High Sensitivity Cardiac Troponin T Versus Cardiac Troponin I on Prediction of Significant Coronary Artery Disease in Patients Hospitalized Due to Symptomatic Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Selection Criteria
2.3. Patient Data
2.4. Assay Data
2.5. Statistical Analysis
3. Results
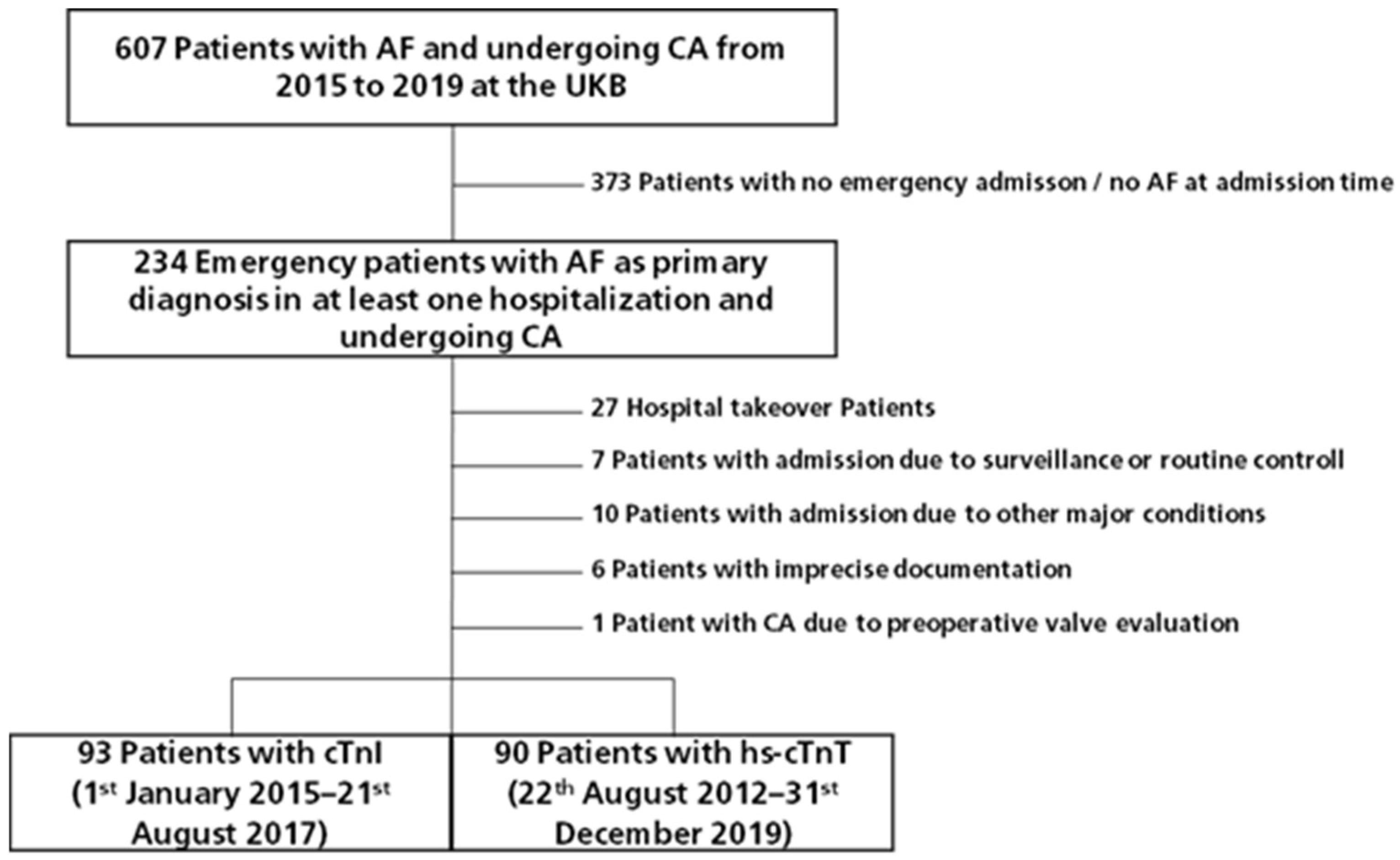
3.1. Study Population
3.2. Baseline Characteristics
3.3. Cardiac Troponin Assays
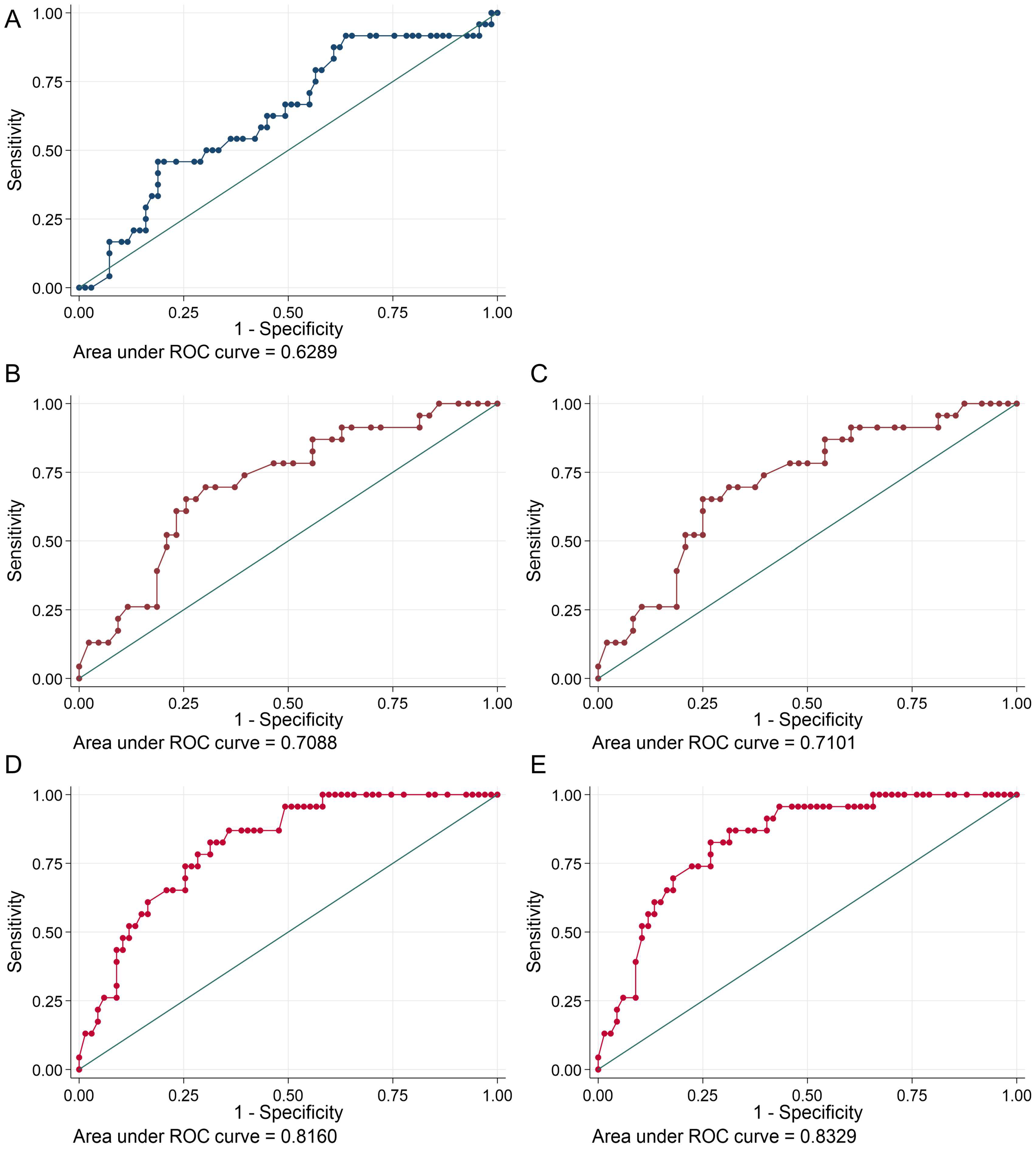
3.4. Predictors for a Significant Coronary Obstruction in AF Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Terminology | Defined Values |
|---|---|---|
| Ejection fraction | “good” | 60% |
| “preserved” | 55% | |
| “slightly reduced” | 50% | |
| “moderately reduced” | 45% | |
| “severely reduced” | 35% | |
| Mitral valve regurgitation | “inconspicuous” | 0 |
| I | ||
| II | ||
| “high grade” | II+ | |
| III | ||
| IV | ||
| Right heart dysfunction | Reduced function of the right ventricle | TAPSE < 2 cm |
| Pulmonary Hypertension | sPAP ≥ 25 mmHg | |
| Right Ventricle Dilatation | ||
| Diameter V. cava | “not dilated”, “congested”, “normal” | Diameter < 2 cm |
| “dilated”, “congested” | Diameter > 2 cm | |
| LA volume | reference range | 22–52 mL → 52 mL (f) |
| 18–58 mL → 58 mL (m) | ||
| mildly abnormal | 53–63 mL → 63 mL (f) | |
| 59–68 mL → 68 mL (m) | ||
| moderately abnormal | 63–72 mL → 72 mL (f) | |
| 69–78 mL → 78 mL (m) | ||
| severely abnormal | ≥73 mL → (f) | |
| ≥79 mL → (m) |
References
- McDonald, A.J.; Pelletier, A.J.; Ellinor, P.T.; Camargo, C.A. Increasing US Emergency Department Visit Rates and Subsequent Hospital Admissions for Atrial Fibrillation from 1993 to 2004. Ann. Emerg. Med. 2008, 51, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lévy, S.; Maarek, M.; Coumel, P.; Guize, L.; Lekieffre, J.; Medvedowsky, J.-L.; Sebaoun, A. Characterization of Different Subsets of Atrial Fibrillation in General Practice in France. Circulation 1999, 99, 3028–3035. [Google Scholar] [CrossRef] [PubMed]
- Krahn, A.D.; Manfreda, J.; Tate, R.B.; Mathewson, F.A.L.; Cuddy, T.E. The Natural History of Atrial Fibrillation: Incidence, Risk Factors, and Prognosis in the Manitoba Follow-up Study. Am. J. Med. 1995, 98, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Kralev, S.; Schneider, K.; Lang, S.; Süselbeck, T.; Borggrefe, M. Incidence and Severity of Coronary Artery Disease in Patients with Atrial Fibrillation Undergoing First-Time Coronary Angiography. PLoS ONE 2011, 6, e24964. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Levy, D.; Vaziri, S.M.; D’Agostino, R.B.; Belanger, A.J.; Wolf, P.A. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort: The Framingham Heart Study. JAMA 1994, 271, 840–844. [Google Scholar] [CrossRef]
- Crenshaw, B.S.; Ward, S.R.; Granger, C.B.; Stebbins, A.L.; Topol, E.J.; Califf, R.M. Atrial Fibrillation in the Setting of Acute Myocardial Infarction: The GUSTO-I Experience fn1fn1This Study Was Funded by Grants from Genentech, South San Francisco, California; Bayer Corporation, New York, New York; CIBA-Corning, Medfield, Massachusetts; ICI Pharmaceuticals, Wilmington, Delaware; and Sanofi Pharmaceuticals, Paris, France. J. Am. Coll. Cardiol. 1997, 30, 406–413. [Google Scholar] [CrossRef]
- Wong, C.-K.; White, H.D.; Wilcox, R.G.; Criger, D.A.; Califf, R.M.; Topol, E.J.; Ohman, E.M. New Atrial Fibrillation after Acute Myocardial Infarction Independently Predicts Death: The GUSTO-III Experience. Am. Heart J. 2000, 140, 878–885. [Google Scholar] [CrossRef]
- Kramer, R.J.; Zeldis, S.M.; Hamby, R.I. Atrial Fibrillation--a Marker for Abnormal Left Ventricular Function in Coronary Heart Disease. Br. Heart J. 1982, 47, 606–608. [Google Scholar] [CrossRef]
- Cameron, A.; Schwartz, M.J.; Kronmal, R.A.; Kosinski, A.S. Prevalence and Significance of Atrial Fibrillation in Coronary Artery Disease (CASS Registry). Am. J. Cardiol. 1988, 61, 714–717. [Google Scholar] [CrossRef]
- Haddad, A.H.; Prchkov, V.K.; Dean, D.C. Chronic Atrial Fibrillation and Coronary Artery Disease. J. Electrocardiol. 1978, 11, 67–69. [Google Scholar] [CrossRef]
- Lokshyn, S.; Mewis, C.; Kuhlkamp, V. Atrial Fibrillation in Coronary Artery Disease. Int. J. Cardiol. 2000, 72, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Erik Otterstad, J.; Kirwan, B.-A.; Lubsen, J.; De Brouwer, S.; Fox, K.A.A.; Corell, P.; Poole-Wilson, P.A. Incidence and Outcome of Atrial Fibrillation in Stable Symptomatic Coronary Disease. Scand. Cardiovasc. J. 2006, 40, 152–159. [Google Scholar] [CrossRef] [PubMed]
- The AFFIRM Investigators Baseline Characteristics of Patients with Atrial Fibrillation: The AFFIRM Study. Am. Heart J. 2002, 143, 991–1001. [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; ESC Scientific Document Group. Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Michniewicz, E.; Mlodawska, E.; Lopatowska, P.; Tomaszuk-Kazberuk, A.; Malyszko, J. Patients with Atrial Fibrillation and Coronary Artery Disease—Double Trouble. Adv. Med. Sci. 2018, 63, 30–35. [Google Scholar] [CrossRef]
- Thygesen, K.; Mair, J.; Giannitsis, E.; Mueller, C.; Lindahl, B.; Blankenberg, S.; Huber, K.; Plebani, M.; Biasucci, L.M.; Tubaro, M.; et al. How to Use High-Sensitivity Cardiac Troponins in Acute Cardiac Care. Eur. Heart J. 2012, 33, 2252–2257. [Google Scholar] [CrossRef]
- Sörensen, N.A.; Shah, A.S.V.; Ojeda, F.M.; Peitsmeyer, P.; Zeller, T.; Keller, T.; Johannsen, S.S.; Lackner, K.J.; Griffiths, M.; Münzel, T.; et al. High-Sensitivity Troponin and Novel Biomarkers for the Early Diagnosis of Non-ST-Segment Elevation Myocardial Infarction in Patients with Atrial Fibrillation. Eur. Heart Journal. Acute Cardiovasc. Care 2016, 5, 419–427. [Google Scholar] [CrossRef]
- Liebetrau, C.; Weber, M.; Tzikas, S.; Palapies, L.; Möllmann, H.; Pioro, G.; Zeller, T.; Beiras-Fernandez, A.; Bickel, C.; Zeiher, A.M.; et al. Identification of Acute Myocardial Infarction in Patients with Atrial Fibrillation and Chest Pain with a Contemporary Sensitive Troponin I Assay. BMC Med. 2015, 13, 169. [Google Scholar] [CrossRef]
- Stoyanov, K.M.; Giannitsis, E.; Biener, M.; Mueller-Hennessen, M.; Arens, K.; Katus, H.A.; Vafaie, M. Prognostic Value of Elevated High-Sensitivity Cardiac Troponin T in Patients Admitted to an Emergency Department with Atrial Fibrillation. Europace 2018, 20, 582–588. [Google Scholar] [CrossRef]
- Roldán, V.; Marín, F.; Díaz, J.; Gallego, P.; Jover, E.; Romera, M.; Manzano-Fernández, S.; Casas, T.; Valdés, M.; Vicente, V.; et al. High Sensitivity Cardiac Troponin T and Interleukin-6 Predict Adverse Cardiovascular Events and Mortality in Anticoagulated Patients with Atrial Fibrillation. J. Thromb. Haemost. 2012, 10, 1500–1507. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Apple, F.S.; Collinson, P.O.; for the IFCC Task Force on Clinical Applications of Cardiac Biomarkers. Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays. Clin. Chem. 2012, 58, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Collinson, P.O.; Saenger, A.K.; Apple, F.S.; on behalf of the IFCC C-CB. High Sensitivity, Contemporary and Point-of-Care Cardiac Troponin Assays: Educational Aids Developed by the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin. Chem. Lab. Med. 2019, 57, 623–632. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment elevationThe Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Rothman, K.J. Epidemiology: An Introduction, 2nd ed.; Oxford University Press (OUP) USA: New York, NY, USA, 2012; ISBN 978-0-19-975455-7. [Google Scholar]
- Babyak, M.A. What You See May Not Be What You Get: A Brief, Nontechnical Introduction to Overfitting in Regression-Type Models. Psychosom. Med. 2004, 66, 411–421. [Google Scholar] [CrossRef]
- Motloch, L.J.; Reda, S.; Larbig, R.; Wolff, A.; Motloch, K.A.; Wernly, B.; Granitz, C.; Lichtenauer, M.; Wolny, M.; Hoppe, U.C. Characteristics of Coronary Artery Disease among Patients with Atrial Fibrillation Compared to Patients with Sinus Rhythm. Hell. J. Cardiol. 2017, 58, 204–212. [Google Scholar] [CrossRef]
- Weber, M.; Bazzino, O.; Navarro Estrada, J.L.; de Miguel, R.; Salzberg, S.; Fuselli, J.J.; Liebetrau, C.; Woelken, M.; Moellmann, H.; Nef, H.; et al. Improved Diagnostic and Prognostic Performance of a New High-Sensitive Troponin T Assay in Patients with Acute Coronary Syndrome. Am. Heart J. 2011, 162, 81–88. [Google Scholar] [CrossRef]
- Vitolo, M.; Malavasi, V.L.; Proietti, M.; Diemberger, I.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.-A.; Kalarus, Z.; et al. 684 Cardiac Troponins and Adverse Outcomes in European Patients with Atrial Fibrillation: A Report from the ESC-EHRA EORP Atrial Fibrillation General Long-Term Registry. Eur. Heart J. Suppl. 2021, 23, suab127.052. [Google Scholar] [CrossRef]
- Parwani, A.S.; Boldt, L.-H.; Huemer, M.; Wutzler, A.; Blaschke, D.; Rolf, S.; Möckel, M.; Haverkamp, W. Atrial Fibrillation-Induced Cardiac Troponin I Release. Int. J. Cardiol. 2013, 168, 2734–2737. [Google Scholar] [CrossRef]
- Tomasdottir, M.; Held, C.; Hadziosmanovic, N.; Westerbergh, J.; Lindbäck, J.; Aylward, P.E.; Budaj, A.; Cannon, C.P.; Engdahl, J.; Granger, C.B.; et al. Risk Markers of Incident Atrial Fibrillation in Patients with Coronary Heart Disease. Am. Heart J. 2021, 233, 92–101. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.; et al. Recommendations for chamber quantification. Eur. J. Echocardiogr. 2006, 7, 79–108. [Google Scholar] [CrossRef]
| I48.0 Paroxysmal atrial fibrillation |
| I48.1 Persistent atrial fibrillation |
| I48.2 Permanent atrial fibrillation |
| I48.9 Unspecified atrial fibrillation and atrial flutter |
| Baseline Characteristics | n | cTnI, n = 93 (51%) | %/ (Quartiles) | hs-cTnT, n = 90 (49%) | %/ (Quartiles) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 183 | 74 | (65–80) | 75 | (66–80) | 0.515 |
| Male | 183 | 53 | 57.0 | 42 | 46.7 | 0.620 |
| Female | 40 | 43.0 | 48 | 53.3 | ||
| First diagnosis of AF | 183 | 41 | 44.1 | 41 | 45.6 | 0.842 |
| Prior CAD diagnosis | 183 | 31 | 33.3 | 33 | 36.7 | 0.637 |
| Family history | 183 | 15 | 16.1 | 8 | 8.9 | 0.141 |
| Diabetes mellitus | 183 | 18 | 19.4 | 24 | 26.7 | 0.241 |
| Smoking | 183 | 18 | 19.4 | 16 | 17.8 | 0.784 |
| Former Smoking | 183 | 26 | 28.0 | 4 | 4.4 | 0.845 |
| Hypertension | 183 | 72 | 77.4 | 80 | 88.9 | 0.039 |
| OSA | 183 | 7 | 7.5 | 8 | 8.9 | 0.738 |
| Clinical parameters | ||||||
| Systolic BP (mmHg) | 128 | 139 | (127–154) | 135 | (112–160) | 0.682 |
| Diastolic BP (mmHg) | 126 | 82 | (68–98) | 85 | (70–98) | 0.866 |
| Tachyarrhythmia | 182 | 74 | 79.6 | 68 | 75.6 | 0.428 |
| Heart rate (bpm) | 182 | 128 | (104–150) | 120 | (95–145) | 0.113 |
| Chest pain | 183 | 33 | 35.5 | 29 | 32.2 | 0.642 |
| Palpitations | 183 | 39 | 41.9 | 28 | 31.1 | 0.130 |
| Cardiac decompensation | 183 | 22 | 23.7 | 27 | 30.0 | 0.334 |
| Syncope | 183 | 6 | 6.5 | 7 | 7.8 | 0.728 |
| Presyncope | 183 | 2 | 2.2 | 1 | 1.1 | 1.000 |
| CA | ||||||
| Indication for Revascularization | 183 | 24 | 25.8 | 23 | 25.6 |
| Baseline Characteristics | n | R-Group, n = 47 (26%) | %/ (Quartiles) | Non-R-Group, n = 136 (74%) | %/ (Quartiles) | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 183 | 77 | (71–82) | 74 | (65–79) | 0.012 |
| Male | 183 | 27 | 57.4 | 74 | 54.4 | 0.719 |
| Female | 183 | 20 | 42.6 | 62 | 45.6 | |
| First diagnosis of AF | 183 | 21 | 44.7 | 61 | 44.9 | 0.984 |
| Prior CAD diagonsis | 183 | 24 | 51.1 | 40 | 29.4 | 0.007 |
| Family history | 183 | 6 | 12.8 | 17 | 12.5 | 0.962 |
| Diabetes mellitus | 183 | 12 | 25.5 | 30 | 22.1 | 0.626 |
| Smoking | 183 | 6 | 12.8 | 28 | 20.6 | 0.236 |
| Former Smoking | 183 | 11 | 23.4 | 39 | 28.7 | 0.486 |
| Hypertension | 183 | 39 | 83.0 | 113 | 83.1 | 0.986 |
| OSA | 183 | 4 | 8.5 | 11 | 8.1 | 1.000 |
| Clinical parameters | ||||||
| Systolic BP (mmHg) | 128 | 150 | (130–170) | 134 | (112–153) | 0.008 |
| Diastolic BP (mmHg) | 126 | 93 | (78–103) | 81 | (68–95) | 0.035 |
| Tachyarrhythmia | 182 | 39 | 83.0 | 103 | 75.7 | 0.342 |
| Heart rate (bpm) | 182 | 130 | (104–146) | 125 | (96–150) | 0.498 |
| Chest pain | 183 | 19 | 40.4 | 43 | 31.6 | 0.273 |
| Palpitations | 183 | 17 | 36.2 | 50 | 36.8 | 0.942 |
| Cardiac decompensation | 183 | 16 | 34.0 | 33 | 24.3 | 0.193 |
| Syncope | 183 | 2 | 4.3 | 11 | 8.1 | 0.520 |
| Presyncope | 183 | 2 | 4.3 | 1 | 0.7 | 0.162 |
| Cardioversion | n | R-Group, n = 47 (26%) | %/ (Quartiles) | Non-R-Group, n = 136 (74%) | %/ (Quartiles) | p Value |
|---|---|---|---|---|---|---|
| ECV performed | 183 | 21 | 44.7 | 69 | 50.7 | 0.475 |
| Primary ECV success | 183 | 17 | 36.2 | 62 | 45.6 | 0.262 |
| ECV success | 183 | 16 | 34.0 | 66 | 48.5 | 0.086 |
| Spontaneous conversion | 182 | 14 | 29.8 | 46 | 33.8 | 0.591 |
| Pharmacological conversion | 182 | 2 | 4.3 | 6 | 4.4 | 1.000 |
| AF recurrence | 180 | 4 | 8.5 | 22 | 16.2 | 0.200 |
| Echocardiography | ||||||
| Hypokinesia focal | 183 | 7 | 14.9 | 18 | 13.2 | 0.776 |
| Hypokinesia global | 183 | 9 | 19.1 | 27 | 19.9 | 0.917 |
| Hypokinesia in total | 183 | 16 | 34.0 | 45 | 33.1 | 0.905 |
| EF (%) | 183 | 52 | (40–58) | 55 | (40–60) | 0.467 |
| EF ≤ 35% | 183 | 9 | 19.1 | 27 | 19.9 | 0.917 |
| V. cava dilatation | 183 | 5 | 10.6 | 12 | 8.8 | 0.772 |
| Right Heart Dysfunction | 183 | 32 | 68.1 | 75 | 55.1 | 0.122 |
| LA size (ml) | 165 | 70 | (52–90) | 60 | (53–80) | 0.258 |
| LA dilatation | 165 | 18 | 38.3 | 36 | 26.5 | 0.106 |
| sPAP (mmHg) | 100 | 35 | (31–46) | 36 | (25–44) | 0.199 |
| sPAP (≥ 25 mmHg) | 100 | 31 | 66.0 | 58 | 42.6 | 0.016 |
| MR | 182 | 0.014 | ||||
| 0 (= 0) | 15 | 2 | 4.3 | 13 | 9.6 | |
| I (= 1) | 72 | 14 | 29.8 | 58 | 42.6 | |
| I–II (=1.5) | 37 | 10 | 21.3 | 27 | 19.9 | |
| II (=2) | 43 | 15 | 31.9 | 28 | 20.6 | |
| II+ (=2.222) | 3 | 2 | 4.3 | 1 | 0.7 | |
| II–III (=2.5) | 8 | 3 | 6.4 | 5 | 3.7 | |
| III (=3) | 4 | 1 | 2.1 | 3 | 2.2 | |
| MR classes | 0.096 | |||||
| 0 (0) | 15 | 2 | 4.3 | 13 | 9.6 | |
| 1 (I, I–II, II) | 152 | 39 | 83.0 | 113 | 83.1 | |
| 2 (II+, II–III, III) | 15 | 6 | 12.8 | 9 | 6.6 | |
| MR > II | 182 | 6 | 12.8 | 9 | 6.6 | 0.220 |
| CA | R-Group, n = 47 (26%) | %/ (Quartiles) | Non-R-Group, n = 136 (74%) | %/ (Quartiles) | p Value |
|---|---|---|---|---|---|
| CAD diagnosis | 47 | 100.0 | 64 | 47.1 | <0.001 |
| Vessels | <0.001 | ||||
| 0 | 0 | 0.0 | 81 | 59.6 | |
| 1 | 11 | 23.4 | 22 | 16.2 | |
| 2 | 10 | 21.3 | 19 | 14.0 | |
| 3 | 26 | 55.3 | 14 | 10.3 |
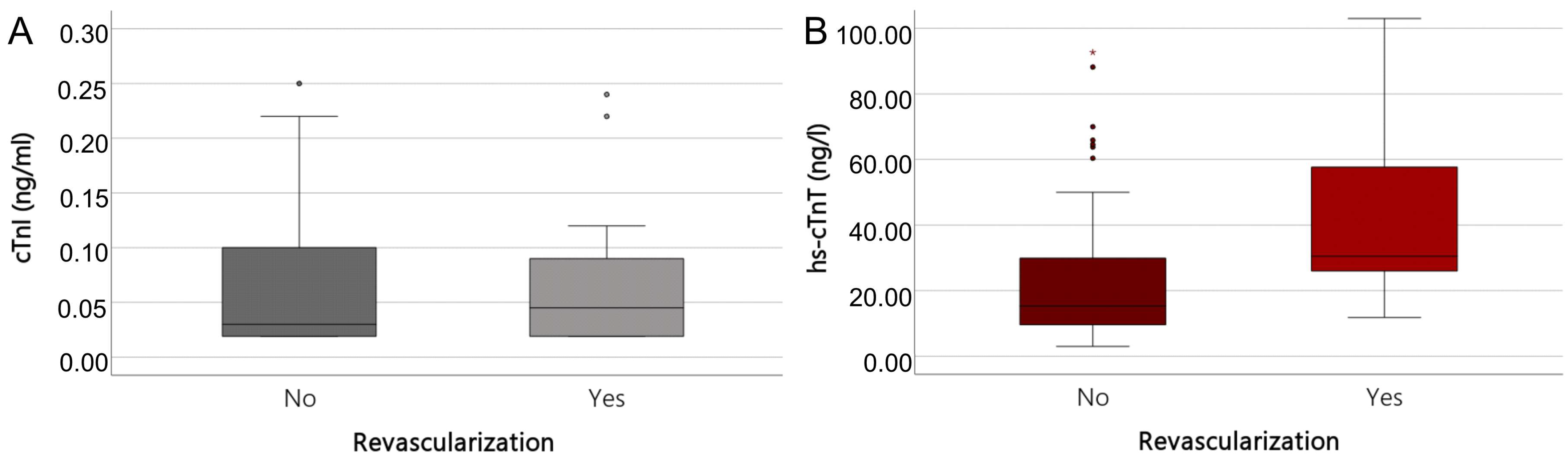
| Troponin Assay | n | R-Group | %/ (Quartiles) | Non-R-Group | %/ (Quartiles) | p Value |
|---|---|---|---|---|---|---|
| cTnI | 93 | n = 24 (26%) | n = 69 (74%) | |||
| cTnI (0 h) | 93 | 0.045 | (< 0.02–0.095) | 0.03 | (<0.02–0.1) | 0.753 |
| cTnI (>0.05 ng/mL) | 93 | 10 | 41.7 | 29 | 42.0 | 0.975 |
| cTnI (3 h) | 75 | 0.12 | (0.053–0.8) | 0.08 | (0.02–0.2) | 0.202 |
| cTnI 3 h (>0.05 ng/mL) | 75 | 20 | 83.3 | 55 | 79.7 | 0.412 |
| ∆0–3 h cTnI | 75 | 0.051 | (0.003–0.293) | 0.01 | (0.001–0.08) | 0.285 |
| ∆0–3 h cTnI time (h) | 75 | 4.7 | (2.9–8.2) | 4,1 | (2.7–7.7) | 0.594 |
| Elevated cTnI | 93 | 15 | 62.5 | 36 | 52.2 | 0.477 |
| hs-cTnT | 90 | n = 23 (26%) | n = 67 (74%) | |||
| hs-cTnT (0 h) | 90 | 30.5 | (25.5–60.6) | 15.3 | (9.6–31.1) | <0.001 |
| hs-cTnT (>14 ng/L) | 90 | 22 | 95.7 | 37 | 55.2 | <0.001 |
| hs-cTnT (1 h) | 71 | 33.7 | (25.8–60.2) | 26.3 | (16.8–47.8) | 0.050 |
| hs-cTnT (1 h) (>14 ng/L) | 71 | 22 | 95.7 | 41 | 61.2 | 0.261 |
| ∆0–1 h hs-cTnT | 71 | 4.5 | (3.9–8.2) | 4.2 | (0.6–10.7) | 0.363 |
| ∆0–1 h hs-cTnT time (h) | 71 | 1.3 | (1.0–2.0) | 1.4 | (1.1–1.8) | 0.936 |
| Elevated hs-cTnT | 90 | 23 | 100.0 | 43 | 64.2 | <0.001 |
| NSTE-ACS guideline algorithm | ||||||
| hs-cTnT rule-in | 90 | 13 | 56.5 | 23 | 34.3 | 0.062 |
| hs-cTnT observe | 90 | 10 | 43.5 | 25 | 37.3 | 0.603 |
| hs-cTnT rule-out | 90 | 0 | 0.0 | 19 | 28.4 | 0.002 |
| hs-cTnT (rule-in + observe) | 90 | 23 | 100.0 | 48 | 71.6 | 0.002 |
| hs-TnT cut-off 22 ng/L | ||||||
| hs-cTnT observe | 90 | 9 | 39.1 | 12 | 17.9 | 0.039 |
| hs-cTnT rule-out | 90 | 1 | 4.3 | 32 | 47.8 | <0.001 |
| hs-cTnT (rule-in + observe) | 90 | 22 | 95.7 | 35 | 52.2 | <0.001 |
| Laboratory | n | R-Group, n = 47 (26%) | %/ (Quartiles) | Non-R-Group, n = 136 (74%) | %/ (Quartiles) | p Value |
|---|---|---|---|---|---|---|
| NT-proBNP | 19 | 8420 | (935–19,929) | 1708 | (727–4003) | 0.195 |
| Lactate | 170 | 1.93 | (1.6–2.64) | 1.91 | (1.48–2.45) | 0.433 |
| Lactate (>1.8 mmol/L) | 170 | 22 | 46.8 | 71 | 52.2 | 0.728 |
| Glucose | 168 | 132 | (115–160.5) | 125 | (107–152) | 0.175 |
| Potassium | 174 | 4.04 | (3.7–4.61) | 4.12 | (3.76–4.43) | 0.975 |
| Potassium pathologic (3.6–4.8 mmol/L reference) | 174 | 14 | 29.8 | 32 | 23.5 | 0.296 |
| Hb | 183 | 13 | (12.2–14.6) | 13.9 | (12.5–14.9) | 0.113 |
| Hb (f < 12 g/dL, m < 14 g/dL) | 183 | 22 | 46.8 | 47 | 34.6 | 0.136 |
| GFR | 183 | 57.42 | (44.6–>70) | 66.98 | (52.66–>70) | 0.016 |
| GFR reduced (<60 mL/min) | 183 | 26 | 55.3 | 43 | 31.6 | 0.004 |
| LDL | 96 | 94.5 | (71.5–123.3) | 100 | (80.8–131.3) | 0.507 |
| LDL (>100 mg/dL) | 96 | 12 | 25.5 | 33 | 24.3 | 0.365 |
| HbA1c | 26 | 6.1 | (5.6–6.8) | 6.2 | (5.5–7) | 0.787 |
| HbA1c (>5.7%) | 26 | 6 | 12.8 | 11 | 8.1 | 1.000 |
| cTnI | hs-TnT 99th Percentile (14 ng/L) | hs-TnT Cut-Off 22 ng/L | |
|---|---|---|---|
| Sensitivity (%) | 62.5 | 100 | 86.96 |
| Specifity (%) | 47.8 | 35.8 | 56.72 |
| False Positive (%) | 52.2 | 64.2 | 52.17 |
| False Negative (%) | 37.5 | 0 | 37.50 |
| LR+ | 1.2 | 1.6 | 2 |
| LR− | 0.78 | - | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomsen, T.; Funken, M.; Nickenig, G.; Becher, M.U. High Sensitivity Cardiac Troponin T Versus Cardiac Troponin I on Prediction of Significant Coronary Artery Disease in Patients Hospitalized Due to Symptomatic Atrial Fibrillation. J. Clin. Med. 2025, 14, 1855. https://doi.org/10.3390/jcm14061855
Thomsen T, Funken M, Nickenig G, Becher MU. High Sensitivity Cardiac Troponin T Versus Cardiac Troponin I on Prediction of Significant Coronary Artery Disease in Patients Hospitalized Due to Symptomatic Atrial Fibrillation. Journal of Clinical Medicine. 2025; 14(6):1855. https://doi.org/10.3390/jcm14061855
Chicago/Turabian StyleThomsen, Tanja, Maximilian Funken, Georg Nickenig, and Marc Ulrich Becher. 2025. "High Sensitivity Cardiac Troponin T Versus Cardiac Troponin I on Prediction of Significant Coronary Artery Disease in Patients Hospitalized Due to Symptomatic Atrial Fibrillation" Journal of Clinical Medicine 14, no. 6: 1855. https://doi.org/10.3390/jcm14061855
APA StyleThomsen, T., Funken, M., Nickenig, G., & Becher, M. U. (2025). High Sensitivity Cardiac Troponin T Versus Cardiac Troponin I on Prediction of Significant Coronary Artery Disease in Patients Hospitalized Due to Symptomatic Atrial Fibrillation. Journal of Clinical Medicine, 14(6), 1855. https://doi.org/10.3390/jcm14061855






