How to Preserve Fertility in Reproductive-Age Women with Cancer
Abstract
1. Introduction
2. Cancer Treatments
2.1. Chemotherapy
2.1.1. Direct Action on DNA
2.1.2. Indirect Action on DNA
2.2. Other Classes of Anticancer Agents
2.3. Radiotherapy
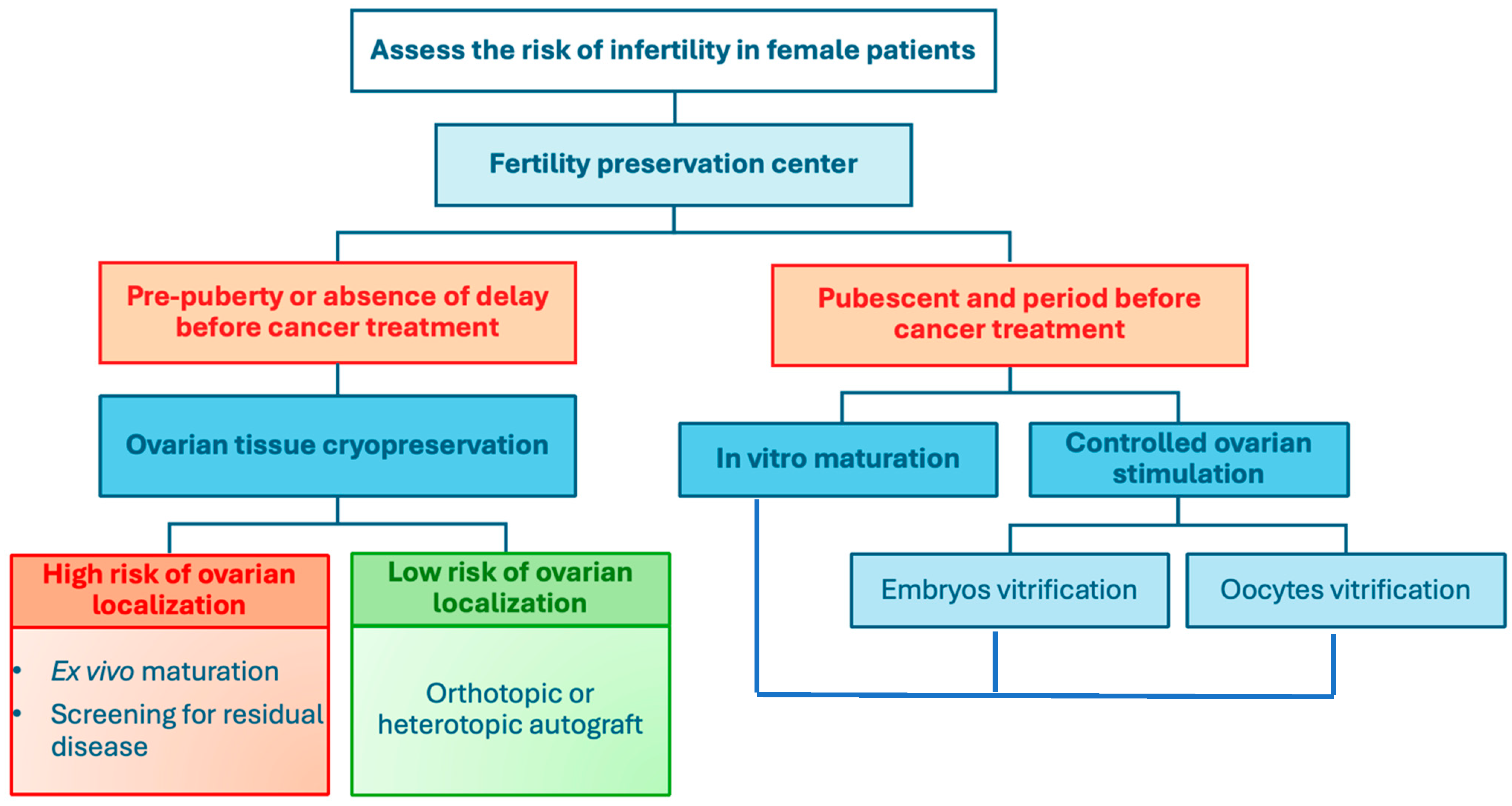
3. Fertility Preservation Techniques
3.1. Oocyte Vitrification After Ovarian Stimulation
3.2. Embryo Vitrification
3.3. In Vitro Maturation
3.4. Ovarian Tissue Cryopreservation
3.5. Combination of Different FP Techniques
3.6. Surgical Techniques for FP
3.7. GnRH Agonists
4. Cancers and Fertility Preservation in Young Women
4.1. Breast Cancer
4.1.1. Treatments
4.1.2. Fertility Preservation
4.1.3. BRCA-Mutated Patients
4.2. Thyroid
4.2.1. Treatment
4.2.2. Fertility Preservation
4.3. Cervical Cancer
4.3.1. Treatment
4.3.2. Fertility Preservation
4.4. Hodgkin’s Lymphoma
4.4.1. Treatment
4.4.2. Fertility Preservation
4.5. Non-Hodgkin’s Lymphoma
4.5.1. Treatment
4.5.2. Fertility Preservation
4.6. Ovarian Cancer
4.6.1. Malignant Tumors
Treatments
Fertility Preservation
4.6.2. Ovarian Germ Cell Tumors
4.6.3. Malignant Tumors of the Sex Cords and Stroma of the Ovary
4.7. Acute Leukemia
4.7.1. Treatment
4.7.2. Fertility Preservation
4.8. Melanoma
4.8.1. Treatment
4.8.2. Fertility Preservation
4.9. Brain Tumors
4.9.1. Treatment
4.9.2. Fertility Preservation
4.10. Primary Malignant Bone Tumors
4.10.1. Treatment
4.10.2. Fertility Preservation
4.11. Digestive Cancers
4.11.1. Treatments
4.11.2. Fertility Preservation
5. Conclusions
Funding
Conflicts of Interest
References
- Cancer. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/cancer (accessed on 29 August 2024).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Lapôtre-Ledoux, B. Incidence des Principaux Cancers en France Métropolitaine en 2023 et Tendances Depuis 1990/Main Cancers Incidence in Metropolitan France in 2023 and Trends Since 1990. Bull. Epidémiol. Hebd.—BEH 2023, 12–13, 188–204. [Google Scholar]
- You, L.; Lv, Z.; Li, C.; Ye, W.; Zhou, Y.; Jin, J.; Han, Q. Worldwide cancer statistics of adolescents and young adults in 2019: A systematic analysis of the Global Burden of Disease Study 2019. ESMO Open 2021, 6, 100255. [Google Scholar] [CrossRef] [PubMed]
- Préservation de la Fertilité chez les Hommes et les Femmes Atteints d’un Cancer/Thésaurus, Janvier 2021. Available online: https://www.cancer.fr/catalogue-des-publications/preservation-de-la-fertilite-et-cancer-thesaurus (accessed on 27 August 2024).
- Bedoschi, G.; Navarro, P.A.; Oktay, K. Chemotherapy-induced damage to ovary: Mechanisms and clinical impact. Future Oncol. 2016, 12, 2333–2344. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Visser, J.A.; Laven, J.S.E.; Broer, S.L.; Themmen, A.P.N.; Fauser, B.C. Anti-Müllerian hormone and ovarian dysfunction. Trends Endocrinol. Metab. 2008, 19, 340–347. [Google Scholar] [CrossRef]
- Decanter, C.; Elefant, E.; Poirot, C.; Courbiere, B. What reproductive follow-up for adolescent and young women after cancer? A review. Reprod. Biomed. Online 2024, 49, 103891. [Google Scholar] [CrossRef]
- Grosbois, J.; Demeestere, I. Dynamics of PI3K and Hippo signaling pathways during in vitro human follicle activation. Hum. Reprod. 2018, 33, 1705–1714. [Google Scholar] [CrossRef]
- Masciangelo, R.; Hossay, C.; Chiti, M.C.; Manavella, D.D.; Amorim, C.A.; Donnez, J.; Dolmans, M.-M. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J. Assist. Reprod. Genet. 2020, 37, 101–108. [Google Scholar] [CrossRef]
- Erden, M.; Oktay, K.H. Does gonadotoxic chemotherapy deplete the ovarian reserve through activation of primordial follicles? Hum. Reprod. 2025, 1–9. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide Triggers Follicle Activation and “Burnout”; AS101 Prevents Follicle Loss and Preserves Fertility. Sci. Transl. Med. 2013, 5, 185ra62. [Google Scholar] [CrossRef]
- Goodman, L.S.; Wintrobe, M.M.; Dameshek, W.; Goodman, M.J.; Gilman, A.; McLennan, M.T. Use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA 1946, 132, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Peccatori, F.A.; Demeestere, I.; Amant, F.; Wyns, C.; Stukenborg, J.-B.; Paluch-Shimon, S.; Halaska, M.J.; Uzan, C.; Meissner, J.; et al. Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO Clinical Practice Guidelines†. Ann. Oncol. 2020, 31, 1664–1678. [Google Scholar] [CrossRef]
- Pourquier, P. Agents alkylants. Bull. Cancer 2011, 98, 1237–1251. [Google Scholar] [CrossRef]
- Green, D.M.; Nolan, V.G.; Goodman, P.J.; Whitton, J.A.; Srivastava, D.; Leisenring, W.M.; Neglia, J.P.; Sklar, C.A.; Kaste, S.C.; Hudson, M.M.; et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the childhood cancer survivor study. Pediatr. Blood Cancer 2014, 61, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Wada, T.; Nishizawa, Y.; Iwanaga, T.; Aoki, Y.; Terasawa, T.; Kosaki, G.; Yamamoto, T.; Wada, A. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer 1977, 39, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, M.H.; Van Dijk, M.; Byrne, J.; Berger, C.; Dirksen, U.; Winther, J.F.; Fossa, S.D.; Grabow, D.; Grandage, V.L.; Haupt, R.; et al. Treatment-related fertility impairment in long-term female childhood, adolescent and young adult cancer survivors: Investigating dose-effect relationships in a European case-control study (PanCareLIFE). Hum. Reprod. 2021, 36, 1561–1573. [Google Scholar] [CrossRef]
- Bisogno, G.; Minard-Colin, V.; Haduong, J.; Zanetti, I.; Ferrari, A.; Chisholm, J.; Heske, C.M.; Hladun, R.; Jenney, M.; Merks, J.H.M.; et al. Implications of Implementing Children’s Oncology Group Risk Stratification to Patients with Rhabdomyosarcoma Treated on European Paediatric Soft Tissue Sarcoma Study Group Clinical Trial. Pediatr. Blood Cancer 2025, 72, e31436. [Google Scholar] [CrossRef]
- ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar]
- Yildiz, S.; Bildik, G.; Benlioglu, C.; Turan, V.; Dilege, E.; Ozel, M.; Kim, S.; Oktem, O. Breast cancer treatment and ovarian function. Reprod. Biomed. Online 2023, 46, 313–331. [Google Scholar] [CrossRef]
- Yin, W.W.; Huang, C.C.; Chen, Y.R.; Yu, D.Q.; Jin, M.; Feng, C. The effect of medication on serum anti-müllerian hormone (AMH) levels in women of reproductive age: A meta-analysis. BMC Endocr. Disord. 2022, 22, 158. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Anderson, R.A.; Irvine, D.S. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Winship, A.L.; Hutt, K.J. Do cancer therapies damage the uterus and compromise fertility? Hum. Reprod. Update 2020, 26, 161–173. [Google Scholar] [CrossRef]
- Crowne, E.; Gleeson, H.; Benghiat, H.; Sanghera, P.; Toogood, A. Effect of cancer treatment on hypothalamic–pituitary function. Lancet Diabetes Endocrinol. 2015, 3, 568–576. [Google Scholar] [CrossRef]
- Mazeron, R.; Maroun, P.; Cao, K.; Mbagui, R.; Slocker-Escarpa, A.; Chargari, C.; Haie-Meder, C. Impact de la radiothérapie sur la fertilité féminine. Bull. Cancer 2015, 102, 470–476. [Google Scholar] [CrossRef]
- Chaput, L.; Grémeau, A.S.; Vorilhon, S.; Pons, H.; Chabrot, C.; Grèze, V.; Pouly, J.-L.; Brugnon, F. Préservation de la fertilité en cancérologie. Bull. Cancer 2018, 105, 99–110. [Google Scholar] [CrossRef]
- Boots, C.E.; Meister, M.; Cooper, A.R.; Hardi, A.; Jungheim, E.S. Ovarian stimulation in the luteal phase: Systematic review and meta-analysis. J. Assist. Reprod. Genet. 2016, 33, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Potdar, N.; Gelbaya, T.A.; Nardo, L.G. Oocyte vitrification in the 21st century and post-warming fertility outcomes: A systematic review and meta-analysis. Reprod. Biomed. Online 2014, 29, 159–176. [Google Scholar] [CrossRef]
- Vajta, G.; Rienzi, L.; Ubaldi, F.M. Open versus closed systems for vitrification of human oocytes and embryos. Reprod. Biomed. Online 2015, 30, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; García-Velasco, J.; Domingo, J.; Pellicer, A.; Remohí, J. Elective and Onco-fertility preservation: Factors related to IVF outcomes. Hum. Reprod. 2018, 33, 2222–2231. [Google Scholar] [CrossRef]
- Fraison, E.; Huberlant, S.; Labrune, E.; Cavalieri, M.; Montagut, M.; Brugnon, F.; Courbiere, B. Live birth rate after female fertility preservation for cancer or haematopoietic stem cell transplantation: A systematic review and meta-analysis of the three main techniques; embryo, oocyte and ovarian tissue cryopreservation. Hum. Reprod. 2023, 38, 489–502. [Google Scholar] [CrossRef]
- Courbiere, B.; Decanter, C.; Bringer-Deutsch, S.; Rives, N.; Mirallié, S.; Pech, J.C.; De Ziegler, D.; Carré-Pigeon, F.; May-Panloup, P.; Sifer, C.; et al. Emergency IVF for embryo freezing to preserve female fertility: A French multicentre cohort study. Hum. Reprod. 2013, 28, 2381–2388. [Google Scholar] [CrossRef]
- Creux, H.; Monnier, P.; Son, W.-Y.; Tulandi, T.; Buckett, W. Immature oocyte retrieval and in vitro oocyte maturation at different phases of the menstrual cycle in women with cancer who require urgent gonadotoxic treatment. Fertil. Steril. 2017, 107, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, M.; Poulain, M.; Le Parco, S.; Sifer, C.; Fanchin, R.; Frydman, N. Similar in vitro maturation rates of oocytes retrieved during the follicular or luteal phase offer flexible options for urgent fertility preservation in breast cancer patients. Hum. Reprod. 2016, 31, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Sermondade, N.; Sonigo, C.; Sifer, C.; Valtat, S.; Ziol, M.; Eustache, F.; Grynberg, M. Serum antimüllerian hormone is associated with the number of oocytes matured in vitro and with primordial follicle density in candidates for fertility preservation. Fertil. Steril. 2019, 111, 357–362. [Google Scholar] [CrossRef]
- Sonigo, C.; Simon, C.; Boubaya, M.; Benoit, A.; Sifer, C.; Sermondade, N.; Grynberg, M. What threshold values of antral follicle count and serum AMH levels should be considered for oocyte cryopreservation after in vitro maturation? Hum. Reprod. 2016, 31, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Sellami, I.; Mayeur, A.; Benoit, A.; Zeghari, F.; Peigné, M.; Roufael, J.; Grynberg, M.; Sonigo, C. Oocyte vitrification for fertility preservation following COS does not delay the initiation of neoadjuvant chemotherapy for breast cancer compared to IVM. J. Assist. Reprod. Genet. 2023, 40, 473–480. [Google Scholar] [CrossRef]
- Mayeur, A.; Puy, V.; Windal, V.; Hesters, L.; Gallot, V.; Benoit, A.; Grynberg, M.; Sonigo, C.; Frydman, N. Live birth rate after use of cryopreserved oocytes or embryos at the time of cancer diagnosis in female survivors: A retrospective study of ten years of experience. J. Assist. Reprod. Genet. 2021, 38, 1767–1775. [Google Scholar] [CrossRef]
- Diaz, A.A.; Kubo, H.; Handa, N.; Hanna, M.; Laronda, M.M. A Systematic Review of Ovarian Tissue Transplantation Outcomes by Ovarian Tissue Processing Size for Cryopreservation. Front. Endocrinol. 2022, 13, 918899. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Donnez, J.; Cacciottola, L. Fertility Preservation: The Challenge of Freezing and Transplanting Ovarian Tissue. Trends Mol. Med. 2021, 27, 777–791. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Von Wolff, M.; Poirot, C.; Diaz-Garcia, C.; Cacciottola, L.; Boissel, N.; Liebenthron, J.; Pellicer, A.; Donnez, J.; Andersen, C.Y. Transplantation of cryopreserved ovarian tissue in a series of 285 women: A review of five leading European centers. Fertil. Steril. 2021, 115, 1102–1115. [Google Scholar] [CrossRef]
- Lotz, L.; Dittrich, R.; Hoffmann, I.; Beckmann, M.W. Ovarian Tissue Transplantation: Experience from Germany and Worldwide Efficacy. Clin. Med. Insights Reprod. Health 2019, 13, 117955811986735. [Google Scholar] [CrossRef] [PubMed]
- Khattak, H.; Malhas, R.; Craciunas, L.; Afifi, Y.; Amorim, C.A.; Fishel, S.; Silber, S.; Gook, D.; Demeestere, I.; Bystrova, O.; et al. Fresh and cryopreserved ovarian tissue transplantation for preserving reproductive and endocrine function: A systematic review and individual patient data meta-analysis. Hum. Reprod. Update 2022, 28, 400–416. [Google Scholar] [CrossRef] [PubMed]
- Duffin, K.; Howie, R.; Kelsey, T.W.; Wallace, H.B.; Anderson, R.A. Long-term follow-up to assess criteria for ovarian tissue cryopreservation for fertility preservation in young women and girls with cancer. Hum. Reprod. 2023, 38, 1076–1085. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; Langendonckt, A.V. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef]
- Ramirez, T.; Pavone, M. Exploring the Frontiers of Ovarian Tissue Cryopreservation: A Review. J. Clin. Med. 2024, 13, 4513. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R.; Lotz, L.; Mueller, A.; Hoffmann, I.; Wachter, D.L.; Amann, K.U.; Beckmann, M.W.; Hildebrandt, T. Oncofertility: Combination of ovarian stimulation with subsequent ovarian tissue extraction on the day of oocyte retrieval. Reprod. Biol. Endocrinol. 2013, 11, 19. [Google Scholar] [CrossRef]
- Dolmans, M.-M. Ovarian tissue cryopreservation followed by controlled ovarian stimulation and pick-up of mature oocytes does not impair the number or quality of retrieved oocytes. J. Ovarian Res. 2014, 7, 80. [Google Scholar] [CrossRef]
- Hourvitz, A.; Yerushalmi, G.M.; Maman, E.; Raanani, H.; Elizur, S.; Brengauz, M.; Orvieto, R.; Dor, J.; Meirow, D. Combination of ovarian tissue harvesting and immature oocyte collection for fertility preservation increases preservation yield. Reprod. Biomed. Online 2015, 31, 497–505. [Google Scholar] [CrossRef]
- Fasano, G.; Dechène, J.; Antonacci, R.; Biramane, J.; Vannin, A.-S.; Van Langendonckt, A.; Devreker, F.; Demeestere, I. Outcomes of immature oocytes collected from ovarian tissue for cryopreservation in adult and prepubertal patients. Reprod. Biomed. Online 2017, 34, 575–582. [Google Scholar] [CrossRef]
- Fastrez, M.; Houba, C.; Vandromme, J.; Rozenberg, S. Fertility-sparing management of gynecological cancers. Maturitas 2015, 82, 141–145. [Google Scholar] [CrossRef]
- Demeestere, I.; Brice, P.; Peccatori, F.A.; Kentos, A.; Dupuis, J.; Zachee, P.; Casasnovas, O.; Van Den Neste, E.; Dechene, J.; De Maertelaer, V.; et al. No Evidence for the Benefit of Gonadotropin-Releasing Hormone Agonist in Preserving Ovarian Function and Fertility in Lymphoma Survivors Treated with Chemotherapy: Final Long-Term Report of a Prospective Randomized Trial. J. Clin. Oncol. 2016, 34, 2568–2574. [Google Scholar] [CrossRef]
- Trama, A.; Botta, L.; Steliarova-Foucher, E. Cancer Burden in Adolescents and Young Adults: A Review of Epidemiological Evidence. Cancer J. 2018, 24, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Rousset-Jablonski, C.; Lortal, B.; Lantheaume, S.; Arnould, L.; Simon, H.; Tuszynski, A.S.; Courtier, M.; Debbah, S.; Lefrançois, M.; Balbin, S.; et al. French national survey on breast cancer care: Caregiver and patient views. Breast Cancer 2024, 31, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Panorama des Cancers en France Édition 2023; Institut national du Cancer (INCA): Boulogne-Billancourt, France, 2023.
- Corey, B.; Smania, M.A.; Spotts, H.; Andersen, M. Young Women with Breast Cancer: Treatment, Care, and Nursing Implications. Clin. J. Oncol. Nurs. 2020, 24, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim, H.A.; Bianchi-Micheli, G.; Cardoso, M.J.; Curigliano, G.; Gelmon, K.A.; Gentilini, O.; et al. ESO–ESMO fifth international consensus guidelines for breast cancer in young women (BCY5). Ann. Oncol. 2022, 33, 1097–1118. [Google Scholar] [CrossRef]
- Tesch, M.E.; Partridge, A.H. Treatment of Breast Cancer in Young Adults; American Society of Clinical Oncology Educational Book: Alexandria, VA, USA, 2022; pp. 795–806. [Google Scholar]
- Boutas, I.; Kontogeorgi, A.; Koufopoulos, N.; Dimas, D.T.; Sitara, K.; Kalantaridou, S.N.; Dimitrakakis, C. Breast Cancer and Fertility Preservation in Young Female Patients: A Systematic Review of the Literature. Clin. Pract. 2023, 13, 1413–1426. [Google Scholar] [CrossRef]
- Sonigo, C.; Sermondade, N.; Calvo, J.; Benard, J.; Sifer, C.; Grynberg, M. Impact of letrozole supplementation during ovarian stimulation for fertility preservation in breast cancer patients. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100049. [Google Scholar] [CrossRef]
- Goldrat, O.; De Cooman, M.; Mailliez, A.; Delbaere, A.; D’Orazio, E.; Demeestere, I.; Decanter, C. Efficacy and safety of controlled ovarian hyperstimulation with or without letrozole for fertility preservation in breast cancer patients: A multicenter retrospective study. Eur. J. Cancer 2022, 174, 134–141. [Google Scholar] [CrossRef]
- Dezellus, A.; Mirallie, S.; Leperlier, F.; Sauterey, B.; Bouet, P.E.; Dessaint, A.; Duros, S.; Gremeau, A.S.; Mouret-Reynier, M.-A.; Durand, L.M.; et al. Use of tamoxifene-controlled ovarian hyperstimulation for fertility preservation before breast cancer treatment: A prospective cohort study with a 5-year follow-up. Breast 2024, 77, 103776. [Google Scholar] [CrossRef]
- Balkenende, E.M.E.; Dahhan, T.; Beerendonk, C.C.M.; Fleischer, K.; Stoop, D.; Bos, A.M.E.; Lambalk, C.B.; Schats, R.; Smeenk, J.M.J.; Louwé, L.A.; et al. Fertility preservation for women with breast cancer: A multicentre randomized controlled trial on various ovarian stimulation protocols. Hum. Reprod. 2022, 37, 1786–1794. [Google Scholar] [CrossRef]
- Kuang, Y.; Chen, Q.; Hong, Q.; Lyu, Q.; Ai, A.; Fu, Y.; Shoham, Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod. Biomed. Online 2014, 29, 684–691. [Google Scholar] [CrossRef]
- Arecco, L.; Borea, R.; Magaton, I.M.; Janković, K.; Mariamizde, E.; Stana, M.; Scavone, G.; Ottonello, S.; Spinaci, S.; Genova, C.; et al. Current practices in oncofertility counseling: Updated evidence on fertility preservation and post-treatment pregnancies in young women affected by early breast cancer. Expert Rev. Anticancer Ther. 2024, 24, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Fleury, A.; Pirrello, O.; Maugard, C.; Mathelin, C.; Linck, C. Breast cancer and ovarian tissue cryopreservation: Review of the literature. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Femmes Porteuses d’une Mutation de BRCA1 ou BRCA2: Recommandations de l’INCA 2017; Institut National Du Cancer: Boulogne-Billancourt, France, 2017.
- Grynberg, M.; Raad, J.; Comtet, M.; Vinolas, C.; Cédrin-Durnerin, I.; Sonigo, C. Fertility preservation in BRCA-mutated women: When and how? Future Oncol. 2018, 14, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Razeti, M.G.; Soldato, D.; Arecco, L.; Levaggi, A.; Puglisi, S.; Solinas, C.; Agostinetto, E.; Spinaci, S.; Lapuchesky, L.; Genova, C.; et al. Approaches to Fertility Preservation for Young Women with Breast Cancer. Clin. Breast Cancer 2023, 23, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.T.; Pisarska, M.D.; Bresee, C.; Chen, Y.-D.I.; Lester, J.; Afshar, Y.; Alexander, C.; Karlan, B.Y. BRCA1 Germline Mutations May Be Associated With Reduced Ovarian Reserve. Fertil. Steril. 2014, 102, 1723–1728. [Google Scholar] [CrossRef]
- Lin, W.T.; Beattie, M.; Chen, L.; Oktay, K.; Crawford, S.L.; Gold, E.B.; Cedars, M.; Rosen, M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non–clinic-based sample of women in northern California. Cancer 2013, 119, 1652–1659. [Google Scholar] [CrossRef]
- Oktay, K.; Kim, J.Y.; Barad, D.; Babayev, S.N. Association of BRCA1 Mutations with Occult Primary Ovarian Insufficiency: A Possible Explanation for the Link Between Infertility and Breast/Ovarian Cancer Risks. J. Clin. Oncol. 2010, 28, 240–244. [Google Scholar] [CrossRef]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef]
- Cordina-Duverger, E.; Leux, C.; Neri, M.; Tcheandjieu, C.; Guizard, A.-V.; Schvartz, C.; Truong, T.; Guénel, P. Hormonal and reproductive risk factors of papillary thyroid cancer: A population-based case-control study in France. Cancer Epidemiol. 2017, 48, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Toro-Wills, M.F.; Imitola-Madero, A.; Alvarez-Londoño, A.; Hernández-Blanquisett, A.; Martínez-Ávila, M.C. Thyroid cancer in women of reproductive age: Key issues for the clinical team. Womens Health 2022, 18, 174550572211363. [Google Scholar] [CrossRef]
- Navarro, P.; Rocher, S.; Miró-Martínez, P.; Oltra-Crespo, S. Radioactive iodine and female fertility. Sci. Rep. 2022, 12, 3704. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical cancer: Epidemiology. Chin. J. Cancer Res. 2020, 32, 720–728. [Google Scholar] [CrossRef]
- Hamers, F.F.; Woronoff, A.S. Réseau français des registres de cancers Francim Cancer du col de l’utérus en France: Tendances de l’incidence et de la mortalité jusqu’en 2018. Bull. Epidémiol. Hebd.—BEH 2019, 22–23, 410–416. [Google Scholar]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; De Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primer 2016, 2, 16086. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Mangili, G.; Martinelli, F.; Noli, S.; Filippi, F.; Bergamini, A.; Bocciolone, L.; Buonomo, B.; Peccatori, F. Fertility preservation in women with cervical cancer. Crit. Rev. Oncol. Hematol. 2020, 154, 103092. [Google Scholar] [CrossRef]
- Drechsel, K.C.E.; Pilon, M.C.F.; Stoutjesdijk, F.; Meivis, S.; Schoonmade, L.J.; Wallace, W.H.B.; Van Dulmen-den Broeder, E.; Beishuizen, A.; Kaspers, G.J.L.; Broer, S.L.; et al. Reproductive ability in survivors of childhood, adolescent, and young adult Hodgkin lymphoma: A review. Hum. Reprod. Update 2023, 29, 486–517. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Hodgkin lymphoma: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2022, 97, 1478–1488. [Google Scholar] [CrossRef]
- Brice, P.; De Kerviler, E.; Friedberg, J.W. Classical Hodgkin lymphoma. Lancet 2021, 398, 1518–1527. [Google Scholar] [CrossRef]
- Santarsieri, A.; Sturgess, K.; Brice, P.; Menne, T.F.; Osborne, W.; Creasey, T.; Ardeshna, K.M.; Behan, S.; Bhuller, K.; Booth, S.; et al. Real World Escalated Beacopdac Delivers Similar Outcomes to Escalated Beacopp and Superior Outcomes to Response-Adapted (RATHL) ABVD, While Potentially Reducing Toxicity Compared with Escalated Beacopp. Blood 2021, 138 (Suppl. S1), 877. [Google Scholar] [CrossRef]
- Santarsieri, A.; Mitchell, E.; Sturgess, K.; Brice, P.; Menne, T.F.; Osborne, W.; Creasey, T.; Ardeshna, K.M.; Behan, S.; Bhuller, K.; et al. Replacing Procarbazine with Dacarbazine in Escalated Beacopp Dramatically Reduces the Post Treatment Haematopoietic Stem and Progenitor Cell Mutational Burden in Hodgkin Lymphoma Patients with No Apparent Loss of Clinical Efficacy. Blood 2022, 140 (Suppl. S1), 1761–1764. [Google Scholar] [CrossRef]
- Decanter, C.; Delepine, J.; Behal, H.; Manier, S.; Bruno, B.; Barbatti, M.; Robin, C.; Labreuche, J.; Morschhauser, F.; Pigny, P. Longitudinal study of AMH variations in 122 Adolescents and Young Adults (AYA) and non-AYA lymphoma patients to evaluate the chemo-induced ovarian toxicity to further personalise fertility preservation counselling. Hum. Reprod. 2021, 36, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Machet, A.; Poudou, C.; Tomowiak, C.; Gastinne, T.; Gardembas, M.; Systchenko, T.; Moya, N.; Debiais, C.; Levy, A.; Gruchet, C.; et al. Hodgkin lymphoma and female fertility: A multicenter study in women treated with doxorubicin, bleomycin, vinblastine, and dacarbazine. Blood Adv. 2023, 7, 3978–3983. [Google Scholar] [CrossRef]
- Amin, M.S.A.; Brunckhorst, O.; Scott, C.; Wrench, D.; Gleeson, M.; Kazmi, M.; Ahmed, K. ABVD and BEACOPP regimens’ effects on fertility in young males with Hodgkin lymphoma. Clin. Transl. Oncol. 2021, 23, 1067–1077. [Google Scholar] [CrossRef]
- Entrop, J.P.; Weibull, C.E.; Smedby, K.E.; Jakobsen, L.H.; Øvlisen, A.K.; Molin, D.; Glimelius, I.; Marklund, A.; Holte, H.; Fosså, A.; et al. Reproduction patterns among classical Hodgkin lymphoma survivors treated with BEACOPP and ABVD in Sweden, Denmark and Norway—A population-based matched cohort study. Int. J. Cancer 2023, 153, 723–731. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, Y.; Fang, X.; Jiang, Y.; Ding, M.; Ge, X.; Yuan, D.; Lu, K.; Li, P.; Li, Y.; et al. The epidemiological patterns of non-Hodgkin lymphoma: Global estimates of disease burden, risk factors, and temporal trends. Front. Oncol. 2023, 13, 1059914. [Google Scholar] [CrossRef]
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-Hodgkin lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Le Guyader-Peyrou, S.; Defossez, G.; Dantony, E.; Mounier, M.; Cornet, E.; Uhry, Z.; Cowppli-Bony, A.; Maynadié, M.; Troussard, X.; Delafosse, P.; et al. Estimations Nationales de l’Incidence et de la Mortalité par Cancer en France Métropolitaine entre 1990 et 2018; Santé publique France: Saint-Maurice, France, 2019; Volume 2, p. 169. [Google Scholar]
- Miranda-Filho, A.; Piñeros, M.; Znaor, A.; Marcos-Gragera, R.; Steliarova-Foucher, E.; Bray, F. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control 2019, 30, 489–499. [Google Scholar] [CrossRef]
- Minoia, C.; Viviani, S.; Silvestris, E.; Palini, S.; Parissone, F.; De Palma, G.; Fedina, A.; Cormio, G.; Guarini, A.; Gini, G.; et al. Fertility preservation and monitoring in adult patients diagnosed with lymphoma: Consensus-based practical recommendations by the Fondazione Italiana Linfomi & Società Italiana della Riproduzione Umana. Front. Oncol. 2023, 13, 1252433. [Google Scholar]
- Berek, J.S.; Renz, M.; Kehoe, S.; Kumar, L.; Friedlander, M. Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int. J. Gynecol. Obstet. 2021, 155, 61–85. [Google Scholar] [CrossRef] [PubMed]
- Bourdel, N.; Huchon, C.; Abdel Wahab, C.; Azaïs, H.; Bendifallah, S.; Bolze, P.-A.; Brun, J.-L.; Canlorbe, G.; Chauvet, P.; Chereau, E.; et al. Borderline ovarian tumors: French guidelines from the CNGOF. Part 2. Surgical management, follow-up, hormone replacement therapy, fertility management and preservation. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101966. [Google Scholar] [CrossRef] [PubMed]
- Conduites à Tenir Initiales Devant des Patientes Atteintes d’un Cancer Épithélial de L’ovaire; Institut National Du Cancer (INCA): Boulogne-Billancourt, France, 2019.
- Uzan, C.; Courbiere, B.; Chabbert-Buffet, N. Tumeurs épithéliales de l’ovaire: Préservation de la fertilité. Article rédigé sur la base de la recommandation nationale de bonnes pratiques cliniques en cancérologie intitulée « Conduites à tenir initiales devant des patientes atteintes d’un cancer épithélial de l’ovaire » élaborée par FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY sous l’égide du CNGOF et labellisée par l’INCa. Gynécol. Obs. Fertil. Sénol. 2019, 47, 180–186. [Google Scholar]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Necula, D.; Istrate, D.; Mathis, J. Fertility preservation in women with early ovarian cancer. Horm. Mol. Biol. Clin. Investig. 2022, 43, 163–169. [Google Scholar] [CrossRef]
- Zapardiel, I.; Diestro, M.D.; Aletti, G. Conservative treatment of early stage ovarian cancer: Oncological and fertility outcomes. Eur. J. Surg. Oncol. EJSO 2014, 40, 387–393. [Google Scholar] [CrossRef]
- Huber, D.; Cimorelli, V.; Usel, M.; Bouchardy, C.; Rapiti, E.; Petignat, P. How many ovarian cancer patients are eligible for fertility-sparing surgery? Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 270–274. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, M.; Del Real-Ordoñez, S.; Gallardo-Alvarado, L.; Cantu-de Leon, D. Fertility-sparing treatment for epithelial ovarian cancer: A literature review. Chin. Clin. Oncol. 2020, 9, 48. [Google Scholar] [CrossRef]
- Liu, D.; Cai, J.; Gao, A.; Wang, Z.; Cai, L. Fertility sparing surgery vs. radical surgery for epithelial ovarian cancer: A meta-analysis of overall survival and disease-free survival. BMC Cancer 2020, 20, 320. [Google Scholar] [CrossRef]
- Canlorbe, G.; Chabbert-Buffet, N.; Uzan, C. Fertility-Sparing Surgery for Ovarian Cancer. J. Clin. Med. 2021, 10, 4235. [Google Scholar] [CrossRef]
- Tumeurs Germinales. Available online: https://www.ovaire-rare.org/Referentiels/Tumeurs_germinales.aspx (accessed on 27 August 2024).
- Rousset-Jablonski, C.; Selle, F.; Adda-Herzog, E.; Planchamp, F.; Selleret, L.; Pomel, C.; Chabbert-Buffet, N.; Daraï, E.; Pautier, P.; Trémollières, F.; et al. Préservation de la fertilité, contraception et traitement hormonal de la ménopause chez les femmes traitées pour tumeurs malignes rares de l’ovaire: Recommandations du réseau national dédié aux cancers gynécologiques rares (TMRG/GINECO). Bull. Cancer 2018, 105, 299–314. [Google Scholar] [CrossRef]
- Tumeurs des Cordons Sexuels. Available online: https://www.ovaire-rare.org/Referentiels/Tumeurs_des_cordons_sexuels.aspx (accessed on 27 August 2024).
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef] [PubMed]
- Maynadié, M.; Troussard, X. Épidémiologie des leucémies aiguës. Rev. Francoph. Lab. 2015, 2015, 29–33. [Google Scholar] [CrossRef]
- Pelcovits, A.; Niroula, R. Acute Myeloid Leukemia: A Review. Rhode Isl. Med. J. 2020, 103, 38–40. [Google Scholar]
- Rytting, M.E.; Jabbour, E.J.; O’Brien, S.M.; Kantarjian, H.M. Acute lymphoblastic leukemia in adolescents and young adults. Cancer 2017, 123, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; Anazodo, A.; Woodruff, T.K. Preserving fertility in female patients with hematological malignancies: A multidisciplinary oncofertility approach. Ann. Oncol. 2019, 30, 1760–1775. [Google Scholar] [CrossRef]
- Chevillon, F.; Rebotier, M.; Dhédin, N.; Bruno, B.; Cacciatore, C.; Charbonnier, A.; Joseph, L.; Le Bourgeois, A.; Talouarn, M.; Magro, L.; et al. Fertilité et greffe de cellules souches hématopoïétiques (SFGM-TC). Bull. Cancer 2025, 112, S24–S35. [Google Scholar] [CrossRef]
- Waseh, S.; Lee, J.B. Advances in melanoma: Epidemiology, diagnosis, and prognosis. Front. Med. 2023, 10, 1268479. [Google Scholar] [CrossRef]
- Epidémiologie des Cancers Cutanés-Détection Précoce des Cancers de la Peau. Available online: https://www.e-cancer.fr/Professionnels-de-sante/Depistage-et-detection-precoce/Detection-precoce-des-cancers-de-la-peau/Epidemiologie (accessed on 28 August 2024).
- Nurla, L.A.; Forsea, A.-M. Melanoma epidemiology in Europe: What is new? Ital. J. Dermatol. Venereol. 2024, 159, 128–134. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Hassel, J.C.; Livingstone, E.; Allam, J.P.; Behre, H.M.; Bojunga, J.; Klein, H.H.; Landsberg, J.; Nawroth, F.; Schüring, A.; Susok, L.; et al. Fertility preservation and management of pregnancy in melanoma patients requiring systemic therapy. ESMO Open 2021, 6, 100248. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bent, M.J.; Geurts, M.; French, P.J.; Smits, M.; Capper, D.; Bromberg, J.E.C.; Chang, S.M. Primary brain tumours in adults. Lancet 2023, 402, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Yamasaki, F. Adolescent and young adult brain tumors: Current topics and review. Int. J. Clin. Oncol. 2022, 27, 457–464. [Google Scholar] [CrossRef]
- Stone, J.B.; Kelvin, J.F.; DeAngelis, L.M. Fertility preservation in primary brain tumor patients. Neuro-Oncol. Pract. 2017, 4, 40–45. [Google Scholar] [CrossRef]
- Ndour, O.; Alumeti, D.M.; Fall, M.; Fall, A.F.; Diouf, C.; Ndoye, N.A.; Ngom, G.; Ndoye, M. Aspects épidémiologiques, diagnostiques et thérapeutiques des ostéosarcomes de l’enfant au CHU Aristide le Dantec de Dakar: À propos de 16 cas. Pan Afr. Med. J. 2013, 14, 104. [Google Scholar] [CrossRef]
- Eaton, B.R.; Schwarz, R.; Vatner, R.; Yeh, B.; Claude, L.; Indelicato, D.J.; Laack, N. Osteosarcoma. Pediatr. Blood Cancer 2021, 68, e28352. [Google Scholar] [CrossRef] [PubMed]
- Weidlinger, S.; Graber, S.; Bratschi, I.; Pape, J.; Kollár, A.; Karrer, T.; Von Wolff, M. A Systematic Review of the Gonadotoxicity of Osteosarcoma and Ewing’s Sarcoma Chemotherapies in Postpubertal Females and Males. J. Adolesc. Young Adult Oncol. 2024, 13, 597–606. [Google Scholar] [CrossRef]
- Strauss, S.J.; Frezza, A.M.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; Bonvalot, S.; et al. Bone sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1520–1536. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21, vii320–vii325. [Google Scholar] [CrossRef]
- Greve, T.; Wielenga, V.T.; Grauslund, M.; Sørensen, N.; Christiansen, D.B.; Rosendahl, M.; Yding Andersen, C. Ovarian tissue cryopreserved for fertility preservation from patients with Ewing or other sarcomas appear to have no tumour cell contamination. Eur. J. Cancer 2013, 49, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Raciborska, A.; Bilska, K.; Filipp, E.; Drabko, K.; Rogowska, E.; Chaber, R.; Pogorzała, M.; Połczyńska, K.; Adrianowska, N.; Rodriguez-Galindo, C.; et al. Ovarian function in female survivors after multimodal Ewing sarcoma therapy. Pediatr. Blood Cancer 2015, 62, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Abir, R.; Feinmesser, M.; Yaniv, I.; Fisch, B.; Cohen, I.J.; Ben-Haroush, A.; Meirow, D.; Felz, C.; Avigad, S. Occasional involvement of the ovary in Ewing sarcoma. Hum. Reprod. 2010, 25, 1708–1712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levine, O.; Zbuk, K. Colorectal cancer in adolescents and young adults: Defining a growing threat. Pediatr. Blood Cancer 2019, 66, e27941. [Google Scholar] [CrossRef]
- Chae-Kim, J.; Hayslip, C.C. Fertility and Endocrine Preservation in the Management of Colorectal Cancer in Women. Dis. Colon Rectum 2020, 63, 723–726. [Google Scholar] [CrossRef]
- Vitale, F.; Dolmans, M.M. Comprehensive Review of In Vitro Human Follicle Development for Fertility Restoration: Recent Achievements, Current Challenges, and Future Optimization Strategies. J. Clin. Med. 2024, 13, 1791. [Google Scholar] [CrossRef]
|
High Risk of Infertility
(>80%) |
Intermediate Risk of Infertility
(20–80%) |
Low Risk of Infertility
(<20%) |
|---|---|---|
| Alkylating Agents | Platinum Salts Spindle Poisons | Splitting Agents Topoisomerase Inhibitors Antimetabolites |
| Molecules: cyclophosphamide, ifosfamide, chlorambucil, carmustine, lomustine, melphalan, thiotepa, chlormethine, busulfan, procarbazine | Molecules: cisplatin, carboplatin, paclitaxel, docetaxel, vincristine, vinblastine, adriamycin | Molecules: bleomycin, irinotecan, topotecan, etoposide, epirubicin, methotrexate, 5-fluorouracil, mercaptopurine, hydroxyurea |
| Risk Category | Type of Gonadotoxic Treatment |
|---|---|
| High risk (>80% risk of treatment-induced amenorrhoea) |
|
| Intermediate risk (40–60% risk of treatment-induced amenorrhoea) |
|
| Low risk (<20% risk of treatment-induced amenorrhoea) |
|
| Very low or no risk |
|
| Unknown risk |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaeck, S.; Depuydt, C.; Bernard, V.; Ammar, O.; Hocké, C.; Carrière, J.; Chansel-Debordeaux, L. How to Preserve Fertility in Reproductive-Age Women with Cancer. J. Clin. Med. 2025, 14, 1912. https://doi.org/10.3390/jcm14061912
Jaeck S, Depuydt C, Bernard V, Ammar O, Hocké C, Carrière J, Chansel-Debordeaux L. How to Preserve Fertility in Reproductive-Age Women with Cancer. Journal of Clinical Medicine. 2025; 14(6):1912. https://doi.org/10.3390/jcm14061912
Chicago/Turabian StyleJaeck, Sébastien, Chloé Depuydt, Valérie Bernard, Omar Ammar, Claude Hocké, Jennifer Carrière, and Lucie Chansel-Debordeaux. 2025. "How to Preserve Fertility in Reproductive-Age Women with Cancer" Journal of Clinical Medicine 14, no. 6: 1912. https://doi.org/10.3390/jcm14061912
APA StyleJaeck, S., Depuydt, C., Bernard, V., Ammar, O., Hocké, C., Carrière, J., & Chansel-Debordeaux, L. (2025). How to Preserve Fertility in Reproductive-Age Women with Cancer. Journal of Clinical Medicine, 14(6), 1912. https://doi.org/10.3390/jcm14061912






