Twelve-Month Estimated Glomerular Filtration Rate Trajectory at the Time of Kidney Replacement Therapy Counseling Predicts Dialysis Initiation Within One Year
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Patients and Data Collection
2.3. Candidate Predictors
2.4. Clinical Endpoint Definition
2.5. Statistical Analysis
2.6. Clinical Implication
2.7. Study Size Considerations
3. Results
3.1. Baseline Characteristics of the Patients
3.2. Factors Influencing the Rate of eGFR Decline
3.3. Factors Associated with the KRT Initiation
3.4. Model Accuracy in the Study Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuROC | Area under the receiver operating characteristic curve |
| BMI | Body mass index |
| CKD | Chronic kidney disease |
| ESKD | End-stage kidney disease |
| eGFR | Estimated glomerular filtration rate |
| eGFRr | Rate of change in eGFR over the 12 months preceding the first KRT counseling session |
| eGFRi | eGFR at the time of the first dialysis initiation |
| KRT | Kidney replacement therapy |
| KFRE-2 | Kidney failure risk equation |
| RAASi | Renin–angiotensin–aldosterone system inhibitors |
| LOWESS | Locally weighted scatterplot smoothing |
| TRIPOD | Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis |
References
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Keith, D.S.; Nichols, G.A.; Gullion, C.M.; Brown, J.B.; Smith, D.H. Longitudinal Follow-up and Outcomes among a Population with Chronic Kidney Disease in a Large Managed Care Organization. Arch. Intern. Med. 2004, 164, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J.T.; Depner, T.A.; Inrig, J.; Mehrotra, R.; Rocco, M.V.; Suri, R.S.; Weiner, D.E.; Greer, N.; Ishani, A.; MacDonald, R.; et al. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am. J. Kidney Dis. 2015, 66, 884–930. [Google Scholar] [CrossRef]
- Nesrallah, G.E.; Mustafa, R.A.; Clark, W.F.; Bass, A.; Barnieh, L.; Hemmelgarn, B.R.; Klarenbach, S.; Quinn, R.R.; Hiremath, S.; Ravani, P.; et al. Canadian Society of Nephrology 2014 Clinical Practice Guideline for Timing the Initiation of Chronic Dialysis. CMAJ 2014, 186, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Heaf, J.; Petersons, A.; Vernere, B.; Heiro, M.; Povlsen, J.V.; Sørensen, A.B.; Rosenberg, M.; Løkkegaard, N.; Alonso-Garcia, F.; Kampmann, J.D.; et al. Why Do Physicians Prescribe Dialysis? A Prospective Questionnaire Study. PLoS ONE 2017, 12, e0188309. [Google Scholar] [CrossRef]
- Lok, C.E.; Huber, T.S.; Lee, T.; Shenoy, S.; Yevzlin, A.S.; Abreo, K.; Allon, M.; Asif, A.; Astor, B.C.; Glickman, M.H.; et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am. J. Kidney Dis. 2020, 75, S1–S164. [Google Scholar] [CrossRef]
- Cooper, B.A.; Branley, P.; Bulfone, L.; Collins, J.F.; Craig, J.C.; Fraenkel, M.B.; Harris, A.; Johnson, D.W.; Kesselhut, J.; Li, J.J.; et al. A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis. N. Engl. J. Med. 2010, 363, 609–619. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Hsu, C.; Parikh, R.V.; Pravoverov, L.N.; Zheng, S.; Glidden, D.V.; Tan, T.C.; Go, A.S. Implication of Trends in Timing of Dialysis Initiation for Incidence of End-Stage Kidney Disease. JAMA Intern. Med. 2020, 180, 1647–1654. [Google Scholar] [CrossRef]
- Chan, C.T.; Blankestijn, P.J.; Dember, L.M.; Gallieni, M.; Harris, D.C.H.; Lok, C.E.; Mehrotra, R.; Stevens, P.E.; Wang, A.Y.-M.; Cheung, M.; et al. Dialysis Initiation, Modality Choice, Access, and Prescription: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019, 96, 37–47. [Google Scholar] [CrossRef]
- Murea, M.; Moossavi, S.; Garneata, L.; Kalantar-Zadeh, K. Narrative Review of Incremental Hemodialysis. Kidney Int. Rep. 2020, 5, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, E.B.; Yang, X.; Thorp, M.L.; Arnold, B.M.; Tabano, D.C.; Petrik, A.F.; Smith, D.H.; Platt, R.W.; Johnson, E.S. Predicting 5-Year Risk of RRT in Stage 3 or 4 CKD: Development and External Validation. Clin. J. Am. Soc. Nephrol. 2017, 12, 87–94. [Google Scholar] [CrossRef]
- Ivory, S.E.; Polkinghorne, K.R.; Khandakar, Y.; Kasza, J.; Zoungas, S.; Steenkamp, R.; Roderick, P.; Wolfe, R. Predicting 6-Month Mortality Risk of Patients Commencing Dialysis Treatment for End-Stage Kidney Disease. Nephrol. Dial. Transpl. 2017, 32, 1558–1565. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Ballew, S.H.; Matsushita, K.; Astor, B.C.; Carrero, J.J.; Chang, A.R.; Inker, L.A.; Kenealy, T.; Kovesdy, C.P.; et al. Evaluating Glomerular Filtration Rate Slope as a Surrogate End Point for ESKD in Clinical Trials: An Individual Participant Meta-Analysis of Observational Data. J. Am. Soc. Nephrol. 2019, 30, 1746. [Google Scholar] [CrossRef]
- O’Hare, A.M.; Batten, A.; Burrows, N.R.; Pavkov, M.E.; Taylor, L.; Gupta, I.; Todd-Stenberg, J.; Maynard, C.; Rodriguez, R.A.; Murtagh, F.E.; et al. Trajectories of Kidney Function Decline in the 2 Years Before Initiation of Long-Term Dialysis. Am. J. Kidney Dis. 2012, 59, 513–522. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Xian, H.; Balasubramanian, S.; Al-Aly, Z. Renal Function Trajectories in Patients with Prior Improved eGFR Slopes and Risk of Death. PLoS ONE 2016, 11, e0149283. [Google Scholar] [CrossRef]
- Khan, M.S.; Bakris, G.L.; Shahid, I.; Weir, M.R.; Butler, J. Potential Role and Limitations of Estimated Glomerular Filtration Rate Slope Assessment in Cardiovascular Trials: A Review. JAMA Cardiol. 2022, 7, 549–555. [Google Scholar] [CrossRef]
- Lim, L.-M.; Lin, M.-Y.; Hwang, S.-J.; Chen, H.-C.; Chiu, Y.-W. Association of Glomerular Filtration Rate Slope with Timely Creation of Vascular Access in Incident Hemodialysis. Sci. Rep. 2021, 11, 13137. [Google Scholar] [CrossRef]
- Inker, L.A.; Collier, W.; Greene, T.; Miao, S.; Chaudhari, J.; Appel, G.B.; Badve, S.V.; Caravaca-Fontán, F.; Del Vecchio, L.; Floege, J.; et al. A Meta-Analysis of GFR Slope as a Surrogate Endpoint for Kidney Failure. Nat. Med. 2023, 29, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Odler, B.; Fu, E.L. eGFR Slope as a Primary Endpoint for Clinical Trials of CKD Progression: One Size Fits All? Clin. Kidney J. 2024, 17, sfae001. [Google Scholar] [CrossRef]
- Orlandi, P.F.; Xie, D.; Yang, W.; Cohen, J.B.; Deo, R.; Ricardo, A.C.; Schrauben, S.; Wang, X.; Hamm, L.L.; He, J.; et al. Slope of Kidney Function and Its Association with Longitudinal Mortality and Cardiovascular Disease among Individuals with CKD. J. Am. Soc. Nephrol. 2020, 31, 2912–2923. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Fukagawa, M. Slope of the Estimated Glomerular Filtration Rate and Its Associated Factors among Individuals with Chronic Kidney Disease in the General Japanese Population. Clin. Exp. Nephrol. 2024, 28, 522–530. [Google Scholar] [CrossRef]
- Flammia, R.S.; Anceschi, U.; Tufano, A.; Tuderti, G.; Ferriero, M.C.; Brassetti, A.; Mari, A.; Di Maida, F.; Minervini, A.; Derweesh, I.H.; et al. Is Hypertension Associated with Worse Renal Functional Outcomes after Minimally Invasive Partial Nephrectomy? Results from a Multi-Institutional Cohort. J. Clin. Med. 2022, 11, 1243. [Google Scholar] [CrossRef]
- Holtkamp, F.A.; De Zeeuw, D.; Thomas, M.C.; Cooper, M.E.; De Graeff, P.A.; Hillege, H.J.L.; Parving, H.-H.; Brenner, B.M.; Shahinfar, S.; Lambers Heerspink, H.J. An Acute Fall in Estimated Glomerular Filtration Rate during Treatment with Losartan Predicts a Slower Decrease in Long-Term Renal Function. Kidney Int. 2011, 80, 282–287. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Coresh, J.; Ballew, S.H.; Woodward, M.; Levin, A.; Naimark, D.M.J.; Nally, J.; Rothenbacher, D.; Stengel, B.; Iseki, K.; et al. Past Decline Versus Current eGFR and Subsequent ESRD Risk. J. Am. Soc. Nephrol. 2016, 27, 2447–2455. [Google Scholar] [CrossRef]
- Thanabalasingam, S.J.; Iliescu, E.A.; Norman, P.A.; Day, A.G.; Akbari, A.; Hundemer, G.L.; White, C.A. Kidney Failure Risk Equation Thresholds for Multidisciplinary Kidney Care Referrals: A Validation Study. Kidney Med. 2024, 6, 100805. [Google Scholar] [CrossRef]

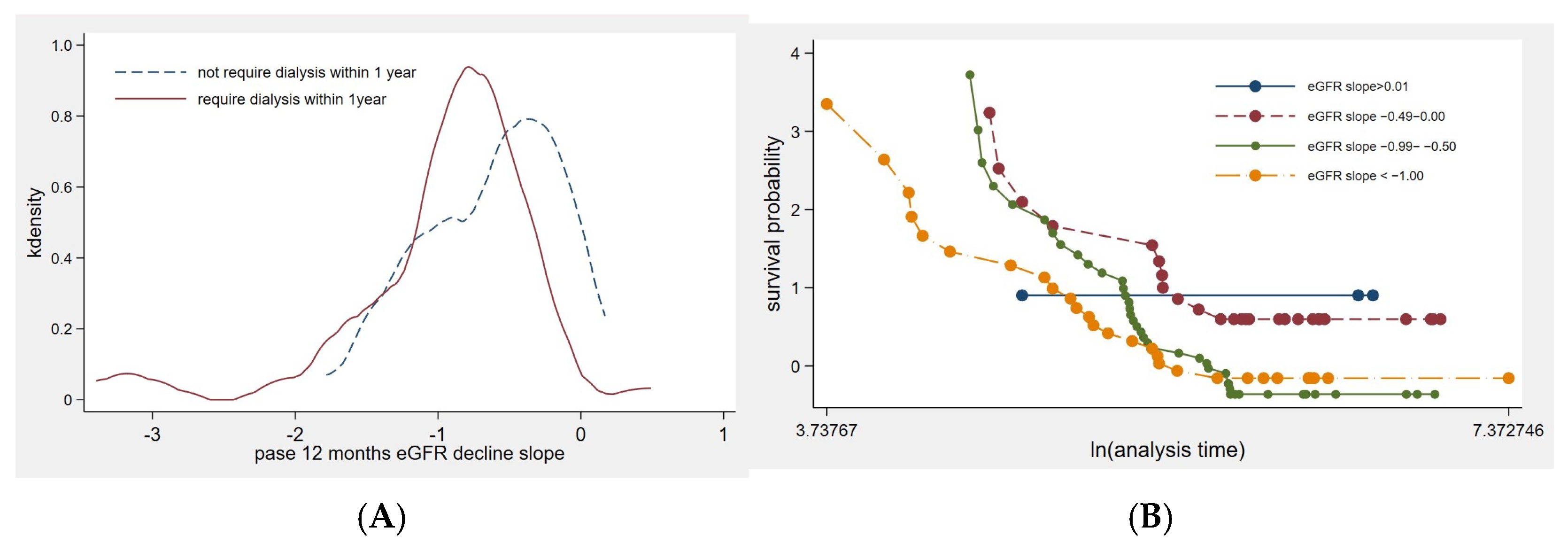
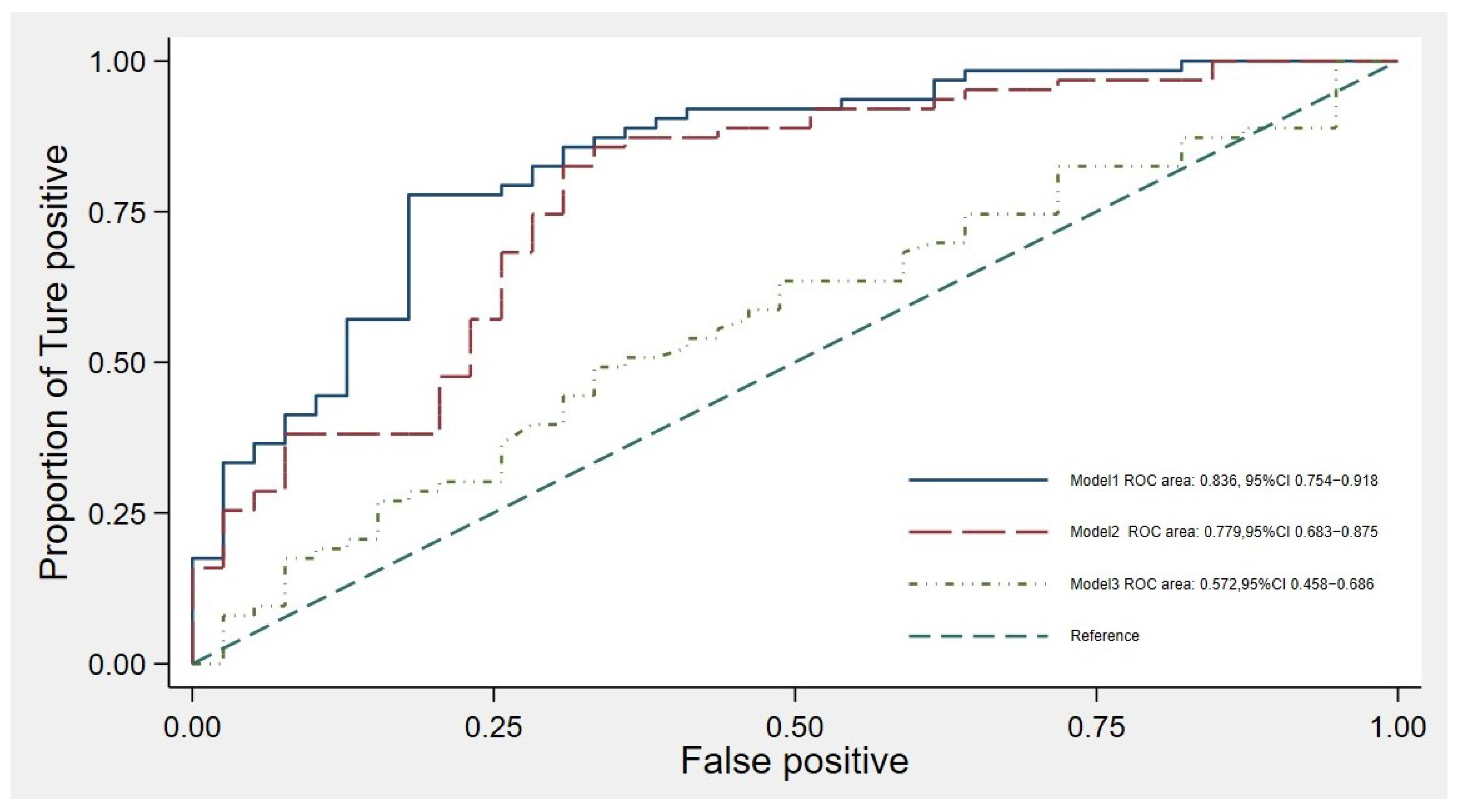
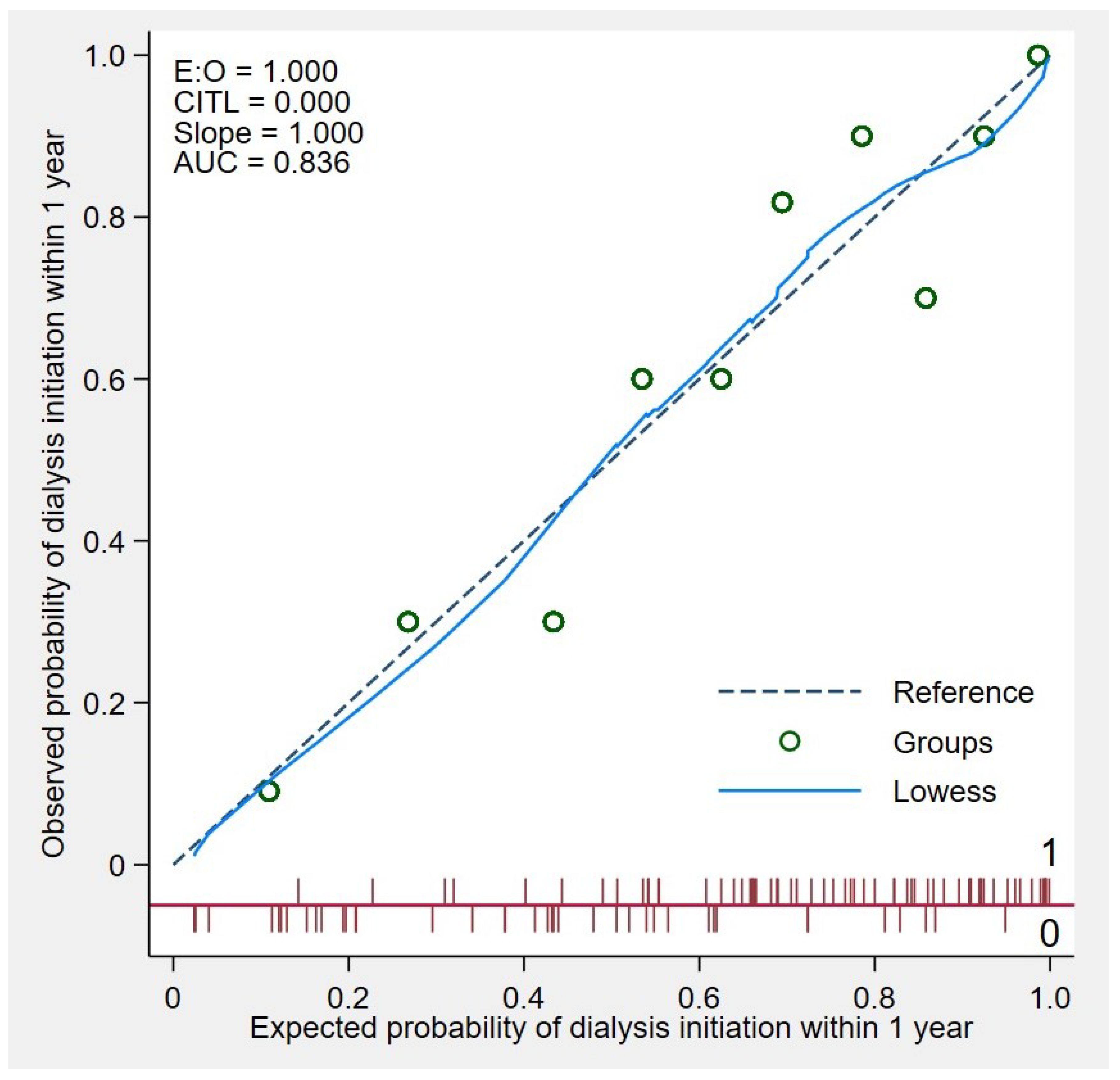
| Clinical Characteristics | Early KRT Initiation with in a Year (n = 64) | No KRT Initiation Within a Year (n = 39) | p-Value |
|---|---|---|---|
| Age, years, mean (SD) | 60.97 (12.38) | 67.21 (9.54) | 0.008 |
| Gender, male, n (%) | 40 (36%) | 23 (63%) | 0.835 |
| Body mass index, kg/m2, mean (SD) | 25.41 (4.57) | 25.05 (4.03) | 0.709 |
| Systolic blood pressure, mmHg, n (%) | 0.031 | ||
| 3 (60%) | 2 (40%) | |
| 15 (43%) | 20 (57%) | |
| 23 (72%) | 9 (28%) | |
| 23 (74%) | 8 (26%) | |
| Causes of ESKD, n (%) | 0.617 | ||
| 42 (65%) | 23 (35%) | |
| 10 (53%) | 9 (47%) | |
| 5 (83%) | 1 (17%) | |
| 2 (67%) | 1 (33%) | |
| 1 (50%) | 1 (50%) | |
| 4 (50%) | 4 (50%) | |
| Mode of KRT | 0.586 | ||
| 12 (70.59) | 5 (29.41) | |
| 52 (60.47) | 34 (39.53) | |
| History of CV events, n (%) | 5 (71%) | 2 (28%) | 0.707 |
| RAASi, n (%) | 8 (100%) | 0 (0%) | 0.019 |
| Hemoglobin, g/dL, mean (SD) | 10.00 (1.48) | 10.38 (1.56) | 0.215 |
| Serum albumin, g/dL, mean (SD) | 3.66 (0.55) | 3.88 (0.46) | 0.035 |
| Serum phosphate, mg/dL, mean (SD) | 4.33 (0.88) | 4.12 (0.67) | 0.218 |
| Serum calcium, mg/dL, mean (SD) | 8.66 (0.62) | 9.06 (0.58) | 0.001 |
| eGFRc, mL/min/1.73 m2, mean (SD) | 10.84 (2.21) | 11.31 (2.29) | 0.304 |
| eGFRi, mL/min/1.73 m2, mean (SD) | 5.67 (1.7) | - | - |
| Average rate of eGFR decline, mL/min/1.73 m2/month, mean (SD) | −0.95 (0.67) | −0.62 (0.48) | 0.007 |
| Variables | Coefficient (SE) | 95% Confident Intervals | p-Value |
|---|---|---|---|
| Age | 0.013 (0.006) | 0.002 to 0.024 | 0.019 |
| Serum albumin < 2.5 mg/dL | −1.157 (0.243) | −1.633 to −0.680 | <0.001 |
| Serum calcium level | −0.330 (0.104) | −0.535 to −0.125 | 0.002 |
| Serum phosphate > 4.5 mg/dL | −0.405 (0.135) | −0.669 to −0.141 | 0.003 |
| RAASi | −0.741 (0.208) | −0.150 to −0.333 | <0.001 |
| Variable | SHR (SE) | 95% Confident Intervals | p-Value |
|---|---|---|---|
| eGFRr, mL/min/1.73 m2/month | 0.656 (0.123) | 0.454 to 0.948 | 0.025 |
| eGFRi, mL/min/1.73 m2 | 0.995 (0.003) | 0.989 to 1.001 | 0.132 |
| Age < 70 year | 1.620 (0.639) | 0.748 to 3.509 | 0.221 |
| Male | 1.482 (0.402) | 0.871 to 2.521 | 0.146 |
| Body mass index, kg/m2 | |||
| 2.056 (2.021) | 0.300 to 14.108 | 0.463 |
| 0.894 (1.902) | 0.265 to 13.547 | 0.524 |
| 12.060 (1.205) | 0.253 to 16.788 | 0.499 |
| Systolic blood pressure, mmHg | |||
| 0.632 (0.553) | 0.114 to 3.514 | 0.600 |
| 1.578 (1.382) | 0.284 to 8.782 | 0.602 |
| 2.588 (2.402) | 0.412 to 15.960 | 0.306 |
| Diabetic nephropathy | 0.726 (0.314) | 0.311 to 1.694 | 0.459 |
| Major cardiovascular events | 1.685 (0.921) | 0.577 to 4.916 | 0.340 |
| RAASi | 0.940 (0.323) | 0.480 to 1.842 | 0.858 |
| Serum albumin, mg/dL | 0.947 (0.039) | 0.874 to 1.025 | 0.179 |
| Serum calcium, mg/dL | 0.969 (0.019) | 0.932 to 1.007 | 0.111 |
| Serum phosphate, mg/dL | 0.977 (0.019) | 0.941 to 1.014 | 0.224 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuemor, P.; Rerkasem, K.; Tantraworasin, A.; Khorana, J.; Thammathiwat, T.; Pichitsiri, W. Twelve-Month Estimated Glomerular Filtration Rate Trajectory at the Time of Kidney Replacement Therapy Counseling Predicts Dialysis Initiation Within One Year. J. Clin. Med. 2025, 14, 1981. https://doi.org/10.3390/jcm14061981
Chuemor P, Rerkasem K, Tantraworasin A, Khorana J, Thammathiwat T, Pichitsiri W. Twelve-Month Estimated Glomerular Filtration Rate Trajectory at the Time of Kidney Replacement Therapy Counseling Predicts Dialysis Initiation Within One Year. Journal of Clinical Medicine. 2025; 14(6):1981. https://doi.org/10.3390/jcm14061981
Chicago/Turabian StyleChuemor, Panuwat, Kittipan Rerkasem, Apichat Tantraworasin, Jiraporn Khorana, Theerachai Thammathiwat, and Watchara Pichitsiri. 2025. "Twelve-Month Estimated Glomerular Filtration Rate Trajectory at the Time of Kidney Replacement Therapy Counseling Predicts Dialysis Initiation Within One Year" Journal of Clinical Medicine 14, no. 6: 1981. https://doi.org/10.3390/jcm14061981
APA StyleChuemor, P., Rerkasem, K., Tantraworasin, A., Khorana, J., Thammathiwat, T., & Pichitsiri, W. (2025). Twelve-Month Estimated Glomerular Filtration Rate Trajectory at the Time of Kidney Replacement Therapy Counseling Predicts Dialysis Initiation Within One Year. Journal of Clinical Medicine, 14(6), 1981. https://doi.org/10.3390/jcm14061981





