Comparison of Bicuspid and Tricuspid Handmade Polytetrafluoroethylene Valved Conduits: Early and Mid-Term Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surgical Technique
2.2. Echocardiographic Evaluation
2.3. Postoperative Antiplatelet/Anticoagulant Therapy
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qian, T.; Yuan, H.; Chen, C.; Liu, Y.; Lu, T.; Huang, C.; Wu, Z. Conduits for Right Ventricular Outflow Tract Reconstruction in Infants and Young Children. Front. Surg. 2021, 8, 719840. [Google Scholar] [CrossRef]
- Tweddell, J.S.; Pelech, A.N.; Frommelt, P.C.; Mussatto, K.A.; Wyman, J.D.; Fedderly, R.T.; Berger, S.; Frommelt, M.A.; Lewis, D.A.; Friedberg, D.Z.; et al. Factors affecting longevity of homograft valves used in right ventricular outflow tract reconstruction for congenital heart disease. Circulation 2000, 102 (Suppl. 3), III130–III135. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.; Sassen, S.; Kostolny, M.; Hörer, J.; Cleuziou, J.; Wottke, M.; Holper, K.; Fend, F.; Eicken, A.; Lange, R. Early graft failure of small-sized porcine valved conduits in reconstruction of the right ventricular outflow tract. Ann. Thorac. Surg. 2006, 82, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yamagishi, M.; Miyazaki, T. Current status of right ventricular outflow tract reconstruction: Complete translation of a review article originally published in KyobuGeka 2014;67:65-77. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 131–141. [Google Scholar] [CrossRef]
- Yamagishi, M.; Kurosawa, H. Outflow reconstruction of tetralogy of Fallot using a Gore-Tex valve. Ann. Thorac. Surg. 1993, 56, 1414–1417. [Google Scholar] [CrossRef]
- Yoshida, M.; Wearden, P.D.; Dur, O.; Pekkan, K.; Morell, V.O. Right ventricular outflow tract reconstruction with bicuspid valved polytetrafluoroethylene conduit. Ann. Thorac. Surg. 2011, 91, 1235–1239. [Google Scholar] [CrossRef]
- Miyazaki, T.; Yamagishi, M.; Maeda, Y.; Taniguchi, S.; Fujita, S.; Hongu, H.; Yaku, H. Long-term outcomes of expanded polytetrafluoroethylene conduits with bulging sinuses and a fan-shaped valve in right ventricular outflow tract reconstruction. J. Thorac. Cardiovasc. Surg. 2018, 155, 2567–2576. [Google Scholar] [CrossRef]
- Yamashita, E.; Yamagishi, M.; Miyazaki, T.; Maeda, Y.; Yamamoto, Y.; Kato, N.; Asada, S.; Hongu, H.; Yaku, H. Smaller-Sized Expanded Polytetrafluoroethylene Conduits With a Fan-Shaped Valve and Bulging Sinuses for Right Ventricular Outflow Tract Reconstruction. Ann. Thorac. Surg. 2016, 102, 1336–1344. [Google Scholar] [CrossRef]
- Quintessenza, J.A. Polytetrafluoroethylene pulmonary valve conduit implantation for chronic pulmonary insufficiency. Cardiol. Young 2014, 24, 1101–1103. [Google Scholar] [CrossRef]
- Costa, F.D.A.D. Conduits for Right Ventricular Outflow Tract Reconstruction in Children: Are We Improving? World J. Pediatr. Congenit. Heart Surg. 2020, 11, 148–149. [Google Scholar] [CrossRef]
- Herrmann, J.L.; Larson, E.E.; Mastropietro, C.W.; Rodefeld, M.D.; Turrentine, M.W.; Nozaki, R.; Brown, J.W. Right Ventricular Outflow Tract Reconstruction in Infant Truncus Arteriosus: A 37-year Experience. Ann. Thorac. Surg. 2020, 110, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Karamlou, T.; Blackstone, E.H.; Hawkins, J.A.; Jacobs, M.L.; Kanter, K.R.; Brown, J.W.; Mavroudis, C.; Caldarone, C.A.; Williams, W.G.; McCrindle, B.W.; et al. Can pulmonary conduit dysfunction and failure be reduced in infants and children less than age 2 years at initial implantation? J. Thorac. Cardiovasc. Surg. 2006, 132, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Cleuziou, J.; Vitanova, K.; Kasnar-Samprec, J.; Hörer, J.; Lange, R.; Schreiber, C. Durability of down-sized homografts for the reconstruction of the right ventricular outflow tract. Eur. J. Cardiothorac. Surg. 2016, 49, 1421–1425. [Google Scholar] [CrossRef]
- Lu, T.; Huang, Y.; Liu, Y.; Shen, Y.; Qiao, Y.; Zhang, Y. Effects of cryopreservation on tracheal allograft antigenicity in dogs. J. Thorac. Dis. 2017, 9, 2038–2047. [Google Scholar] [CrossRef]
- Boethig, D.; Thies, W.R.; Hecker, H.; Breymann, T. Mid term course after pediatric right ventricular outflow tract reconstruction: A comparison of homografts, porcine xenografts and Contegras. Eur. J. Cardiothorac. Surg. 2005, 27, 58–66. [Google Scholar] [CrossRef]
- Homann, M.; Haehnel, J.C.; Mendler, N.; Paek, S.U.; Holper, K.; Meisner, H.; Lange, R. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur. J. Cardiothorac. Surg. 2000, 17, 624–630. [Google Scholar] [CrossRef]
- Sierra, J.; Christenson, J.T.; Lahlaidi, N.H.; Beghetti, M.; Kalangos, A. Right ventricular outflow tract reconstruction: What conduit to use? Homograft or Contegra? Ann. Thorac. Surg. 2007, 84, 606–611. [Google Scholar] [CrossRef]
- Baskett, R.J.; Ross, D.B.; Nanton, M.A.; Murphy, D.A. Factors in the early failure of cryopreserved homograft pulmonary valves in children: Preserved immunogenicity? J. Thorac. Cardiovasc. Surg. 1996, 112, 1170–1179. [Google Scholar] [CrossRef]
- Mercer, C.W.; West, S.C.; Sharma, M.S.; Yoshida, M.; Morell, V.O. Polytetrafluoroethylene conduits versus homografts for right ventricular outflow tract reconstruction in infants and young children: An institutional experience. J. Thorac. Cardiovasc. Surg. 2018, 155, 2082–2091.e1. [Google Scholar] [CrossRef]
- Hongu, H.; Yamagishi, M.; Maeda, Y.; Itatani, K.; Fujita, S.; Nakatsuji, H.; Yaku, H. Expanded Polytetrafluoroethylene Conduits with Bulging Sinuses and a Fan-Shaped Valve in Right Ventricular Outflow Tract Reconstruction. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 972–980. [Google Scholar] [CrossRef]
- Chang, T.I.; Hsu, K.H.; Li, S.J.; Chuang, M.K.; Luo, C.W.; Chen, Y.J.; Chang, C.I. Evolution of pulmonary valve reconstruction with focused review of expanded polytetrafluoroethylene handmade valves. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Nistal, F.; García-Martínez, V.; Arbe, E.; Fernández, D.; Artiñano, E.; Mazorra, F.; Gallo, I. In vivo experimental assessment of polytetrafluoroethylene trileaflet heart valve prosthesis. J. Thorac. Cardiovasc. Surg. 1990, 99, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Perri, G.; Polito, A.; Esposito, C.; Albanese, S.B.; Francalanci, P.; Pongiglione, G.; Carotti, A. Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur. J. Cardiothorac. Surg. 2012, 41, 1320–1325. [Google Scholar] [CrossRef]
- Voges, I.; Bräsen, J.H.; Entenmann, A.; Scheid, M.; Scheewe, J.; Fischer, G.; Hart, C.; Andrade, A.; Pham, H.M.; Kramer, H.H. Adverse results of a decellularized tissue-engineered pulmonary valve in humans assessed with magnetic resonance imaging. Eur. J. Cardiothorac. Surg. 2013, 44, e272–e279. [Google Scholar] [CrossRef] [PubMed]
- Christ, T.; Paun, A.C.; Grubitzsch, H.; Holinski, S.; Falk, V.; Dushe, S. Long-term results after the Ross procedure with the decellularized AutoTissue Matrix P® bioprosthesis used for pulmonary valve replacement. Eur. J. Cardiothorac. Surg. 2019, 55, 885–892. [Google Scholar] [CrossRef]
- Selcuk, A.; Kilic, Y.; Korun, O.; Yurdakok, O.; Cicek, M.; Altin, H.F.; Altuntas, Y.; Yilmaz, E.H.; Sasmazel, A.; Aydemir, N.A. High incidence of fever in patients after biointegral pulmonic valved conduit implantation. J. Card. Surg. 2021, 36, 3147–3152. [Google Scholar] [CrossRef]
- Shebani, S.O.; McGuirk, S.; Baghai, M.; Stickley, J.; De Giovanni, J.V.; Bu’lock, F.A.; Barron, D.J.; Brawn, W.J. Right ventricular outflow tract reconstruction using Contegra valved conduit: Natural history and conduit performance under pressure. Eur. J. Cardiothorac. Surg. 2006, 29, 397–405. [Google Scholar] [CrossRef]
- Dave, H.; Mueggler, O.; Comber, M.; Enodien, B.; Nikolaou, G.; Bauersfeld, U.; Jenni, R.; Bettex, D.; Prêtre, R. Risk factor analysis of 170 single-institutional contegra implantations in pulmonary position. Ann. Thorac. Surg. 2011, 91, 195–203. [Google Scholar] [CrossRef]
- Delmo-Walter, E.M.; Alexi-Meskishvili, V.; Abdul-Khaliq, H.; Meyer, R.; Hetzer, R. Aneurysmal dilatation of the Contegra bovine jugular vein conduit after reconstruction of the right ventricular outflow tract. Ann. Thorac. Surg. 2007, 83, 682–684. [Google Scholar] [CrossRef]
- Hirai, K.; Baba, K.; Goto, T.; Ousaka, D.; Kondo, M.; Eitoku, T.; Kotani, Y.; Kasahara, S.; Ohtsuki, S.; Tsukahara, H. Outcomes of Right Ventricular Outflow Tract Reconstruction in Children: Retrospective Comparison Between Bovine Jugular Vein and Expanded Polytetrafluoroethylene Conduits. Pediatr. Cardiol. 2021, 42, 100–108. [Google Scholar] [CrossRef]
- Beckerman, Z.; De León, L.E.; Zea-Vera, R.; Mery, C.M.; Fraser, C.D., Jr. High incidence of late infective endocarditis in bovine jugular vein valved conduits. J. Thorac. Cardiovasc. Surg. 2018, 156, 728–734.e2. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.M.; Tan, C.; Srivastava, N.; Herrmann, J.L.; Rodefeld, M.D.; Turrentine, M.W.; Brown, J.W. Bovine Jugular Vein Conduit: A Mid- to Long-Term Institutional Review. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ugaki, S.; Rutledge, J.; Al Aklabi, M.; Ross, D.B.; Adatia, I.; Rebeyka, I.M. An increased incidence of conduit endocarditis in patients receiving bovine jugular vein grafts compared to cryopreserved homograft for right ventricular outflow reconstruction. Ann. Thorac. Surg. 2015, 99, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Mery, C.M.; Guzmán-Pruneda, F.A.; De León, L.E.; Zhang, W.; Terwelp, M.D.; Bocchini, C.E.; Adachi, I.; Heinle, J.S.; McKenzie, E.D.; Fraser, C.D., Jr. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J. Thorac. Cardiovasc. Surg. 2016, 151, 432–441.e4412. [Google Scholar] [CrossRef]
- Choi, K.H.; Sung, S.C.; Kim, H.; Lee, H.D.; Kim, G.; Ko, H.; Byun, J.H.; Kim, W.H.; Kim, E.R.; Park, H.K. Simplified Tricuspid Polytetrafluoroethylene Valved Conduit: Midterm Results of Multicenter Study. Ann. Thorac. Surg. 2019, 108, 1228–1233. [Google Scholar] [CrossRef]
- Choi, K.H.; Sung, S.C.; Kim, H.; Lee, H.D.; Kim, G.; Ko, H. Late results of right ventricular outflow tract reconstruction with a bicuspid expanded polytetrafluoroethylene valved conduit. J. Card. Surg. 2018, 33, 36–40. [Google Scholar] [CrossRef]
- Quintessenza, J.A.; Jacobs, J.P.; Chai, P.J.; Morell, V.O.; Lindberg, H. Polytetrafluoroethylene bicuspid pulmonary valve implantation: Experience with 126 patients. World J. Pediatr. Congenit. Heart Surg. 2010, 1, 20–27. [Google Scholar] [CrossRef]
- Seese, L.M.; Turbendian, H.K.; Castrillon, C.E.D.; Morell, V.O. The Fate of Homograft Versus Polytetrafluoroethylene Conduits After Neonatal Truncus Arteriosus Repair. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 141–147. [Google Scholar] [CrossRef]
- Matsushima, S.; Matsuhisa, H.; Wakita, K.; Tsujimoto, T.; Takagaki, N.; Honda, I.; Oshima, Y.; Kawanami, O.; Okada, K. Expanded polytetrafluoroethylene conduits with curved and handsewn bileaflet designs for right ventricular outflow tract reconstruction. J. Thorac. Cardiovasc. Surg. 2024, 167, 439–449.e6. [Google Scholar] [CrossRef]
- Ootaki, Y.; Welch, A.S.; Walsh, M.J.; Quartermain, M.D.; Williams, D.A.; Ungerleider, R.M. Medium-Term Outcomes After Implantation of Expanded Polytetrafluoroethylene Valved Conduit. Ann. Thorac. Surg. 2018, 105, 843–850. [Google Scholar] [CrossRef]
- Dur, O.; Yoshida, M.; Manor, P.; Mayfield, A.; Wearden, P.D.; Morell, V.O.; Pekkan, K. In vitro evaluation of right ventricular outflow tract reconstruction with bicuspid valved polytetrafluoroethylene conduit. Artif. Organs 2010, 34, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Shinkawa, T.; Tang, X.; Gossett, J.M.; Mustafa, T.; Hategekimana, F.; Watanabe, F.; Miyazaki, T.; Yamagishi, M.; Imamura, M. Valved Polytetrafluoroethylene Conduits for Right Ventricular Outflow Tract Reconstruction. Ann. Thorac. Surg. 2015, 100, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Kurosawa, H.; Nomura, K.; Kitamura, N. Fan-shaped expanded polytetrafluoroethylene valve in the pulmonary position. J. Cardiovasc. Surg. 2002, 43, 779–786. [Google Scholar]
- Miyazaki, T.; Yamagishi, M.; Maeda, Y.; Yamamoto, Y.; Taniguchi, S.; Sasaki, Y.; Yaku, H. Expanded polytetrafluoroethylene conduits and patches with bulging sinuses and fan-shaped valves in right ventricular outflow tract reconstruction: Multicenter study in Japan. J. Thorac. Cardiovasc. Surg. 2011, 142, 1122–1129. [Google Scholar] [CrossRef]
- Miyazaki, T.; Yamagishi, M.; Nakashima, A.; Fukae, K.; Nakano, T.; Yaku, H.; Kado, H. Expanded polytetrafluoroethylene valved conduit and patch with bulging sinuses in right ventricular outflow tract reconstruction. J. Thorac. Cardiovasc. Surg. 2007, 134, 327–332. [Google Scholar] [CrossRef]
- Askovich, B.; Hawkins, J.A.; Sower, C.T.; Minich, L.L.; Tani, L.Y.; Stoddard, G.; Puchalski, M.D. Right ventricle-to-pulmonary artery conduit longevity: Is it related to allograft size? Ann. Thorac. Surg. 2007, 84, 907–912. [Google Scholar] [CrossRef]
- Kim, W.H.; Min, S.K.; Choi, C.H.; Lee, J.R.; Kim, Y.J.; Bae, E.J.; Noh, C.I. Follow-up of Shelhigh porcine pulmonic valve conduits. Ann. Thorac. Surg. 2007, 84, 2047–2050. [Google Scholar] [CrossRef]
- Yamagishi, M. Right ventricular outflow reconstruction using a polytetrafluoroethylene conduit with bulging sinuses and tricuspid fan-shaped polytetrafluoroethylene valve. Oper. Tech. Thorac. Cardiovasc. Surg. 2017, 21, 211–229. [Google Scholar] [CrossRef]
- Iyer, K.S. Valved conduits in the right ventricular outflow-the Achilles heel of congenital heart surgery! Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 127–128. [Google Scholar] [CrossRef]
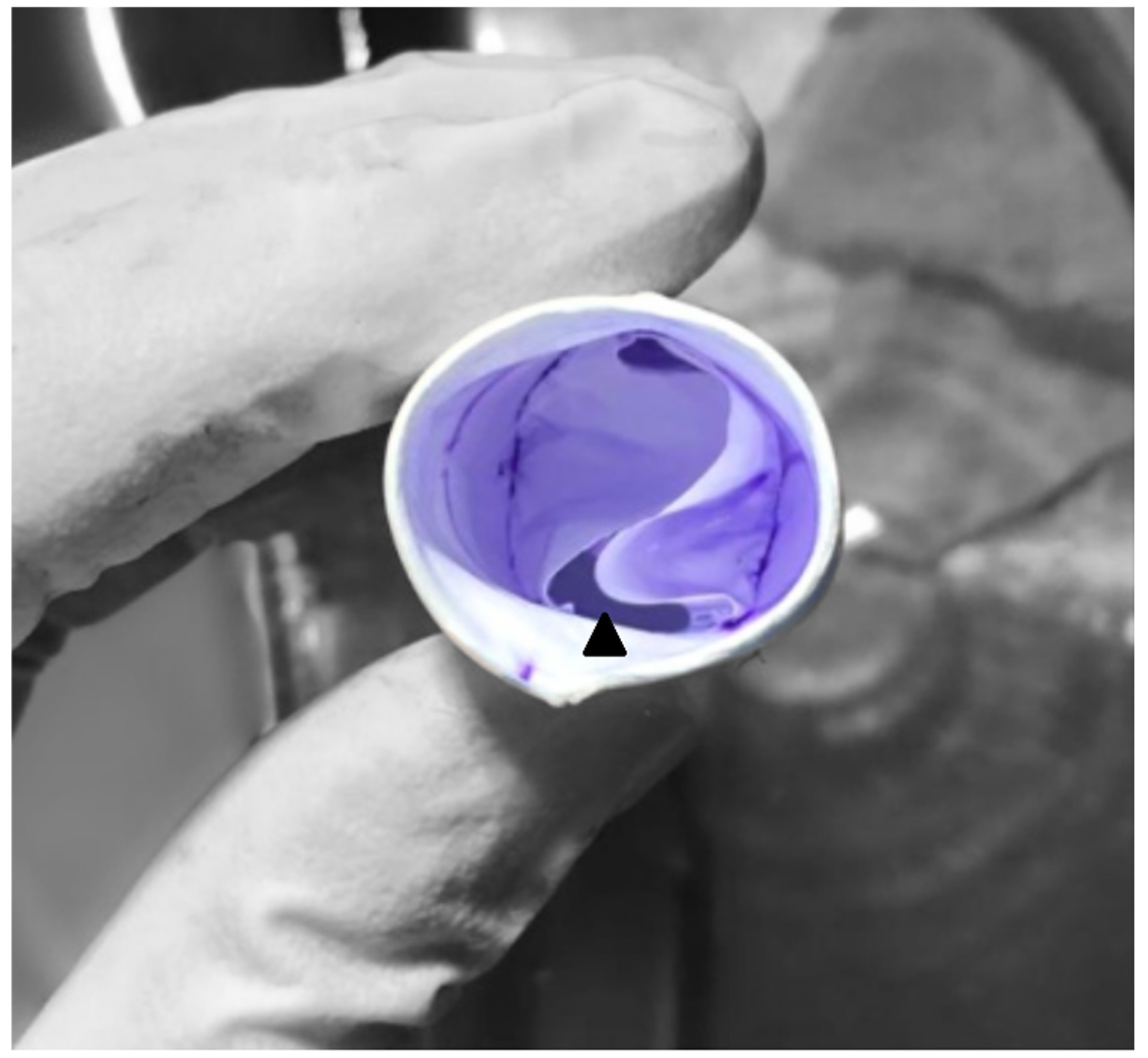
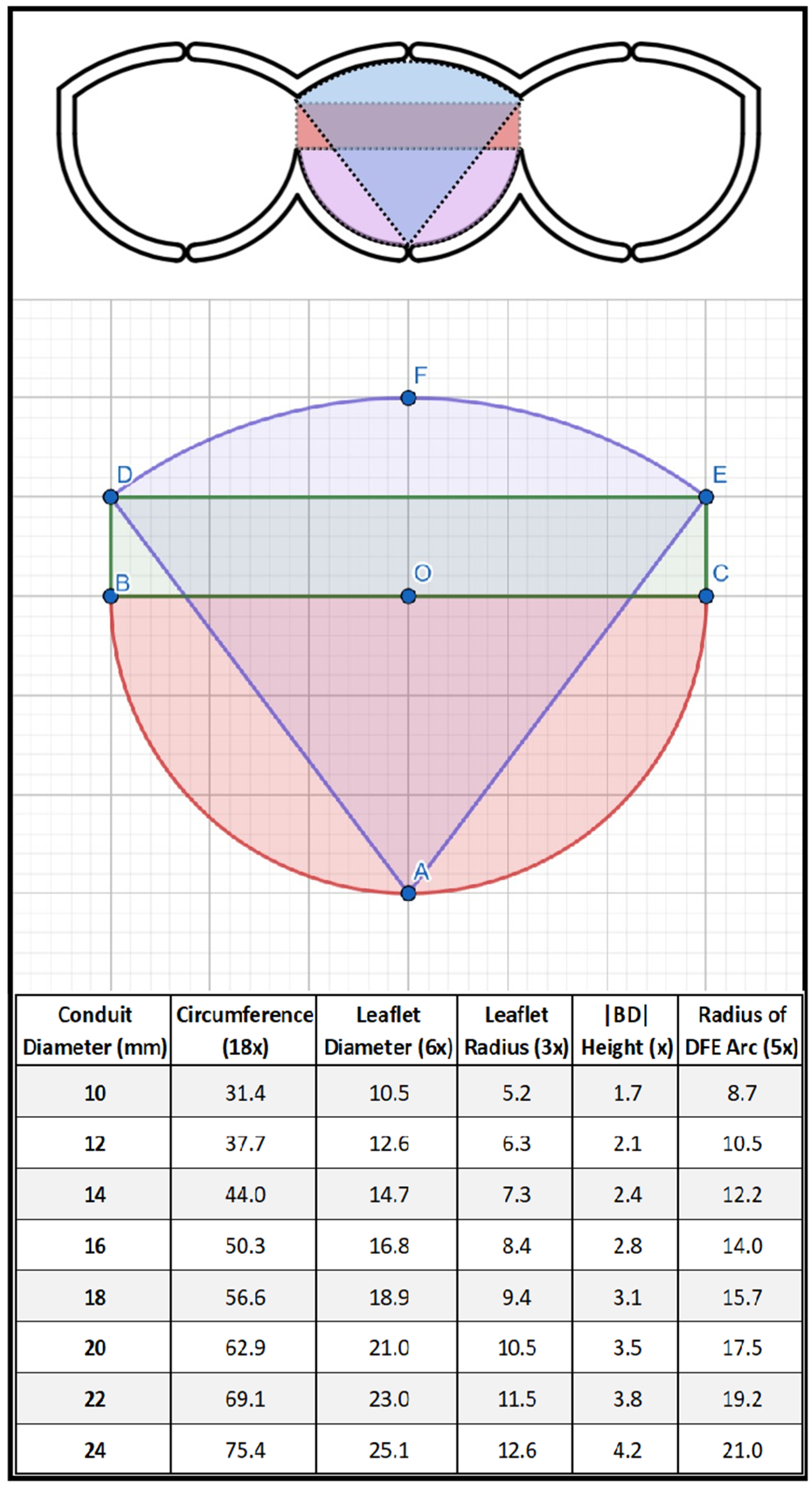
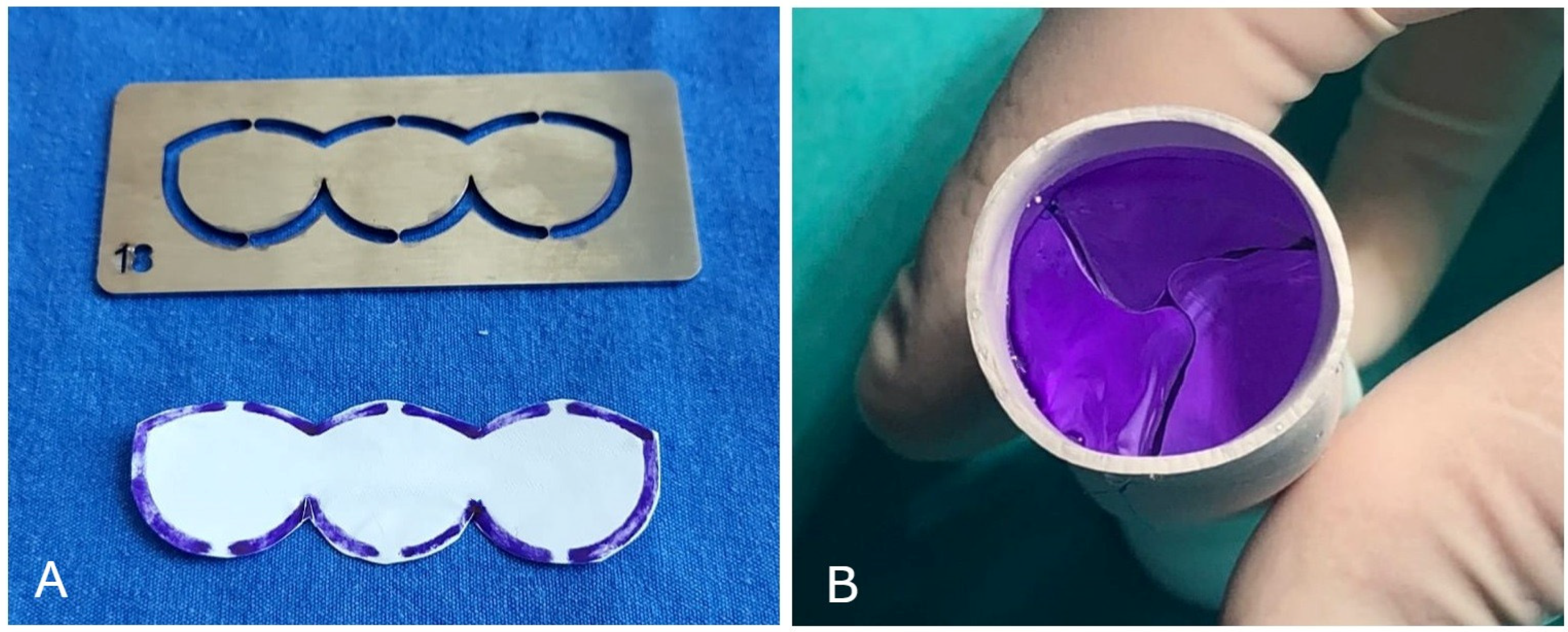

| All Patients (n = 72) | Bicuspid (n = 30) | Tricuspid (n = 42) | ||
|---|---|---|---|---|
| Variables | n (%)/mean ± SD/med (IQR) | n/mean ± SD/med (IQR)/% | n/mean ± SD/med (IQR)/% | p |
| Gender | ||||
| Female | 30 (42%) | 13 (43.3%) | 17 (40.5%) | 0.808 |
| Male | 42 (58%) | 17 (56.7%) | 25 (59.5%) | |
| Age (months) | 69 (26–123) | 43 (13–80) | 87 (46–149) | 0.001 * |
| Body weight (kg) | 21.5 ± 15.1 | 13.9 ± 6.2 | 27.1 ± 17.7 | <0.001 * |
| Diagnosis of the Patients’ | ||||
| Truncus arteriosus | 7 (9.7%) | 3 (10%) | 4 (9.5%) | 0.863 |
| Pulmonary atresia with VSD | 36 (50%) | 15 (50%) | 21 (50%) | |
| TGA with VSD and PS | 6 (8.3) | 2 (6.7%) | 4 (9.5%) | |
| Tetralogy of Fallot | 12 (16.7) | 5 (16.7%) | 7 (16.7%) | |
| Tetralogy of Fallot with APV | 3 (4.2%) | 1 (3.3%) | 2 (4.8%) | |
| LVOTO | 4 (5.6%) | 1 (3.3%) | 3 (7.1%) | |
| Others | 4 (5.6%) | 3 (10%) | 1 (2.4%) | |
| CPB time (min) | 213 ± 67 | 191 ± 56 | 228 ± 70 | 0.02 * |
| CC time (min) | 118 ± 53 | 102 ± 51 | 131 ± 53 | 0.08 |
| Diameter of PTFE Conduits | 16 (16–19) | 16 (16–18) | 18 (16–20) | 0.264 |
| 14 mm | 7 (9.7%) | 5 (16.7%) | 2 (4.8%) | |
| 16 mm | 31 (43%) | 13 (43.3%) | 18 (42.9%) | |
| 18 mm | 16 (22.2%) | 5 (16.7%) | 11 (26.2%) | |
| 20 mm | 12 (27.8%) | 4 (13.3%) | 8 (19%) | |
| 22 mm | 6 (8.3%) | 3 (10%) | 3 (7.1%) |
| All Patients (n = 72) | Bicuspid (n = 30) | Tricuspid (n = 42) | ||
|---|---|---|---|---|
| Variables | n (%)/mean ± SD/med (IQR) | n/mean ± SD/med (IQR)/% | n/mean ± SD/med (IQR)/% | p |
| Conduit Insufficiency | ||||
| Grade 1 | 18 (25%) | 11 (36.7%) | 7 (16.7%) | 0.004 * |
| ≥Grade 2 | 3 (4.2%) | 3 (10%) | 0 | |
| Conduit Stenozis | ||||
| Mild | 25 (34.7%) | 10 (33.3%) | 15 (35.7%) | 0.834 |
| Moderate | 0 | 0 | 0 | |
| Severe | 0 | 0 | 0 | |
| Peak Gradient on Conduit (mmHg) | 0 (0–15) | 0 (0–15) | 0 (0–20) | 0, 467 |
| RV systolic pressure (mmHg) | 40 ± 16 | 39 ± 17 | 41 ± 14 | 0.215 |
| All Patients (n = 72) | Bicuspid (n = 30) | Tricuspid (n = 42) | ||
|---|---|---|---|---|
| Variables | n (%)/mean ± SD/med (IQR) | n/mean ± SD/med (IQR)/% | n/mean ± SD/med (IQR)/% | p |
| Open Sternum | 22 (30.6%) | 6 (20%) | 16 (38.1%) | 0.100 |
| Re-exploration for Bleeding | 13 (18.1%) | 5 (16.7%) | 8 (19.1%) | 0.796 |
| Pnömonia | 8 (11.1%) | 4 (13.3%) | 4 (9.5%) | 0.612 |
| Mediastinitis | 2 (2.8%) | 1 (3.3%) | 1 (2.4%) | 0.808 |
| Septicemia | 4 (5.6%) | 1 (3.3%) | 3 (7.1%) | 0.487 |
| Neurologic Event | 5 (6.9%) | 1 (3.3%) | 4 (9.5%) | 0.308 |
| Arrhythmia | 6 (8.3%) | 3 (10%) | 3 (7.1%) | 0.665 |
| ECMO | 8 (11.1%) | 4 (13.3%) | 4 (9.5%) | 0.612 |
| Duration of ECMO (days) | 3.5 (2.5–4.5) | 4 (2.5–18.5) | 3.5 (2.5–4) | 0.557 |
| ICU stay (days) | 6 (4–11.5) | 6 (4–10) | 7 (5–13) | 0.183 |
| Hospital stay (days) | 14 (9–20) | 13 (8–15) | 15 (11–27) | 0.020 * |
| In-Hospital Mortality | 4 (5.6%) | 3 (10%) | 1 (2.4%) | 0.164 |
| Surviving Patients (n = 68) | Bicuspid (n = 27) | Tricuspid (n = 41) | ||
|---|---|---|---|---|
| Variables | n (%)/mean ± SD/med (IQR) | n/mean ± SD/med (IQR)/% | n/mean ± SD/med (IQR)/% | p |
| Follow-up duration (months) | 22.4 ± 11 | 29.6 ± 11.5 | 17.3 ± 7.4 | 0.001 * |
| Conduit Insufficiency | ||||
| Grade 1 | 21 (30.9%) | 11 (40.7%) | 10 (24.4%) | 0.049 * |
| ≥Grade 2 | 3 (4.4%) | 3 (11.1%) | 0 | |
| Conduit Stenozis | ||||
| Mild | 37 (54.4%) | 14 (51.9%) | 23 (56.1%) | 0.280 |
| Moderate | 2 (2.9%) | 0 | 2 (4.9%) | |
| Severe | 0 | 0 | 0 | |
| Peak Gradient on conduit (mmHg) | 15 (0–25) | 0 (0–15) | 15 (0–25) | 0.032 * |
| RV systolic pressure (mmHg) | 45 ± 17 | 40 ± 14 | 46 ± 15 | 0.024 * |
| Baloon angioplasty for peripheral PAS | 4 (5.9%) | 3 (11.1%) | 1 (2.4%) | 0.301 |
| Reoperations | 2 (2.9%) | 0 | 2 (4.9%) | 0.360 |
| Late Mortality | 1 (1.5%) | 0 | 1 (2.4%) | 0.603 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çiçek, M.; Özdemir, F.; Yurdakök, O.; Korun, O.; Önalan, M.A.; Hekim Yılmaz, E.; Kudsioğlu, T.; Aydemir, N.A. Comparison of Bicuspid and Tricuspid Handmade Polytetrafluoroethylene Valved Conduits: Early and Mid-Term Results. J. Clin. Med. 2025, 14, 1957. https://doi.org/10.3390/jcm14061957
Çiçek M, Özdemir F, Yurdakök O, Korun O, Önalan MA, Hekim Yılmaz E, Kudsioğlu T, Aydemir NA. Comparison of Bicuspid and Tricuspid Handmade Polytetrafluoroethylene Valved Conduits: Early and Mid-Term Results. Journal of Clinical Medicine. 2025; 14(6):1957. https://doi.org/10.3390/jcm14061957
Chicago/Turabian StyleÇiçek, Murat, Fatih Özdemir, Okan Yurdakök, Oktay Korun, Mehmet Akif Önalan, Emine Hekim Yılmaz, Türkan Kudsioğlu, and Numan Ali Aydemir. 2025. "Comparison of Bicuspid and Tricuspid Handmade Polytetrafluoroethylene Valved Conduits: Early and Mid-Term Results" Journal of Clinical Medicine 14, no. 6: 1957. https://doi.org/10.3390/jcm14061957
APA StyleÇiçek, M., Özdemir, F., Yurdakök, O., Korun, O., Önalan, M. A., Hekim Yılmaz, E., Kudsioğlu, T., & Aydemir, N. A. (2025). Comparison of Bicuspid and Tricuspid Handmade Polytetrafluoroethylene Valved Conduits: Early and Mid-Term Results. Journal of Clinical Medicine, 14(6), 1957. https://doi.org/10.3390/jcm14061957





