Peripheral Arterial Disease in Diabetic Foot: One Disease with Multiple Patterns
Abstract
1. Introduction
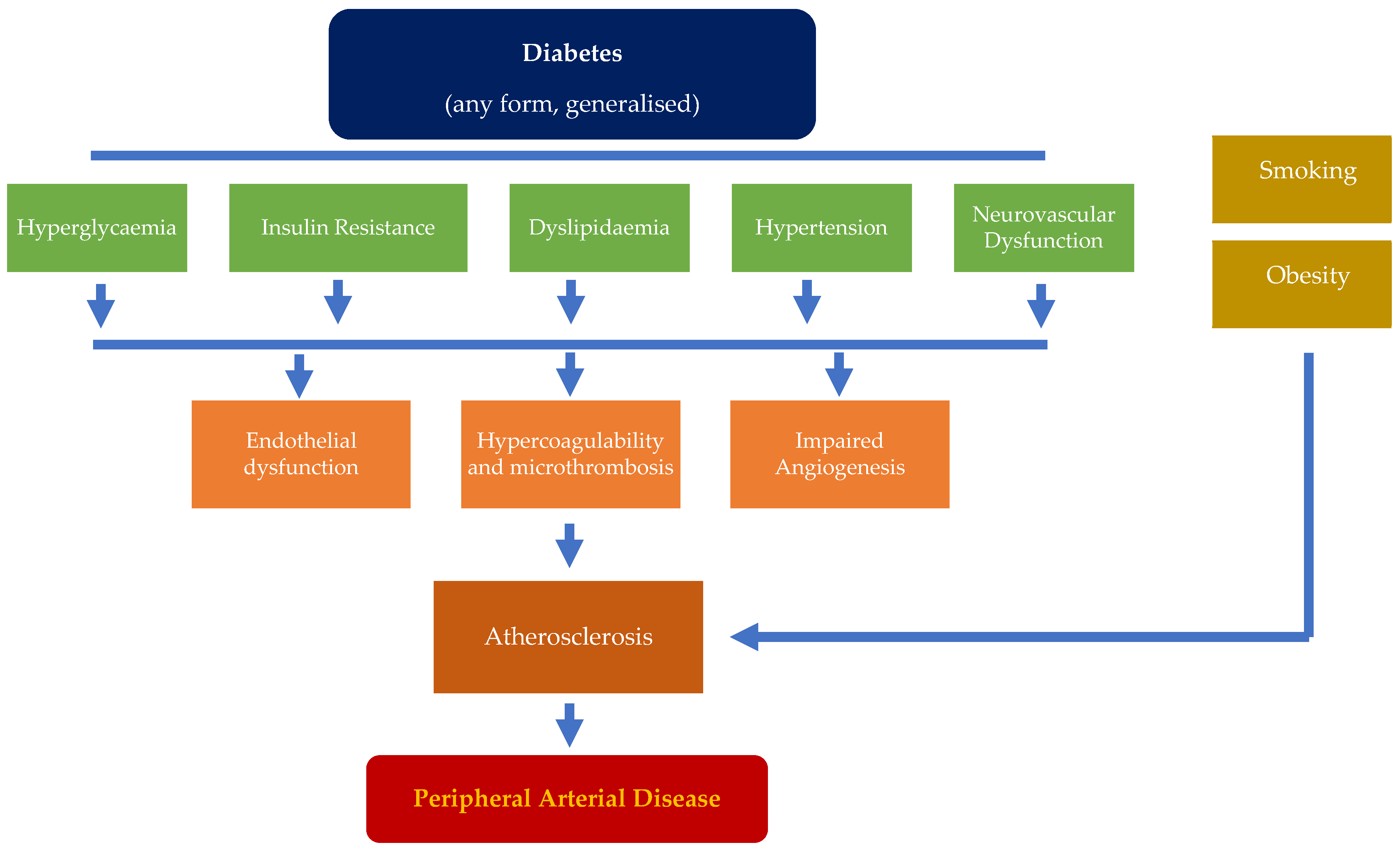
2. Pathophysiology in the Development of PAD
3. Common Characteristics and Anatomical Distribution of PAD in Persons with Diabetic Foot
4. Evolution and New Pattern of PAD in Patients with Diabetic Foot Ulcers
5. The Small Artery Disease: A Mysterious Pattern of PAD
6. Diagnostic Assessment
7. Treatment
8. Early Detection of PAD and Global Management
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armstrong, D.G.; Cohen, K.; Courric, S.; Bharara, M.; Marston, W. Diabetic foot ulcers and vascular insufficiency: Our population has changed, but our methods have not. J. Diabetes Sci. Technol. 2011, 5, 1591–1595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meloni, M.; Piaggesi, A.; Uccioli, L. From a Spark to a Flame: The Evolution of Diabetic Foot Disease in the Last Two Decades. Int. J. Low. Extrem. Wounds, 2024; ahead of print. 15347346241238480. [Google Scholar] [CrossRef]
- Meloni, M.; Izzo, V.; Giurato, L.; Lázaro-Martínez, J.L.; Uccioli, L. Prevalence, Clinical Aspects and Outcomes in a Large Cohort of Persons with Diabetic Foot Disease: Comparison between Neuropathic and Ischemic Ulcers. J. Clin. Med. 2020, 9, 1780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faglia, E.; Clerici, G.; Clerissi, J.; Gabrielli, L.; Losa, S.; Mantero, M.; Caminiti, M.; Curci, V.; Quarantiello, A.; Lupattelli, T.; et al. Long-term prognosis of diabetic patients with critical limb ischemia: A population-based cohort study. Diabetes Care 2009, 32, 822–827, Erratum in Diabetes Care 2009, 32, 1355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meloni, M.; Izzo, V.; Giurato, L.; Cervelli, V.; Gandini, R.; Uccioli, L. Impact of heart failure and dialysis in the prognosis of diabetic patients with ischemic foot ulcers. J. Clin. Transl. Endocrinol. 2018, 11, 31–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fitridge, R.; Chuter, V.; Mills, J.; Hinchliffe, R.; Azuma, N.; Behrendt, C.A.; Boyko, E.J.; Conte, M.S.; Humphries, M.; Kirksey, L.; et al. The intersocietal IWGDF, ESVS, SVS guidelines on peripheral artery disease in people with diabetes and a foot ulcer. Diabetes Metab. Res. Rev. 2024, 40, e3686. [Google Scholar] [CrossRef] [PubMed]
- Soyoye, D.O.; Abiodun, O.O.; Ikem, R.T.; Kolawole, B.A.; Akintomide, A.O. Diabetes and peripheral artery disease: A review. World J. Diabetes 2021, 12, 827–838. [Google Scholar] [CrossRef]
- Rask-Madsen, C.; King, G.L. Mechanisms of Disease: Endothelial dysfunction in insulin resistance and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 46–56. [Google Scholar] [CrossRef]
- Drexel, H.; Aczel, S.; Marte, T.; Benzer, W.; Langer, P.; Moll, W.; Saely, C.H. Is atherosclerosis in diabetes and impaired fasting glucose driven by elevated LDL cholesterol or by decreased HDL cholesterol? Diabetes Care 2005, 28, 101–107. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Scirica, B.M.; Bonaca, M.P.; Steg, P.G.; Mosenzon, O.; Hirshberg, B.; Im, K.; Raz, I.; Braunwald, E.; Bhatt, D.L. Prevalence and outcomes of polyvascular (coronary, peripheral, or cerebrovascular) disease in patients with diabetes mellitus (from the SAVOR-TIMI 53 trial). Am. J. Cardiol. 2019, 123, 145–152. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of peripheral artery disease and polyvascular disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Jandeleit-Dahm, K.A.M. Atherosclerosis in diabetes mellitus: Novel mechanisms and mechanism-based therapeutic approaches. Nat. Rev. Cardiol. 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Magri, C.J.; Mintoff, D.; Camilleri, L.; Xuereb, R.G.; Galea, J.; Fava, S. Relationship of hyperglycaemia, hypoglycaemia, and glucose variability to atherosclerotic disease in type 2 diabetes. J. Diabetes Res. 2018, 2018, 7464320. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, R.; Brownrigg, J.; Hinchliffe, R. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes. Metab. 2015, 17, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Beverly, J.K.; Budoff, M.J. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J. Diabetes 2020, 12, 102–104. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Domínguez-Montero, A.; Funcasta-Calderón, R. Insulin resistance is associated with subclinical vascular disease in humans. World J. Diabetes 2019, 10, 63. [Google Scholar] [CrossRef]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of cardiovascular diseases: New insights into molecular mechanisms of atherosclerosis, arterial hypertension, and coronary artery disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Hurtubise, J.; McLellan, K.; Durr, K.; Onasanya, O.; Nwabuko, D.; Ndisang, J.F. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr. Atheroscler. Rep. 2016, 18, 1–12. [Google Scholar] [CrossRef]
- Murakami, T. Atherosclerosis and arteriosclerosis. Hypertens. Res. 2023, 46, 1810–1811. [Google Scholar] [CrossRef]
- Owens, C.D.; Mukli, P.; Csipo, T.; Lipecz, A.; Silva-Palacios, F.; Dasari, T.W.; Tarantini, S.; Gardner, A.W.; Montgomery, P.S.; Waldstein, S.R.; et al. Microvascular dysfunction and neurovascular uncoupling are exacerbated in peripheral artery disease, increasing the risk of cognitive decline in older adults. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H924–H935. [Google Scholar] [CrossRef]
- Forst, T.; Pfützner, A.; Kunt, T.; Pohlmann, T.; Schenk, U.; Bauersachs, R.; Küstner, E.; Beyer, J. Skin microcirculation in patients with type I diabetes with and without neuropathy after neurovascular stimulation. Clin. Sci. 1998, 94, 255–261. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Mota, A.P.; de Castro Santos, M.E.; Lima e Silva Fd de Carvalho Schachnik, N.C.; de Oliveira Sousa, M.; das Graças Carvalho, M. Hypercoagulability markers in patients with peripheral arterial disease: Association to ankle-brachial index. Angiology 2009, 60, 529–535. [Google Scholar]
- Vaidya, A.R.; Wolska, N.; Vara, D.; Mailer, R.K.; Schröder, K.; Pula, G. Diabetes and thrombosis: A central role for vascular oxidative stress. Antioxidants 2021, 10, 706. [Google Scholar] [CrossRef]
- Weissberg, P.L. Atherogenesis: Current understanding of the causes of atheroma. Heart 2000, 83, 247. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.-I.; Matsui, T. Role of hyperglycemia-induced advanced glycation end product (AGE) accumulation in atherosclerosis. Ann. Vasc. Dis. 2018, 11, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Vyavahare, N.R. High-glucose levels and elastin degradation products accelerate osteogenesis in vascular smooth muscle cells. Diabetes Vasc. Dis. Res. 2013, 10, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, M.S.; Cortizo, A.M.; Sedlinsky, C. Effects of advanced glycation end-products, diabetes and metformin on the osteoblastic transdifferentiation capacity of vascular smooth muscle cells: In Vivo and In Vitro studies. J. Diabetes Its Complicat. 2023, 37, 108626. [Google Scholar] [CrossRef]
- Ndip, A.; Williams, A.; Jude, E.B.; Serracino-Inglott, F.; Richardson, S.; Smyth, J.V.; Boulton, A.J.; Alexander, M.Y. The RANKL/RANK/OPG Signaling Pathway Mediates Medial Arterial Calcification in Diabetic Charcot Neuroarthropathy. Diabetes 2011, 60, 2187–2196. [Google Scholar] [CrossRef]
- Edmonds, M. Medial arterial calcification and diabetes mellitus. Z. Für Kardiol. 2000, 89 (Suppl. 2), S101–S104. [Google Scholar] [CrossRef]
- Veves, A.; Akbari, C.M.; Primavera, J.; Donaghue, V.M.; Zacharoulis, D.; Chrzan, J.S.; DeGirolami, U.; LoGerfo, F.W.; Freeman, R. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 1998, 47, 457–463. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Skeik, N.; Elejla, S.A.; Sethi, A.; Manunga, J.; Mirza, A. Effects of SGLT2 inhibitors and GLP1-receptor agonists on cardiovascular and limb events in peripheral artery disease: A review. Vasc. Med. 2023, 28, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Lamacchia, O.; Sorrentino, M.R. Diabetes mellitus, arterial stiffness and cardiovascular disease: Clinical implications and the influence of SGLT2i. Curr. Vasc. Pharmacol. 2021, 19, 233–240. [Google Scholar] [CrossRef]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef] [PubMed]
- Low Wang, C.C.; Blomster, J.I.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; Norgren, L.; Jones, W.S.; et al. Cardiovascular and Limb Outcomes in Patients with Diabetes and Peripheral Artery Disease: The EUCLID Trial. J. Am. Coll. Cardiol. 2018, 72, 3274–3284. [Google Scholar] [CrossRef]
- Ix, J.H.; Miller, R.G.; Criqui, M.H.; Orchard, T.J. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J. Vasc. Surg. 2012, 56, 721–727. [Google Scholar] [CrossRef]
- Jude, E.B.; Oyibo, S.O.; Chalmers, N.; Boulton, A.J.M. Peripheral arterial disease in diabetic and nondiabetic patient A comparison of severity and outcome. Diabetes Care 2001, 24, 1433–1437. [Google Scholar] [CrossRef]
- Ciavarella, A.; Silletti, A.; Mustacchio, A.; Gargiulo, M.; Galaverni, M.C.; Stella, A.; Vannini, P. Angiographic evaluation of the anatomic pattern of arterial obstructions in diabetic patients with critical limb ischemia. Diabet. Med. 1993, 19, 586–589. [Google Scholar]
- Graziani, L.; Silvestro, A.; Bertone, V.; Manara, E.; Andreini, R.; Sigala, A.; Mingardi, R.; De Giglio, R. Vascular involvement in diabetic subjects with ischemic foot ulcer: A new morphologic categorization of disease severity. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 453–460. [Google Scholar] [CrossRef]
- Ferraresi, R.; Mauri, G.; Losurdo, F.; Troisi, N.; Brancaccio, D.; Caravaggi, C.; Neri, L. BAD transmission and SAD distribution: A new scenario for critical limb ischemia. J. Cardiovasc. Surg. 2018, 59, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Izzo, V.; Giurato, L.; Gandini, R.; Uccioli, L. Below-the-ankle arterial disease severely impairs the outcomes of diabetic patients with ischemic foot ulcers. Diabetes Res. Clin. Pract. 2019, 152, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Bellia, A.; Giurato, L.; Lauro, D.; Uccioli, L. Below-the-ankle arterial disease: A new marker of coronary artery disease in patients with diabetes and foot ulcers. Acta Diabetol. 2022, 59, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi, R.; Ucci, A.; Pizzuto, A.; Losurdo, F.; Caminiti, M.; Minnella, D.; Casini, A.; Clerici, G.; Montero-Baker, M.; Mills, J. A Novel Scoring System for Small Artery Disease and Medial Arterial Calcification Is Strongly Associated with Major Adverse Limb Events in Patients with Chronic Limb-Threatening Ischemia. J. Endovasc. Ther. 2021, 28, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Leibson, C.L.; Ransom, J.E.; Olson, W.; Zimmerman, B.R.; O’Fallon, W.M.; Palumbo, P.J. Peripheral arterial disease, diabetes, and mortality. Diabetes Care 2004, 27, 2843–2849. [Google Scholar] [CrossRef]
- Chuter, V.; Schaper, N.; Mills, J.; Hinchliffe, R.; Russell, D.; Azuma, N.; Behrendt, C.A.; Boyko, E.J.; Conte, M.S.; Humphries, M.; et al. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: A systematic review. Diabetes Metab. Res. Rev. 2024, 40, e3683. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Adams, J.E.; Anderson, G.F.; Boulton, A.J.; Cavanagh, P.R. Medial arterial calcification in the feet of diabetic patients and matched non-diabetic control subjects. Diabetologia 1993, 36, 10. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar]
- Pickwell, K.M.; Siersma, V.D.; Kars, M.; Holstein, P.E.; Schaper, N.C. Diabetic foot disease: Impact of ulcer location on ulcer healing. Diabetes Metab. Res. Rev. 2013, 29, 377–383. [Google Scholar] [CrossRef]
- Kalani, M.; Brismar, K.; Fagrell, B.; Ostergren, J.; Jörneskog, G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers. Diabetes Care 1999, 22, 147–151. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef] [PubMed]
- Barshes, N.R.; Flores, E.; Belkin, M.; Kougias, P.; Armstrong, D.G.; Mills, J.L.S. The accuracy and cost-effectiveness of strategies used to identify peripheral artery disease among patients with diabetic foot ulcers. YMVA 2016, 64, 1682. [Google Scholar] [CrossRef] [PubMed]
- Izzo, V.; Meloni, M.; Fabiano, S.; Morosetti, D.; Giurato, L.; Chiaravalloti, A.; Ruotolo, V.; Gandini, R.; Uccioli, L. Rearfoot Transcutaneous Oximetry is a Useful Tool to Highlight Ischemia of the Heel. Cardiovasc. Interv. Radiol. 2017, 40, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.; Luigi, U.; Valeria, R.; Ermanno, B.; Carlo, M.; Maria, R.; Aikaterini, A.; Laura, G.; Alfonso, B.; Davide, L. Effectiveness of autologous mononuclear cells as adjuvant therapy in patients with ischaemic diabetic foot ulcers receiving indirect lower limb revascularization. Acta Diabetol. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ragghianti, B.; Berardi, B.M.; Mannucci, E.; Monami, M. Autologous Peripheral Blood Mononuclear Cells in Patients with Small Artery Disease and Diabetic Foot Ulcers: Efficacy, Safety, and Economic Evaluation. J. Clin. Med. 2023, 12, 4148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berchiolli, R.; Bertagna, G.; Adami, D.; Piaggesi, A.; Iacopi, E.; Giangreco, F.; Torri, L.; Troisi, N. Peripheral Interventional Strategy Assessment (PISA) for Diabetic Foot Ulcer Revascularization: Preliminary Outcomes of a Multidisciplinary Pilot Study. Diagnostics 2023, 13, 2879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collins, R.; Burch, J.; Cranny, G.; Aguiar-Ibáñez, R.; Craig, D.; Wright, K.; Berry, E.; Gough, M.; Kleijnen, J.; Westwood, M. Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: Systematic review. BMJ 2007, 334, 1257e66. [Google Scholar] [CrossRef]
- Bradbury, A.W.; Adam, D.J. Diagnosis of peripheral arterial disease of the lower limb. BMJ 2007, 334, 1229–1230. [Google Scholar] [CrossRef]
- Aiello, A.; Anichini, R.; Brocco, E.; Caravaggi, C.; Chiavetta, A.; Cioni, R.; Da Ros, R.; De Feo, M.E.; Ferraresi, R.; Florio, F.; et al. Treatment of peripheral arterial disease in diabetes: A consensus of the Italian Societies of Diabetes (SID, AMD), Radiology (SIRM) and Vascular Endovascular Surgery (SICVE). Nutr. Metab. Cardiovasc. Dis. 2014, 24, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.; Lilja, E.; Orneholm, H.; Gottsäter, A.; Eneroth, M.; Acosta, S. Amputation-Free Survival in Patients with Diabetes Mellitus and Peripheral Arterial Disease with Heel Ulcer: Open Versus Endovascular Surgery. Vasc. Endovasc. Surg. 2019, 53, 118–125. [Google Scholar] [CrossRef]
- Butt, T.; Lilja, E.; Elgzyri, T.; Apelqvist, J.; Gottsäter, A.; Engström, G.; Acosta, S. Amputation-free Survival in Patients With Diabetic Foot Ulcer and Peripheral Arterial Disease: Endovascular Versus Open Surgery in a Propensity Score Adjusted Analysis. J. Diabetes Complicat. 2020, 34, 107551. [Google Scholar] [CrossRef]
- Silvestro, M.; Palena, L.M.; Manzi, M.; Gómez-Jabalera, E.; Vishwanath, D.; Casini, A.; Ferraresi, R. Anterolateral RetrogradeAccess to the Distal Popliteal Artery and to the Tibioperoneal Trunk for Recanalization of Femoropopliteal Chronic Total Occlusions. J. Vasc. Surg. 2018, 68, 1824–1832. [Google Scholar] [CrossRef]
- Gandini, R.; Del Giudice, C.; Merolla, S.; Morosetti, D.; Pampana, E.; Simonetti, G. Treatment of Chronic SFA In-Stent Occlusion with Combined Laser Atherectomy and Drug-Eluting Balloon Angioplasty in Patients with Critical Limb Ischemia: A Single-Center, Prospective, Randomized Study. J. Endovasc. Ther. 2013, 20, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Faglia, E.; Clerici, G.; Clerissi, J.; Mantero, M.; Caminiti, M.; Quarantiello, A.; Curci, V.; Lupattelli, T.; Morabito, A. When is a technically successful peripheral angioplasty effective in preventing above-the-ankle amputation in diabetic patients with critical limb ischaemia? Diabet. Med. 2007, 24, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Huizing, E.; Schreve, M.A.; de Vries, J.P.M.; Ferraresi, R.; Kum, S.; Ünlü, Ç. Below-the-Ankle Angioplasty in Patients With Critical Limb Ischemia: A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2019, 30, 1361–1368.e2. [Google Scholar] [CrossRef]
- Meloni, M.; Morosetti, D.; Giurato, L.; Stefanini, M.; Loreni, G.; Doddi, M.; Panunzi, A.; Bellia, A.; Gandini, R.; Brocco, E.; et al. Foot Revascularization Avoids Major Amputation in Persons with Diabetes and Ischaemic Foot Ulcers. J. Clin. Med. 2021, 10, 3977. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gandini, R.; Uccioli, L.; Spinelli, A.; Del Giudice, C.; Da Ros, V.; Volpi, T.; Meloni, M.; Simonetti, G. Alternative techniques for treatment of complex below-the knee arterial occlusions in diabetic patients with critical limb ischemia. Cardiovasc. Interv. Radiol. 2013, 36, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nezzo, M.; Meloni, M.; Carini, A.; Carreri, B.; Nicita, F.; Garaci, F.; Morosetti, D. Efficacy of retrograde revascularization in diabetic patients with chronic limb-threatening ischemia after a failed antegrade approach. Vascular, 2024; ahead of print. 17085381241256022. [Google Scholar] [CrossRef] [PubMed]
- Adami, D.; Marconi, M.; Piaggesi, A.; Mocellin, D.M.; Berchiolli, R.N.; Ferrari, M. Bifurcated bypass in severe chronic limb threatening ischaemia. Vascular 2022, 30, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Troisi, N.; Adami, D.; Piaggesi, A.; Canovaro, F.; Pieruzzi, L.; Torri, L.; Ferrari, M.; Berchiolli, R. Non-reversed bifurcated vein graft improves time to healing in ischemic patients undergoing lower limb distal bypass. Int. Angiol. 2023, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.; Slim, H.; Zayed, H.; Huang, D.Y.; Wilkins, C.J.; Evans, D.R.; Sidhu, P.S.; Edmonds, M. The impact of arterial pedal arch quality and angiosome revascularization on foot tissue loss healing and infrapopliteal bypass outcome. J. Vasc. Surg. 2013, 57, 1219–1226. [Google Scholar] [CrossRef]
- Nakama, T.; Watanabe, N.; Haraguchi, T.; Sakamoto, H.; Kamoi, D.; Tsubakimoto, Y.; Ogata, K.; Satoh, K.; Urasawa, K.; Andoh, H.; et al. Clinical outcomes of pedal artery angioplasty for patients with ischemic wounds: Results from the multicenter RENDEZVOUS registry. JACC Cardiovasc. Interv. 2017, 10, 79–90. [Google Scholar] [CrossRef]
- Palena, L.M.; Brocco, E.; Manzi, M. The clinical utility of below-the-ankle angioplasty using “transmetatarsal artery access” in complex cases of CLI. Catheter. Cardiovasc. Interv. 2014, 83, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ucci, A.; Perini, P.; Freyrie, A.; Schreve, M.A.; Ünlü, Ç.; Huizing, E.; van den Heuvel, D.A.; Kum, S.; Shishehbor, M.H.; Ferraresi, R. Endovascular and Surgical Venous Arterialization for No-Option Patients with Chronic Limb-Threatening Ischemia: A Systematic Review and Meta-Analysis. J. Endovasc. Ther. 2023; ahead of print. 15266028231210220. [Google Scholar] [CrossRef] [PubMed]
- Migliara, B.; Feriani, G.; Mirandola, M.; Griso, A.; Cappellari, T.F.; Nicoletti, C. Percutaneous Deep Venous Arterialization Using an IVUS-Guided Technique in no-Option Patients with Chronic Limb-Threatening Ischemia: 24-Month Results. Cardiovasc. Interv. Radiol. 2024, 47, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- So, S.E.; Chan, Y.C.; Cheng, S.W. Efficacy and Durability of Percutaneous Deep Vein Arterialization: A Systematic Review. Ann. Vasc. Surg. 2024, 105, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Acín, F.; Varela, C.; López de Maturana, I.; de Haro, J.; Bleda, S.; Rodriguez-Padilla, J. Results of infrapopliteal endovascular procedures performed in diabetic patients with critical limb ischaemia and tissue loss from the perspective of an angiosome-oriented revascularization strategy. Int. J. Vasc. Med. 2014, 2014, 270539. [Google Scholar]
- Alexandrescu, V.A.; Brochier, S.; Limgba, A.; Balthazar, S.; Khelifa, H.; De Vreese, P.; Azdad, K.; Nodit, M.; Pottier, M.; Van Espen, D.; et al. Healing of Diabetic Neuroischemic Foot Wounds with vs Without Wound-Targeted Revascularization: Preliminary Observations From an 8-Year Prospective Dual-Center Registry. J. Endovasc. Ther. 2020, 27, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Bekeny, J.C.; Alfawaz, A.; Day, J.; Naz, I.; Attinger, C.E.; Fan, K.L.; Evans, K.K.; Akbari, C.M. Indirect Endovascular Revascularization via Collaterals: A New Classification to Predict Wound Healing and Limb Salvage. Ann. Vasc. Surg. 2021, 73, 264–272. [Google Scholar] [CrossRef]
- Zheng, X.T.; Zeng, R.C.; Huang, J.Y.; Pan, L.M.; Su, X.; Wu, Z.H.; Yu, G.F. The Use of the Angiosome Concept for Treating Infrapopliteal Critical Limb Ischemia through Interventional Therapy and Determining the Clinical Significance of Collateral Vessels. Ann. Vasc. Surg. 2016, 32, 41–49. [Google Scholar] [CrossRef]
- Špillerová, K.; Settembre, N.; Biancari, F.; Albäck, A.; Venermo, M. Angiosome Targeted PTA Is More Important in Endovascular Revascularisation than in Surgical Revascularisation: Analysis of 545 Patients with Ischaemic Tissue Lesions. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 567–575. [Google Scholar] [CrossRef]
- Meloni, M.; Izzo, V.; Da Ros, V.; Morosetti, D.; Stefanini, M.; Brocco, E.; Giurato, L.; Gandini, R.; Uccioli, L. Characteristics and Outcome for Persons with Diabetic Foot Ulcer and No-Option Critical Limb Ischemia. J. Clin. Med. 2020, 9, 3745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sojakova, D.; Husakova, J.; Fejfarova, V.; Nemcova, A.; Jarosikova, R.; Kopp, S.; Lovasova, V.; Jude, E.B.; Dubsky, M. The Use of Autologous Cell Therapy in Diabetic Patients with Chronic Limb-Threatening Ischemia. Int. J. Mol. Sci. 2024, 25, 10184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dubský, M.; Husáková, J.; Bem, R.; Jirkovská, A.; Němcová, A.; Fejfarová, V.; Sutoris, K.; Kahle, M.; Jude, E.B. Comparison of the impact of autologous cell therapy and conservative standard treatment on tissue oxygen supply and course of the diabetic foot in patients with chronic limb-threatening ischemia: A randomized controlled trial. Front. Endocrinol. 2022, 13, 888809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dubský, M.; Jirkovská, A.; Bem, R.; Nemcová, A.; Fejfarová, V.; Jude, E.B. Cell therapy of critical limb ischemia in diabetic patients—State of art. Diabetes Res. Clin. Pract. 2017, 126, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Scatena, A.; Apicella, M.; Mantuano, M.; Ragghianti, B.; Silverii, A.; Miranda, C.; Monge, L.; Uccioli, L.; Scevola, G.; Stabile, E.; et al. Autologous cell therapy for ischemic diabetic foot: A meta-analysis of randomized controlled trials for the development of the Italian guidelines for the treatment of diabetic foot syndrome. Acta Diabetol. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Scatena, A.; Petruzzi, P.; Maioli, F.; Lucaroni, F.; Ambrosone, C.; Ventoruzzo, G.; Liistro, F.; Tacconi, D.; Di Filippi, M.; Attempati, N.; et al. Autologous Peripheral Blood Mononuclear Cells for Limb Salvage in Diabetic Foot Patients with No-Option Critical Limb Ischemia. J. Clin. Med. 2021, 10, 2213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panunzi, A.; Madotto, F.; Sangalli, E.; Riccio, F.; Sganzaroli, A.B.; Galenda, P.; Bertulessi, A.; Barmina, M.F.; Ludovico, O.; Fortunato, O.; et al. Results of a prospective observational study of autologous peripheral blood mononuclear cell therapy for no-option critical limb-threatening ischemia and severe diabetic foot ulcers. Cardiovasc. Diabetol. 2022, 21, 196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meloni, M.; Giurato, L.; Andreadi, A.; Bellizzi, E.; Bellia, A.; Lauro, D.; Uccioli, L. Peripheral Blood Mononuclear Cells: A New Frontier in the Management of Patients with Diabetes and No-Option Critical Limb Ischaemia. J. Clin. Med. 2023, 12, 6123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoberock, K.; Kaschwich, M.; Nicolay, S.S.; Mahmoud, N.; Heidemann, F.; Rieß, H.C.; Debus, E.S.; Behrendt, C.A. The interrelationship between diabetes mellitus and peripheral arterial disease. Vasa 2021, 50, 323–330. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Charbonnel, B.; Eckland, D.J.A.; Erdmann, E.; Massi-Benedetti, M.; Moules, I.K.; Skene, A.M.; Tan, M.H.; Lefèbvre, P.J.; Murray, G.D. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): A randomised controlled trial. Lancet 2005, 366, 1279–1289. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willigendael, M.; Teijink, J.A.; Bartelink, M.L.; Peters, R.J.; Buller, H.R.; Prins, M.H. Smoking and the patency of lower extremity bypass grafts: A meta-analysis. J. Vasc. Surg. 2005, 42, 67–74. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Wu, J.; Singh, G.D.; Dawson, D.L.; Pevec, W.C.; Amsterdam, E.A.; Laird, J.R. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J. Vasc. Surg. 2014, 60, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020, 41, 255–323, Erratum in Eur. Heart J. 2020, 41, 4317. [Google Scholar] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2018, 36, 2284–2309, Erratum in J. Hypertens. 2019, 37, 456. [Google Scholar]
- Faglia, E.; Clerici, G.; Scatena, A.; Caminiti, M.; Curci, V.; Morabito, A.; Prisco, V.; Greco, R.; Edmonds, M. Effectiveness of combined therapy with angiotensin-converting enzyme inhibitors and statins in reducing mortality in diabetic patients with critical limb ischemia: An observational study. Diabetes Res. Clin. Pract. 2014, 103, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Giurato, L.; Monge, L.; Miranda, C.; Scatena, A.; Ragghianti, B.; Silverii, G.A.; Vermigli, C.; De Cassai, A.; Volpe, A.; et al. Effect of a multidisciplinary team approach in patients with diabetic foot ulcers on major adverse limb events (MALEs): Systematic review and meta-analysis for the development of the Italian guidelines for the treatment of diabetic foot syndrome. Acta Diabetol. 2024, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
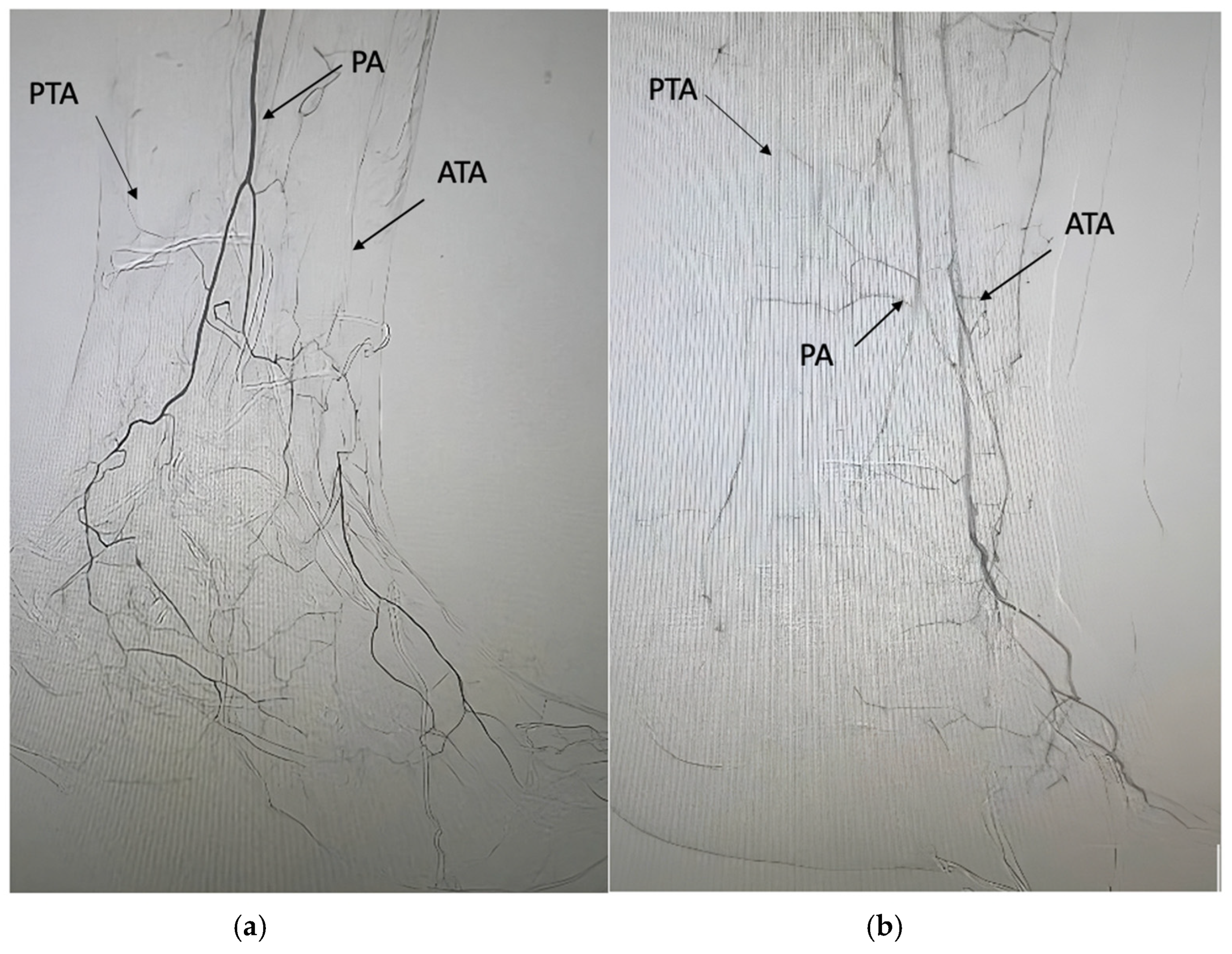
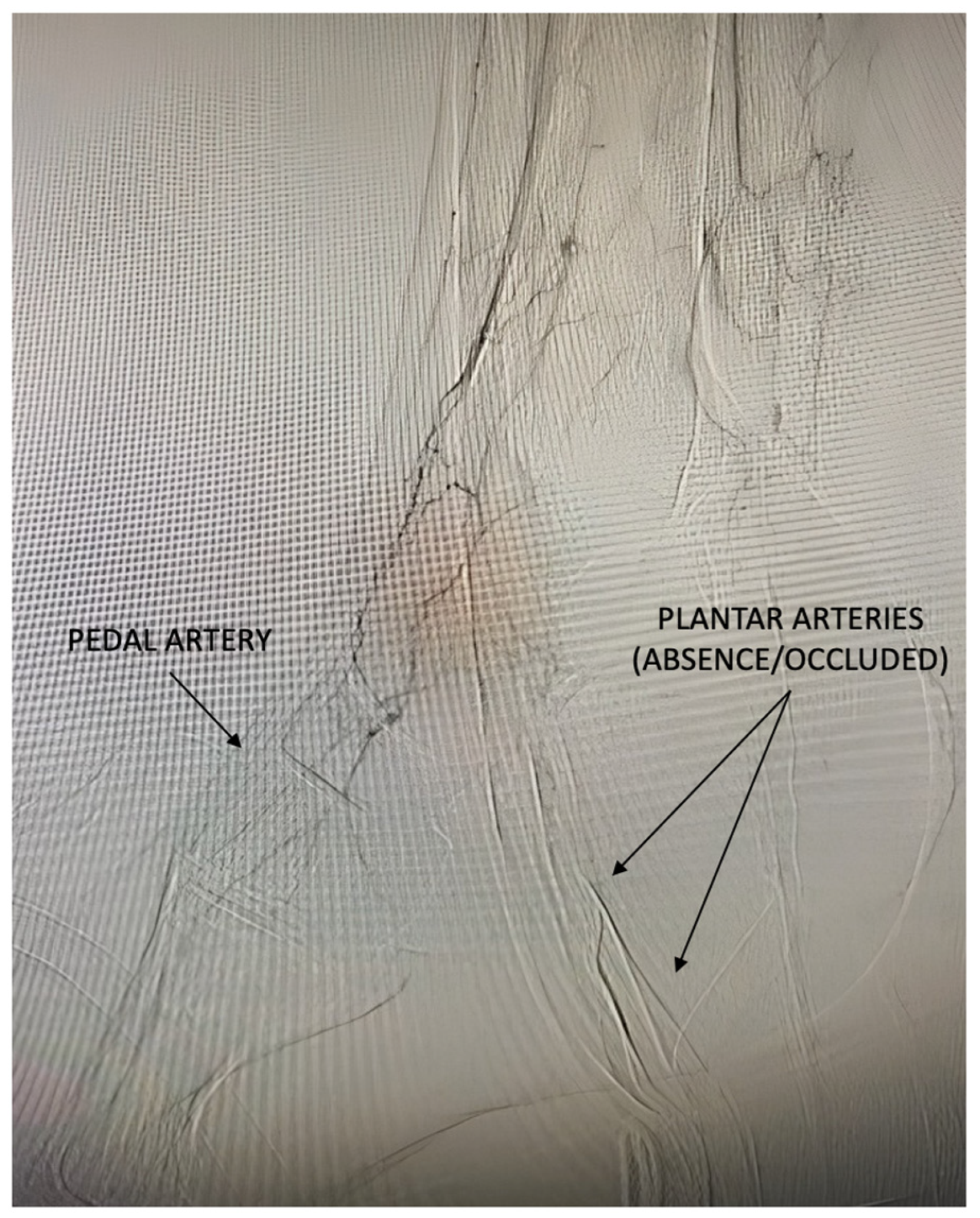
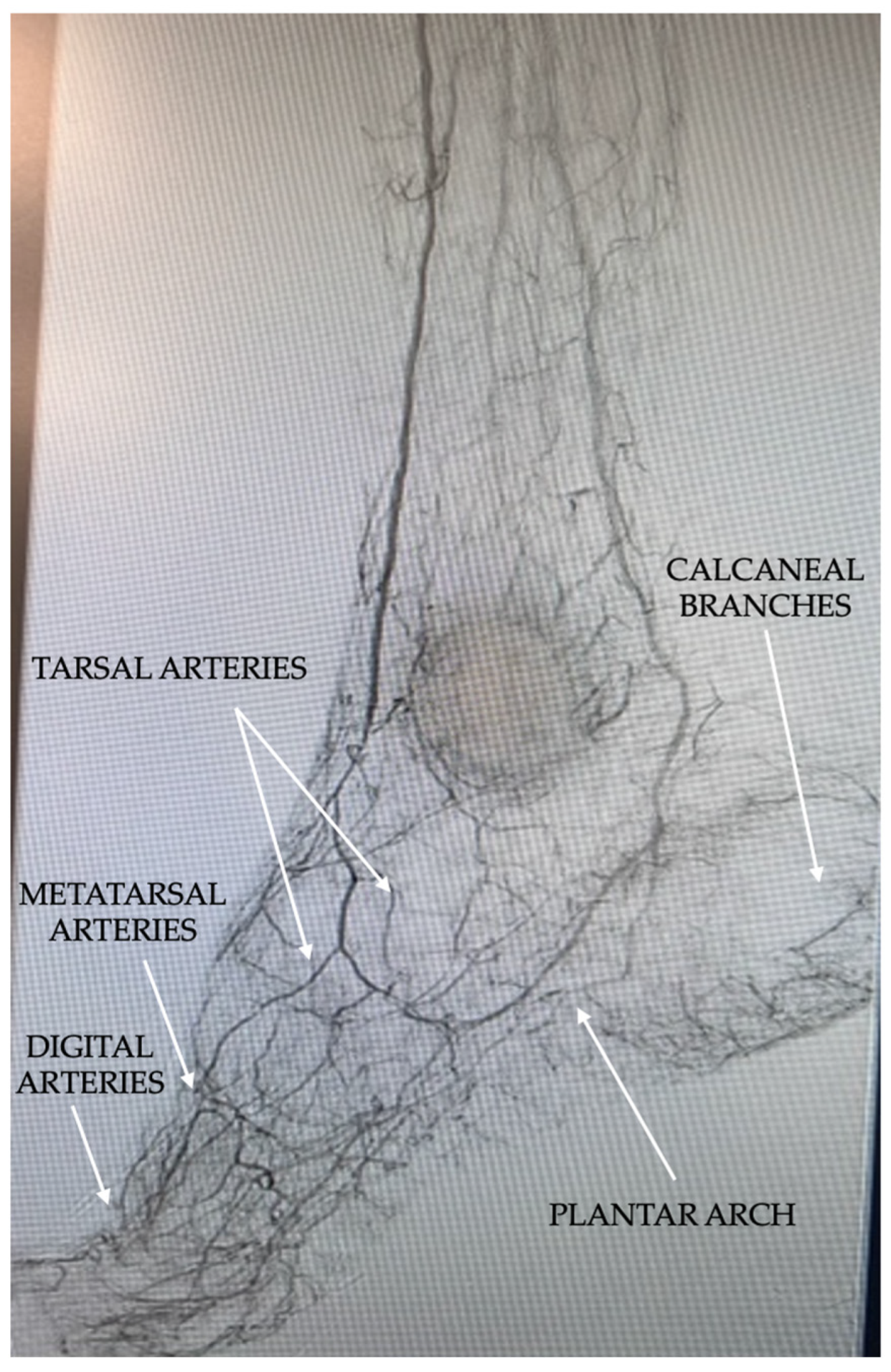

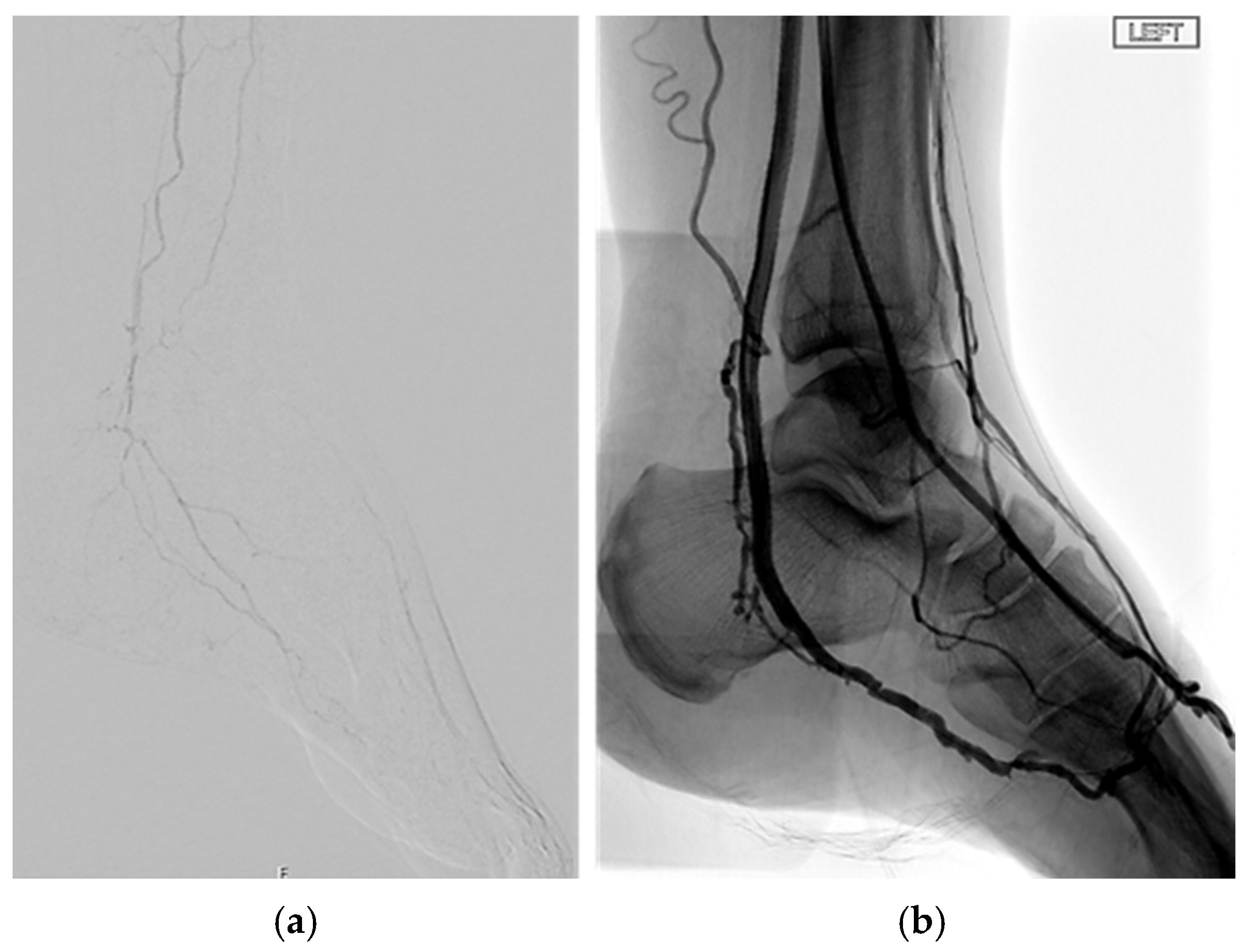
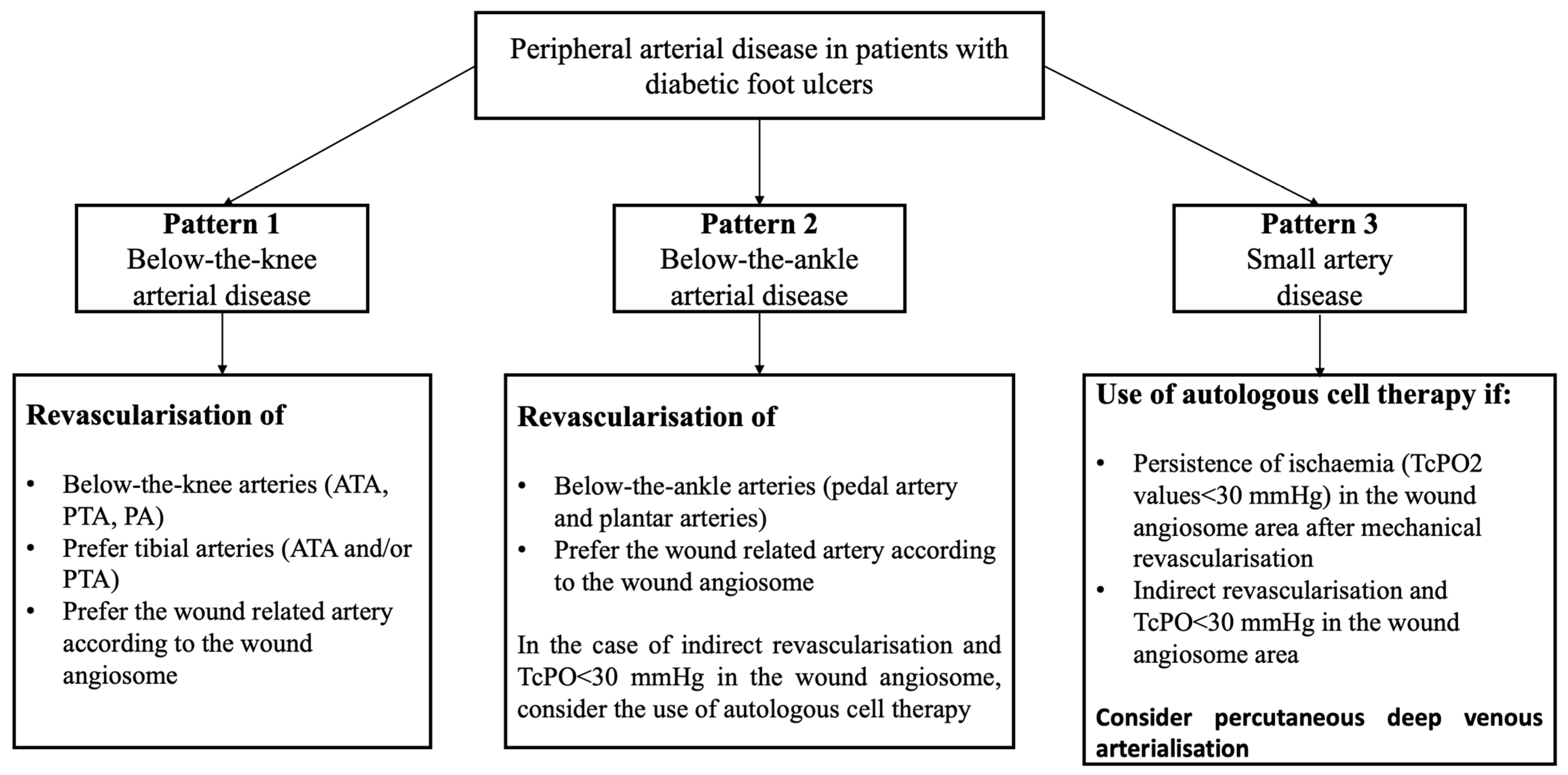
| Pattern 1 | Pattern 2 | Pattern 3 |
|---|---|---|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, M.; Vas, P.R.J. Peripheral Arterial Disease in Diabetic Foot: One Disease with Multiple Patterns. J. Clin. Med. 2025, 14, 1987. https://doi.org/10.3390/jcm14061987
Meloni M, Vas PRJ. Peripheral Arterial Disease in Diabetic Foot: One Disease with Multiple Patterns. Journal of Clinical Medicine. 2025; 14(6):1987. https://doi.org/10.3390/jcm14061987
Chicago/Turabian StyleMeloni, Marco, and Prashanth R. J. Vas. 2025. "Peripheral Arterial Disease in Diabetic Foot: One Disease with Multiple Patterns" Journal of Clinical Medicine 14, no. 6: 1987. https://doi.org/10.3390/jcm14061987
APA StyleMeloni, M., & Vas, P. R. J. (2025). Peripheral Arterial Disease in Diabetic Foot: One Disease with Multiple Patterns. Journal of Clinical Medicine, 14(6), 1987. https://doi.org/10.3390/jcm14061987







