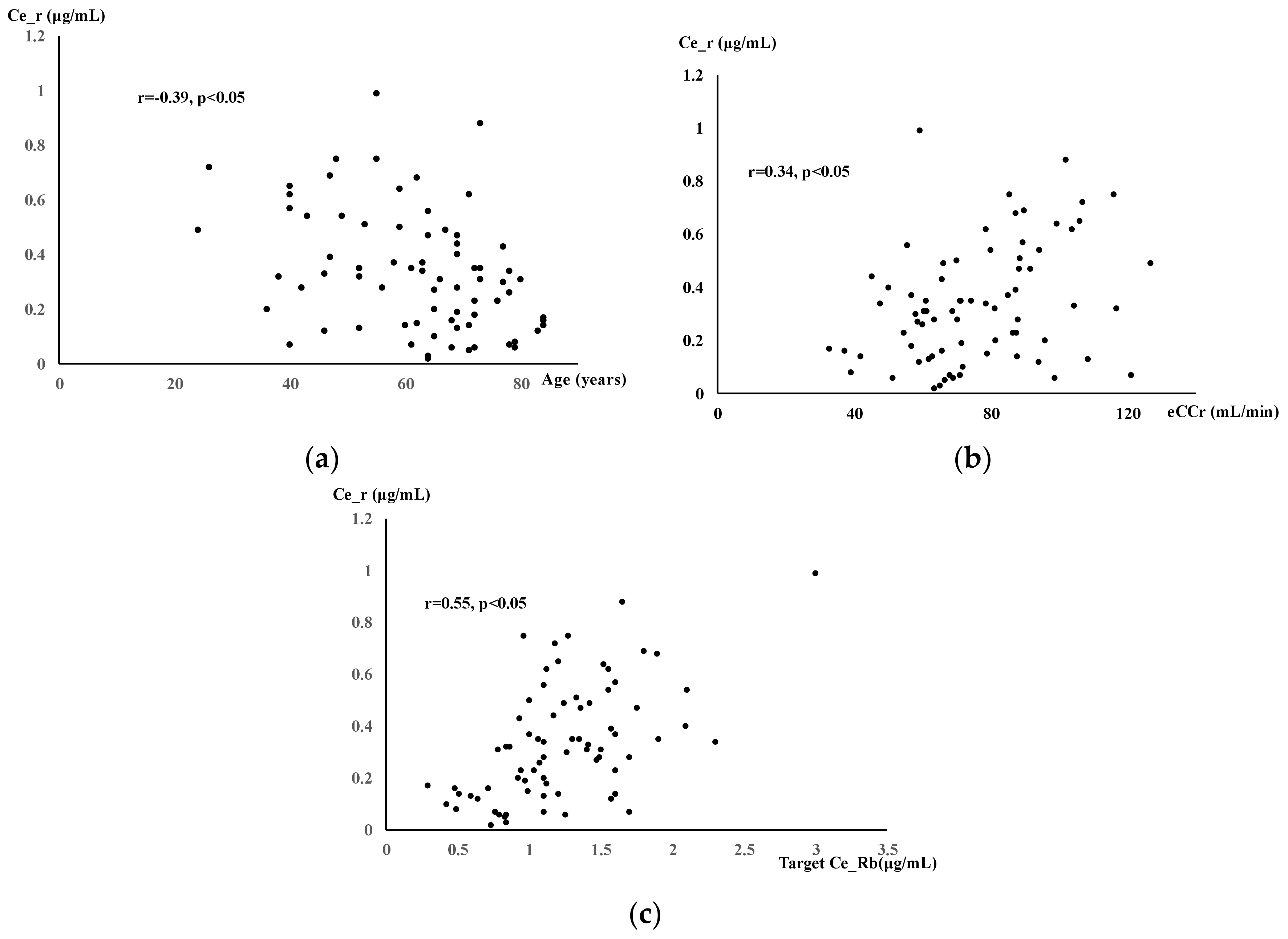
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA-PS | American Society of Anesthesiologists–physical status |
| BIS | Bispectal Index |
| GCRP | Good clinical research practice |
| SaMD | Software as a Medical Device |
| TIVA | Total intravenous anesthesia |
| TOF | Train-of-four |
| TOFC | Train-of-four count |
| TOFR | Train-of-four ratio |
References
- Kleijn, H.J.; Zollinger, D.P.; van den Heuvel, M.W.; Kerbusch, T. Population pharmacokinetic-pharmacodynamic analysis for sugammadex-mediated reversal of rocuronium-induced neuromuscular blockade. Br. J. Clin. Pharmacol. 2011, 72, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Yasuma, F.; Nagata, O.; Sekiguchi, S.; Maehara, Y.; Matsuki, Y.; Shigemi, K. Effect-site concentration of rocuronium at the time of recovery and risk of recurarization in the presence of antagonism of neuromuscular block by sugammadex. Nihon Rinshou Masui Gakkaishi (J. Jpn Soc. Clin. Anesth.) 2022, 42, 7–12. (In Japanese) [Google Scholar] [CrossRef]
- Nagata, O.; Matsuki, Y.; Matsuda, S.; Hazama, K.; Fukunaga, S.; Nakatsuka, H.; Yasuma, F.; Maehara, Y.; Fujioka, S.; Tajima, K.; et al. Anesthesia management via an automated control system for propofol, remifentanil, and rocuronium compared to management by anesthesiologists: An investigator-initiated study. J. Clin. Med. 2023, 12, 6611. [Google Scholar] [CrossRef]
- Wierda, J.M.; Kleef, U.W.; Lambalk, L.M.; Kloppenburg, W.D.; Agoston, S. The pharmacodynamics and pharmacokinetics of Org 9426, a new non-depolarizing neuromuscular blocking agent, in patients anaesthetized with nitrous oxide, halothane and fentanyl. Can. J. Anaesth. 1991, 38, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Ploeger, B.A.; Smeets, J.; Strougo, A.; Drenth, H.J.; Ruigt, G.; Houwing, N.; Danhof, M. Pharmacokinetic-pharmacodynamic model for the reversal of neuromuscular blockade by sugammadex. Anesthesiology 2009, 110, 95–105. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Matsuki, Y.; Nagata, O.; Matsuda, S.; Shigemi, K. The relationship between the measured blood concentration of rocuronium in stable muscle relaxation with a closed-loop control and the estimated blood concentration from a pharmacokinetic simulation. J. Clin. Med. 2024, 13, 3139. [Google Scholar] [CrossRef]
- Kajiura, A.; Nagata, O.; Takizawa, Y.; Nakatomi, T.; Kodera, S.; Murayama, T. A large individual variation in both the infusion rate and the blood concentration of rocuronium necessary for obtain adequate surgical muscle relaxation during total intravenous anesthesia with propofol and remifentanil. J. Anesth. 2015, 29, 9–14. [Google Scholar] [CrossRef]
- Takagi, S.; Suzuki, T.; Nakatsuka, H.; Sasakawa, T.; Iwasaki, H.; Kotake, Y.; Nagata, E.; Kanmura, Y. Comparison of a new EMG module, AF-201P, with acceleromyography using the post-tetanic counts during rocuronium-induced deep neuromuscular block: A prospective, multicenter study. J. Clin. Monit. Comput. 2022, 36, 1347–1353. [Google Scholar] [CrossRef]
- Iwasaki, H.; Sato, H.; Takagi, S.; Kitajima, O.; Luthe, S.K.; Suzuki, T. A comparison between the adductor pollicis muscle and the abductor digiti minimi muscle using electromyography AF-201P in rocuronium-induced neuromuscular block: A prospective comparative study. BMC Anesthesiol. 2022, 22, 117. [Google Scholar] [CrossRef]
- Pühringer, F.K.; Gordon, M.; Demeyer, I.; Sparr, H.J.; Ingimarsson, J.; Klarin, B.; van Duijnhoven, W.; Heeringa, M. Sugammadex rapidly reverses moderate rocuronium- or vecuronium-induced neuromuscular block during sevoflurane anaesthesia: A dose-response relationship. Br. J. Anaesth. 2010, 105, 610–619. [Google Scholar] [CrossRef]
- Duvaldestin, P.; Kuizenga, K.; Saldien, V.; Claudius, C.; Servin, F.; Klein, J.; Debaene, B.; Heeringa, M. A randomized, dose-response study of sugammadex given for the reversal of deep rocuronium- or vecuronium-induced neuromuscular blockade under sevoflurane anesthesia. Anesth. Analg. 2010, 110, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Otomo, S.; Iwasaki, H.; Takahoko, K.; Onodera, Y.; Sasakawa, T.; Kunisawa, T.; Iwasaki, H. Prediction of optimal reversal dose of sugammadex after rocuronium administration in adult surgical patients. Anesthesiol. Res. Pract. 2014, 2014, 848051. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, D.L.; Benedict, P.E.; Kovac, A.L.; Drover, D.R.; Brister, N.W.; Morte, J.B.; Monk, T.G. Efficacy, safety, and pharmacokinetics of sugammadex for the reversal of rocuronium-induced neuromuscular blockade in elderly patients. Anesthesiology 2011, 114, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Carron, M.; Bertoncello, F.; Ieppariello, G. Profile of sugammadex for reversal of neuromuscular blockade in the elderly: Current perspectives. Clin. Interv. Aging 2018, 13, 13–24. [Google Scholar] [CrossRef]
- Yazar, E.; Yılmaz, C.; Bilgin, H.; Karasu, D.; Bayraktar, S.; Apaydın, Y.; Sayan, H.E. A comparison of the effect of sugammadex on the recovery period and postoperative residual block in young elderly and middle-aged elderly patients. Balk. Med. J. 2016, 33, 181–187. [Google Scholar] [CrossRef]
- Suzuki, T.; Kitajima, O.; Ueda, K.; Kondo, Y.; Kato, J.; Ogawa, S. Reversibility of rocuronium-induced profound neuromuscular block with sugammadex in younger and older patients. Br. J. Anaesth. 2011, 106, 823–826. [Google Scholar] [CrossRef]
- Staals, L.M.; Snoeck, M.M.; Driessen, J.J.; Flockton, E.A.; Heeringa, M.; Hunter, J.M. Multicentre, parallel-group, comparative trial evaluating the efficacy and safety of sugammadex in patients with end-stage renal failure or normal renal function. Br. J. Anaesth. 2008, 101, 492–497. [Google Scholar] [CrossRef]
- Min, K.C.; Lasseter, K.C.; Marbury, T.C.; Wrishko, R.E.; Hanley, W.D.; Wolford, D.G.; Udo de Haes, J.U.; Reitmann, C.; Gutstein, D.E. Pharmacokinetics of sugammadex in subjects with moderate and severe renal impairment. Int. J. Clin. Pharmacol. Ther. 2017, 55, 746–752. [Google Scholar] [CrossRef]
- Oh, S.K.; Lim, B.G. Sugammadex administration in patients with end-stage renal disease: A narrative review with recommendations. Anesth. Pain Med. 2023, 18, 11–20. [Google Scholar] [CrossRef]
- de Souza, C.M.; Tardelli, M.A.; Tedesco, H.; Garcia, N.N.; Caparros, M.P.; Alvarez-Gomez, J.A.; de Oliveira Junior, I.S. Efficacy and safety of sugammadex in the reversal of deep neuromuscular blockade induced by rocuronium in patients with end-stage renal disease: A comparative prospective clinical trial. Eur. J. Anaesthesiol. 2015, 32, 681–686. [Google Scholar] [CrossRef]
- Magorian, T.; Wood, P.; Caldwell, J.; Fisher, D.; Segredo, V.; Szenohradszky, J.; Sharma, M.; Gruenke, L.; Miller, R. The pharmacokinetics and neuromuscular effects of rocuronium bromide in patients with liver disease. Anesthe. Analg. 1995, 80, 754–759. [Google Scholar] [CrossRef]
- Leykin, Y.; Pellis, T.; Lucca, M.; Lomangino, G.; Marzano, B.; Gullo, A. The pharmacodynamic effects of rocuronium when dosed according to real body weight or ideal body weight in morbidly obese patients. Anesthe. Analg. 2004, 99, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Armendáriz, B.I.; Lobato, S.F.; Aguilera, C.L.; Morros, D.E.; Fraile, J.E.; Vera, B.J. Residual neuromascular block in type II diabetes mellitus after rocuronium: A prospective observational study. Eur. J. Anaesthesiol. 2014, 31, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Spacek, A.; Neiger, F.X.; Krenn, C.G.; Hoerauf, K.; Kress, H.G. Rocuronium-induced neuromuscular block is affected by chronic carbamazepine therapy. Anesthesiology 1999, 90, 109–112. [Google Scholar] [CrossRef]
- Fuchs-Buder, T.; Brull, S.J.; Fagerlund, M.J.; Renew, J.R.; Cammu, G.; Murphy, G.S.; Warlé, M.; Vested, M.; Fülesdi, B.; Nemes, R.; et al. Good clinical research practice (GCRP) in pharmacodynamic studies of neuromuscular blocking agents III: The 2023 Geneva revision. Acta Anaesthesiol. Scand. 2023, 67, 994–1017. [Google Scholar] [CrossRef]
| Background Factors | Values | |
|---|---|---|
| Sex | Male/Female | 25:49 |
| Age (years) | Mean ± SD | 62.2 ± 14.0 |
| Height (cm) | Mean ± SD | 159.0 ± 7.8 |
| Weight (kg) | Mean ± SD | 56.3 ± 8.1 |
| BMI (kg/m2) | Mean ± SD | 22.3 ± 2.2 |
| ASA-PS | I:II:III | 21:51:2 |
| eCCr (mL/min) | Mean ± SD | 75.9 ± 20.6 |
| Operation time (h) | Mean ± SD | 2.5 ± 1.0 |
| Rb dose (mg/operation time (h)) | Mean ± SD | 43.8 ± 16.1 |
| Total SGX dose (mg/kg) | Mean ± SD | 2.2 ± 0.4 |
| Recovery time (min) (from SGX administration till the TOFR reached ≥0.9) | Mean ± SD | 2.9 ± 1.1 |
| Factors | Group NA n = 50 | Group A n = 24 | p-Value |
|---|---|---|---|
| Age (years) | 60.1 ± 15.1 | 66.5 ± 9.9 | 0.067 |
| Actual body weight (kg) | 56.8 ± 8.3 | 55.6 ± 7.8 | 0.557 |
| eCCr (mL/min) | 77.4 ± 20.7 | 72.8 ± 19.9 | 0.380 |
| Rb dose (mg/h) | 44.2 ± 17.6 | 42.1 ± 12.5 | 0.602 |
| Target Ce_Rb (μg/mL) | 1.25 ± 0.48 | 1.18 ± 0.41 | 0.531 |
| Ce_r (μg/mL) | 0.42 ± 0.2 | 0.16 ± 0.11 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuma, F.; Nagata, O.; Matsuki, Y.; Shigemi, K. Pharmacokinetic–Pharmacodynamic Simulation of Muscle Relaxation Antagonistic Conditions for Post-Operative Recurarization Prevention. J. Clin. Med. 2025, 14, 2043. https://doi.org/10.3390/jcm14062043
Yasuma F, Nagata O, Matsuki Y, Shigemi K. Pharmacokinetic–Pharmacodynamic Simulation of Muscle Relaxation Antagonistic Conditions for Post-Operative Recurarization Prevention. Journal of Clinical Medicine. 2025; 14(6):2043. https://doi.org/10.3390/jcm14062043
Chicago/Turabian StyleYasuma, Fumiyo, Osamu Nagata, Yuka Matsuki, and Kenji Shigemi. 2025. "Pharmacokinetic–Pharmacodynamic Simulation of Muscle Relaxation Antagonistic Conditions for Post-Operative Recurarization Prevention" Journal of Clinical Medicine 14, no. 6: 2043. https://doi.org/10.3390/jcm14062043
APA StyleYasuma, F., Nagata, O., Matsuki, Y., & Shigemi, K. (2025). Pharmacokinetic–Pharmacodynamic Simulation of Muscle Relaxation Antagonistic Conditions for Post-Operative Recurarization Prevention. Journal of Clinical Medicine, 14(6), 2043. https://doi.org/10.3390/jcm14062043





