Association of Endothelial Nitric Oxide Synthase Polymorphisms with Clinical Severity in Patients with COVID-19
Abstract
1. Introduction
2. Materials and Methods
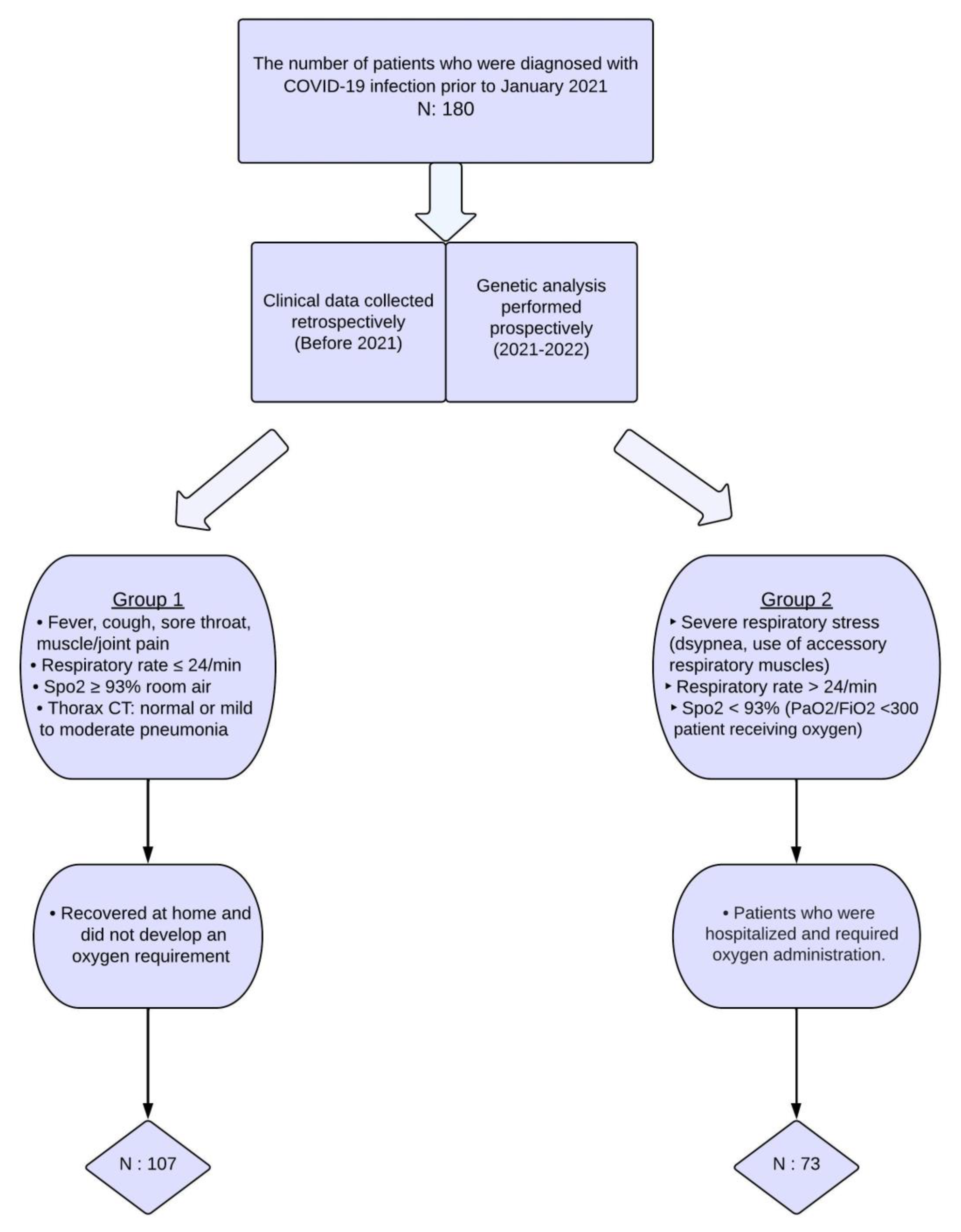
2.1. Selection of Patients and Acquisition of Samples
2.2. Genotyping
2.3. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-2 | Angiotensin-Converting Enzyme-2 |
| COPD | chronic obstructive pulmonary disease |
| COVID-19 | coronavirus disease 2019 |
| NOS3 | endothelial nitric oxide synthase |
| IVIG | intravenous immunoglobulin |
| LDH | lactate dehydrogenase |
| NIMV | non-invasive mechanical ventilation |
| NO | nitric oxide |
| PCR | polymerase chain reaction |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
References
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe COVID-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, A.; Sen, C.; Garcia, G., Jr.; Langerman, J.; Shia, D.W.; Meneses, L.K.; Vijayaraj, P.; Durra, A.; Koloff, C.R.; Freund, D.R.; et al. Direct exposure to SARS-CoV-2 and cigarette smoke increases infection severity and alters the stem cell-derived airway repair response. Cell Stem Cell 2020, 27, 869–875.e4. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Gkizarioti, Z.; Patrinos, G.P.; Tsakris, A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum. Genom. 2020, 14, 40. [Google Scholar] [CrossRef]
- Severe COVID-19 GWAS Group; Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; et al. Genomewide association study of severe COVID-19 with respiratory failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Ji, X.S.; Chen, B.; Ze, B.; Zhou, W.H. Human genetic basis of severe or critical illness in COVID-19. Front. Cell. Infect. Microbiol. 2022, 12, 963239. [Google Scholar] [CrossRef]
- Delgado-Wicke, P.; Fernández de Córdoba-Oñate, S.; Roy-Vallejo, E.; Alegría-Carrasco, E.; Rodríguez-Serrano, D.A.; Lamana, A.; Montes, N.; Nicolao-Gómez, A.; Carracedo-Rodríguez, R.; Marcos-Jiménez, A.; et al. Genetic Variants Regulating the Immune Response Improve the Prediction of COVID-19 Severity Provided by Clinical Variables. Sci. Rep. 2024, 14, 20728. [Google Scholar] [CrossRef]
- Demirci, M.; Ünlü, Ö.; Yiğin, A.; Zeyrek, F.Y. Pathogenesis of SARS-CoV-2 and immune response in COVID-19. Turk. Mikrobiyol. Cem. Derg. 2020, 50, 183–191. [Google Scholar] [CrossRef]
- Ombrello, M.J.; Schulert, G.S. COVID-19 and cytokine storm syndrome: Are there lessons from macrophage activation syndrome? Transl. Res. 2021, 232, 1–12. [Google Scholar] [CrossRef]
- Khan, M.; Shah, N.; Mushtaq, H.; Jehanzeb, V. Profiling laboratory biomarkers associated with COVID-19 disease progression: A single-center experience. Int. J. Microbiol. 2021, 2021, 6643333. [Google Scholar] [CrossRef]
- Ponti, G.; Maccaferri, M.; Ruini, C.; Tomasi, A.; Ozben, T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020, 57, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Babaoglu, M.O.; Dikmenoglu, N.; Ileri-Gurel, E.; Seringec, N.; Zoto, T.; Yasar, U.; Kayaalp, S.O.; Bozkurt, A. Functional effects of endothelial nitric oxide synthase genetic polymorphisms on haemorheological parameters in healthy human individuals. Basic Clin. Pharmacol. Toxicol. 2011, 108, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S. Nitric oxide. J. Hypertens. Suppl. 1994, 12, S35–S39. [Google Scholar] [PubMed]
- Özkan, M.; Günay, N.; Sener, E.F.; Karcıoglu, Ö.; Tahtasakal, R.; Dal, F.; Günay, N.E.; Demiryürek, A.T. Variants in TNF and NOS3 (NOS3) genes associated with sepsis in adult patients. J. Gene Med. 2021, 23, e3323. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic effect of enhancing endothelial nitric oxide synthase (NOS3) expression and preventing NOS3 uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Marín, J.; Rodríguez-Martínez, M.A. Role of vascular nitric oxide in physiological and pathological conditions. Pharmacol. Ther. 1997, 75, 111–134. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, J. Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol. Genet. Metab. 2000, 70, 241–251. [Google Scholar] [CrossRef]
- Godfrey, V.; Chan, S.-L.; Cassidy, A.; Butler, R.; Choy, A.; Fardon, T.; Struthers, A.; Lang, C. The functional consequence of the Glu298Asp polymorphism of the endothelial nitric oxide synthase gene in young healthy volunteers. Cardiovasc. Drug Rev. 2007, 25, 280–288. [Google Scholar] [CrossRef]
- İlhan, N.; Ateş, K.; İlhan, N.; Kaman, D.; Çeliker, H. NOS3 Glu298Asp polymorphism and endothelial dysfunction in patients with and without end-stage renal disease. Balkan Med. J. 2016, 33, 128–137. [Google Scholar] [CrossRef]
- Konar, S.K.; Ramesh, S.; Christopher, R.; Prasanthi, A.; Bhat, D.I.; Shukla, D.; Bharath, R. The correlation of endothelial nitric oxide synthase (NOS3) polymorphism and other risk factors with aneurysmal subarachnoid hemorrhage: A case-control study. Neurol. India 2019, 67, 1006–1012. [Google Scholar] [CrossRef]
- Ma, Z.J.; Chen, R.; Ren, H.-Z.; Guo, X.; Guo, J.; Chen, L.M. Association between NOS3 4b/a polymorphism and the risk of diabetic retinopathy in type 2 diabetes mellitus: A meta-analysis. J. Diabetes Res. 2014, 2014, 549747. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Bhatnagar, M.K.; Bhattacharjee, J. Association of endothelial dysfunction with endothelin, nitric oxide and NOS3 Glu298Asp gene polymorphism in coronary artery disease. Dis. Markers 2011, 31, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Liu, S.; Guo, Y.; Liu, S.; Xu, J.; Pan, L.; Hu, Y.; Liu, Y.; Cheng, Y. Association between NOS3 rs1799983 polymorphism and hypertension: A meta-analysis involving 14,185 cases and 13,407 controls. BMC Cardiovasc. Disord. 2021, 21, 385. [Google Scholar] [CrossRef] [PubMed]
- Dieter, C.; Brondani, L.A.; Leitão, C.B.; Gerchman, F.; Lemos, N.E.; Crispim, D. Genetic Polymorphisms Associated with Susceptibility to COVID-19 Disease and Severity: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0270627. [Google Scholar] [CrossRef]
- Kaltoum, A.B.O. Mutations and polymorphisms in genes involved in the infections by COVID-19: A Review. Gene Rep. 2021, 23, 101062. [Google Scholar] [CrossRef]
- Eid, M.A.; Aleksandrova, A.A.; Shkurat, M.A.; Shkurat, T.P. The Association of PON1 and NOS3 Genetic Variants with the Severity of COVID-19. Gene Rep. 2023, 33, 101814. [Google Scholar] [CrossRef]
- Tanus-Santos, J.E.; Desai, M.; Deak, L.R.; Pezzullo, J.C.; Abernethy, D.R.; Flockhart, D.A.; Freedman, J.E. Effects of endothelial nitric oxide synthase gene polymorphisms on platelet function, nitric oxide release, and interactions with estradiol. Pharmacogenetics 2002, 12, 407–413. [Google Scholar] [CrossRef]
- Xu, S.-W.; Ilyas, I.; Weng, J.-P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Nägele, M.P.; Haubner, B.; Tanner, F.C.; Ruschitzka, F.; Flammer, A.J. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis 2020, 314, 58–62. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Dzúrová, D. Body mass index and risk for COVID-19-related hospitalization in adults aged 50 and older in Europe. Nutrients 2022, 14, 4001. [Google Scholar] [CrossRef] [PubMed]
- Ghaznavi-Rad, E.; Khosravi, M.; Sayyadi, M. The importance of using routine laboratory tests in the diagnosis and prognosis of patients with coronavirus disease 2019: Shedding light on clinical laboratory data in COVID-19. J. Clin. Lab. Anal. 2022, 36, e24713. [Google Scholar] [CrossRef] [PubMed]
- Akerström, S.; Mousavi-Jazi, M.; Klingström, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef]
- Guan, S.P.; Seet, R.C.S.; Kennedy, B.K. Does NOS3 derived nitric oxide protect the young from severe COVID-19 complications? Ageing Res. Rev. 2020, 64, 101201. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Carmeliet, P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 2019, 30, 414–433. [Google Scholar] [CrossRef]
- Rossaint, R.; Gerlach, H.; Schmidt-Ruhnke, H.; Pappert, D.; Lewandowski, K.; Steudel, W.; Falke, K. Efficacy of inhaled nitric oxide in patients with severe ARDS. Chest 1995, 107, 1107–1115. [Google Scholar] [CrossRef]
- Konradt, C.; Hunter, C.A. Pathogen interactions with endothelial cells and the induction of innate and adaptive immunity. Eur. J. Immunol. 2018, 48, 1607–1620. [Google Scholar] [CrossRef]
- Papadopoulos, K.I.; Sutheesophon, W.; Aw, T.-C. The influence of renin angiotensin aldosterone system (RAAS), endothelial nitric oxide synthase (NOS3) and erythropoietin (EPO) on COVID-19 complications. Chem. Biol. Interact. 2022, 354, 109834. [Google Scholar] [CrossRef]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Endothelial nitric oxide synthase: From biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene 2016, 575, 584–599. [Google Scholar] [CrossRef]
- Smith, L.M.; Cuthbertson, B.; Harvie, J.; Webster, N.; Robins, S.; Ralston, S.H. Increased bone resorption in the critically ill: Association with sepsis and increased nitric oxide production. Crit. Care Med. 2002, 30, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Cotta Filho, C.K.; Oliveira-Paula, G.H.; Rondon Pereira, V.C.; Lacchini, R. Clinically relevant endothelial nitric oxide synthase polymorphisms and their impact on drug response. Expert Opin. Drug Metab. Toxicol. 2020, 16, 927–951. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, E.; Kofteridis, D.; Stratigi, K.; Petraki, E.; Vazgiourakis, V.; Fragouli, E.; Mamoulakis, D.; Boumpas, D.T.; Goulielmos, G.N. Intron 4 a/b polymorphism of the endothelial nitric oxide synthase gene is associated with both type 1 and type 2 diabetes in a genetically homogeneous population. Hum. Immunol. 2008, 69, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Souza-Costa, D.C.; Belo, V.A.; Silva, P.S.; Sertorio, J.T.; Metzger, I.F.; Lanna, C.M.; Machado, M.A.; Tanus-Santos, J.E. NOS3 haplotype associated with hypertension in obese children and adolescents. Int. J. Obes. 2011, 35, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Staalsø, J.M.; Edsen, T.; Kotinis, A.; Romner, B.; Springborg, J.B.; Olsen, N.V. Association of the NOS3 intron-4 VNTR polymorphism with aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2014, 121, 587–592. [Google Scholar] [CrossRef]
- Ozturk, E.; Balat, O.; Pehlivan, S.; Ugur, M.G.; Ozcan, C.; Sever, T.; Kul, S. Endothelial nitric oxide synthase gene polymorphisms in preeclampsia with or without eclampsia in a Turkish population. J. Obstet. Gynaecol. Res. 2011, 37, 1778–1783. [Google Scholar] [CrossRef]
- Sljivancanin Jakovljevic, T.; Kontic-Vucinic, O.; Nikolic, N.; Nikolic, N.; Carkic, J.; Stamenkovic, J.; Soldatovic, I.; Milasin, J. Association between endothelial nitric oxide synthase (NOS3) −786 T/C and 27-bp VNTR 4b/a polymorphisms and preeclampsia development. Reprod. Sci. 2021, 28, 3529–3539. [Google Scholar] [CrossRef]
- Yanamandra, K.; Boggs, P.B.; Thurmon, T.F.; Lewis, D.; Bocchini, J.A.; Dhanireddy, R. Novel allele of the endothelial nitric oxide synthase gene polymorphism in Caucasian asthmatics. Biochem. Biophys. Res. Commun. 2005, 335, 545–549. [Google Scholar] [CrossRef]
- Silva, B.M.; Neves, F.J.; Rocha, N.G.; Sales, A.R.; Medeiros, R.F.; Barbosa, T.C.; Pereira, F.S.; Cardoso, F.T.; da Nóbrega, A.C.L. Endothelial nitric oxide gene haplotype reduces the effect of a single bout of exercise on the vascular reactivity in healthy subjects. Transl. Res. 2013, 161, 15–25. [Google Scholar] [CrossRef][Green Version]
- Alshaikh, F.S.; Godman, B.; Sindi, O.N.; Seaton, R.A.; Kurdi, A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0272375. [Google Scholar] [CrossRef]
| Genetic Polymorphism | PCR a Primers | PCR Product Length (bp) | Restriction Enzyme | Restriction Fragment Length (bp) | Allele |
|---|---|---|---|---|---|
| NOS3 G894T rs1799983 | 5′–AAGGCAGGAGACAGTGGATGGA–3′ | 248 | BanII | 163 + 85 | G |
| 5′–CCCAGTCAATCCCTTTGGTGCTCA–3′ | 248 | T | |||
| NOS3 27-bp VNTR 4b/a | 5′–AGGCCCTATGGTAGTGCCTTT–3′ | No restriction. The lengths of PCR products for 4a, 4b, and 4c alleles are 447, 420, and 393 bp, respectively. | |||
| 5′–TCTCTTAGTGCTGTGGTCAC–3′ | |||||
| Group 1 (Mild) (n = 107) | Group 2 (Severe) (n = 73) | p-Value | |
|---|---|---|---|
| Age a (years) | 41.9 ± 14.7 | 60.2 ± 16.9 | <0.001 |
| Sex (n) (%) Female b Male b | |||
| 75 (70.1%) | 37 (50.7%) | 0.008 | |
| 32 (29.9%) | 36 (49.3%) | ||
| Cigarettes b (box/years) | 6.9 ± 11.9 | 10.8 ± 15.6 | 0.114 |
| Diagnostic method (n) (%) PCR test b Thorax CT (PCR negative) b | |||
| 105 (98.1%) | 71 (97.3%) | ||
| 2 (1.9%) | 2 (2.7%) | 1.000 | |
| Pulmonary embolism (COVID-19 related) b | 0 | 0 | |
| Comorbidities (n) (%) | |||
| Type 2 diabetes b | 19 (17.8%) | 25 (34.2%) | 0.011 |
| Hypertension b | 23 (21.5%) | 28 (38.4%) | 0.014 |
| Coronary artery disease b | 5 (4.7%) | 18 (24.7%) | <0.001 |
| Heart failure b | 7 (6.5%) | 22 (30.1%) | <0.001 |
| Cardiac arrhythmia b | 2 (1.9%) | 9 (12.3%) | 0.008 |
| COPD b | 6 (5.6%) | 14 (19.2%) | 0.009 |
| Asthma b | 15 (14.0%) | 14 (19.2%) | 0.473 |
| GI diseases b | 8 (7.5%) | 1 (1.4%) | 0.086 |
| Liver diseases b | 3 (2.8%) | 2 (2.7%) | 0.980 |
| Kidney diseases b | 3 (2.8%) | 6 (8.2%) | 0.161 |
| Hyperthyroidism b | 1 (0.9%) | 0 | |
| Hypothyroidism b | 13 (12.1%) | 14 (19.2%) | 0.278 |
| Hyperlipidemia b | 5 (4.7%) | 5 (6.8%) | 0.768 |
| Connective tissue disease b | 5 (4.7%) | 6 (8.2%) | 0.510 |
| Dementia b | 3 (2.8%) | 14 (19.2%) | <0.001 |
| Obesity b | 9 (8.4%) | 13 (17.8%) | 0.097 |
| Group-1 (n = 107) | Group-2 (n = 73) | p-Value | |
|---|---|---|---|
| D-dimer a (mg/L) | 0.55 ± 0.40 | 1.86 ± 2.97 | 0.001 |
| C-reactive protein a (mg/dL) | 1.48 ± 1.59 | 14.16 ± 23.49 | 0.001 |
| Lactate dehydrogenase a (U/L) | 166.79 ± 59.42 | 316.90 ± 148.58 | 0.001 |
| Troponin-I a (ng/L) | 4.94 ± 5.16 | 147.06 ± 775.47 | 0.024 |
| Ferritin a (µg/L) | 101.0 ± 84.1 | 273.1 ± 351.5 | 0.004 |
| Creatine phosphokinase a (U/L) | 112.8 ± 142.8 | 151.5 ± 159.1 | 0.275 |
| Hemoglobin a (g/dL) | 13.9 ± 1.3 | 12.8 ± 1.9 | 0.001 |
| Platelet count a (103/µL) | 248.3 ± 94.9 | 222.6 ± 113.5 | 0.221 |
| Lymphocyte numbers a (103/µL) | 1.643 ± 0.6 | 1.296 ± 1.2 | 0.092 |
| Neutrophil/lymphocyte ratio b | 3.05 (2.26–3.59) | 4.45 (2.58–6.18) | 0.001 |
| DRUGS ADMINISTERED | |||
| Corticosteroid c | 6 (5.6%) | 58 (79.5%) | 0.001 |
| Enoxaparin c | 13 (12.1%) | 69 (94.5%) | 0.001 |
| Tocilizumab | 0 | 0 | |
| Anakinra | 0 | 2 (2.7%) | |
| Favipiravir c | 42 (39.3%) | 37 (50.7%) | 0.129 |
| Remdesivir | 0 | 1 (1.4%) | |
| Hydroxychloroquine c | 6 (5.6%) | 8 (11.0%) | 0.302 |
| IVIG | 0 | 3 (4.1%) | |
| Plasma exchange | 0 | 3 (4.1%) | |
| Paracetamol/NSAIDs c | 50 (46.7%) | 52 (71.2%) | 0.002 |
| Acetylsalicylic acid c | 23 (21.5%) | 18 (24.7%) | 0.752 |
| Antibiotic use c | 4 (3.7%) | 34 (46.6%) | 0.001 |
| Low-flow oxygen c | 0 | 73 (100%) | 0.001 |
| High-flow oxygen c | 0 | 23 (31.5%) | 0.001 |
| NIMV c | 0 | 18 (24.7%) | 0.001 |
| Vasopressor | 0 | 4 (5.5%) |
| Genetic Polymorphism | Group 1 (n = 107) (Patients with Mild COVID-19) | Group 2 (n = 73) Patients with Severe COVID-19 | p-Value; (χ2, df) | ||
|---|---|---|---|---|---|
| NOS3 G894T rs1799983 | n | (%) | n | (%) | |
| Genotypes | |||||
| GG | 55 | (51.4) | 40 | (54.8) | 0.85; (0.31, 2) |
| GT | 38 | (35.5) | 23 | (31.5) | |
| TT | 14 | (13.1) | 10 | (13.7) | |
| Alleles | |||||
| G | 148 | (69.2) | 103 | (70.5) | 0.78; (0.08, 1) |
| T | 66 | (30.8) | 43 | (29.5) | |
| NOS3 27-bp VNTR 4b/a | |||||
| Genotypes | |||||
| 4b/4b | 79 | (73.8) | 56 | (78.9) | 0.44; (0.59, 1) |
| 4b/4a and 4a/4a | 28 | (26.2) | 15 | (21.1) | |
| Alleles | |||||
| 4b | 183 | (85.5) | 127 | (89.4) | 0.28; (1.17, 1) |
| 4a | 31 | (14.5) | 15 | (10.6) | |
| Genetic Polymorphism | Subgroup 1 (n = 96) (Patients with Mild COVID-19) | Subgroup 2 (n = 20) (Patients with Severe COVID-19) | p-Value; (χ2, df) | ||
|---|---|---|---|---|---|
| NOS3 G894T rs1799983 | n | (%) | n | (%) | |
| Genotypes | |||||
| GG | 57 | (59.4) | 11 | (55.0) | 0.68; (0.79, 2) |
| GT | 27 | (28.1) | 5 | (25.0) | |
| TT | 12 | (12.5) | 4 | (20.0) | |
| Alleles | |||||
| G | 141 | (73.4) | 27 | (67.5) | 0.45; (0.58, 1) |
| T | 51 | (26.6) | 13 | (32.5) | |
| NOS3 27-bp VNTR 4b/a | p-value (Fisher’s exact test) | ||||
| Genotypes | |||||
| 4b/4b | 71 | (74.0) | 18 | (94.7) | 0.07 |
| 4b/4a and 4a/4a | 25 | (26.0) | 1 | (5.3) | |
| Alleles | |||||
| 4b | 164 | (85.4) | 37 | (97.4) | 0.06 |
| 4a | 28 | (14.6) | 1 | (2.6) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İdikut, A.; Değer, İ.; Göktaş, G.; Karahan, S.; Sarınç, S.; Köksal, D.; Babaoğlu, M.O.; Babaoğlu, E. Association of Endothelial Nitric Oxide Synthase Polymorphisms with Clinical Severity in Patients with COVID-19. J. Clin. Med. 2025, 14, 1931. https://doi.org/10.3390/jcm14061931
İdikut A, Değer İ, Göktaş G, Karahan S, Sarınç S, Köksal D, Babaoğlu MO, Babaoğlu E. Association of Endothelial Nitric Oxide Synthase Polymorphisms with Clinical Severity in Patients with COVID-19. Journal of Clinical Medicine. 2025; 14(6):1931. https://doi.org/10.3390/jcm14061931
Chicago/Turabian Styleİdikut, Aytekin, İlter Değer, Gamze Göktaş, Sevilay Karahan, Sevinç Sarınç, Deniz Köksal, Melih O. Babaoğlu, and Elif Babaoğlu. 2025. "Association of Endothelial Nitric Oxide Synthase Polymorphisms with Clinical Severity in Patients with COVID-19" Journal of Clinical Medicine 14, no. 6: 1931. https://doi.org/10.3390/jcm14061931
APA Styleİdikut, A., Değer, İ., Göktaş, G., Karahan, S., Sarınç, S., Köksal, D., Babaoğlu, M. O., & Babaoğlu, E. (2025). Association of Endothelial Nitric Oxide Synthase Polymorphisms with Clinical Severity in Patients with COVID-19. Journal of Clinical Medicine, 14(6), 1931. https://doi.org/10.3390/jcm14061931







