Anterior Segment Characteristics and Quality of Life of Patients with Central Serous Chorioretinopathy
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Recruitment
2.2. Ocular Examination
2.3. Optical Coherence Tomography (OCT) Imaging
2.4. Q-LES-Q and Beck Anxiety Inventory (BAI)
2.5. Statistical Analysis
3. Results
3.1. Ocular Characteristics
3.2. Q-LES-Q and Beck Anxiety Inventory Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VA | Visual Acuity |
| CSCR | Central Serous Chorioretinopathy |
| DR | Diabetic Retinopathy |
| OCT | Optical Coherence Tomography |
| AS-OCT | Anterior Segment Optical Coherence Tomography |
| Q-LES-Q | Quality-of-Life Enjoyment and Satisfaction Questionnaire |
| BAI | Beck Anxiety Inventory |
References
- Sesar, A.P.; Sesar, A.; Bucan, K.; Sesar, I.; Cvitkovic, K.; Cavar, I. Personality Traits, Stress, and Emotional Intelligence Associated with Central Serous Chorioretinopathy. Med. Sci. Monit. 2021, 27, e928677. [Google Scholar] [CrossRef]
- Liew, G.; Quin, G.; Gillies, M.; Fraser-Bell, S. Central Serous Chorioretinopathy: A Review of Epidemiology and Pathophysiology. Clin. Exp. Ophthalmol. 2013, 41, 201–214. [Google Scholar] [CrossRef]
- Sartini, F.; Figus, M.; Nardi, M.; Casini, G.; Posarelli, C. Non-Resolving, Recurrent and Chronic Central Serous Chorioretinopathy: Available Treatment Options. Eye 2019, 33, 1035–1043. [Google Scholar] [CrossRef]
- Kanda, P.; Gupta, A.; Gottlieb, C.; Karanjia, R.; Coupland, S.G.; Bal, M.S. Pathophysiology of Central Serous Chorioretinopathy: A Literature Review with Quality Assessment. Eye 2021, 36, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Berger, L.; Bühler, V.; Yzer, S. Central Serous Chorioretinopathy—An Overview. Klin. Monbl. Augenheilkd. 2021, 238, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Haimovici, R.; Koh, S.; Gagnon, D.R.; Lehrfeld, T.; Wellik, S. Risk Factors for Central Serous Chorioretinopathy: A Case-Control Study. Ophthalmology 2004, 111, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Dudani, A.I.; Hussain, N.; Ramakrishnan, M.; Telang, O.; Patil, V.M.; Dudani, K.; Jadhav, B.; Gholap, V. Psychiatric Evaluation in Patients with Central Serous Chorioretinopathy in Asian Indians. Indian J. Ophthalmol. 2021, 69, 1204–1207. [Google Scholar] [CrossRef]
- Yannuzzi, L.A. Type-a Behavior and Central Serous Chorioretinopathy. Retina 1987, 7, 111–131. [Google Scholar] [CrossRef]
- Sirri, L.; Fava, G.A.; Guidi, J.; Porcelli, P.; Rafanelli, C.; Bellomo, A.; Grandi, S.; Grassi, L.; Pasquini, P.; Picardi, A.; et al. Type A Behaviour: A Reappraisal of Its Characteristics in Cardiovascular Disease. Int. J. Clin. Pract. 2012, 66, 854–861. [Google Scholar] [CrossRef]
- Al-Asadi, N. Type A Behaviour Pattern: Is It a Risk Factor for Hypertension? East. Mediterr. Health J. 2010, 16, 740–745. [Google Scholar] [CrossRef]
- Lee, J.M.; Watanuki, S. Cardiovascular Responses of Type A and Type B Behavior Patterns to Visual Stimulation during Rest, Stress and Recovery. J. Physiol. Anthropol. 2007, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, D.S.; Kulikov, A.N.; Vasiliev, A.S.; Chhablani, J. Accommodation Is Decreased in Eyes with Acute Central Serous Chorioretinopathy. Optom. Vis. Sci. 2022, 99, 687–691. [Google Scholar] [CrossRef]
- Chen, W.; Coorough, R. Effect of EMG-Biofeedback Training in Reduction of Muscle Tension for Individuals Displaying Type A Behavior Patterns. Percept. Mot. Ski. 1986, 62, 841–842. [Google Scholar] [CrossRef]
- McDougal, D.H.; Gamlin, P.D. Autonomic Control of the Eye. Compr. Physiol. 2015, 5, 439–473. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eli, H.; Kurland, D.; Lender, R.; Doron, R.; Shneor, E. The Effect of Type-A and Type-B Behavior Patterns on Refractive and Ocular Findings. In Proceedings of the American Academy of Optometry, Orlando, FL, USA, 23–28 October 2019. [Google Scholar]
- Suraida, A.R.; Ibrahim, M.; Zunaina, E. Correlation of the Anterior Ocular Segment Biometry with HbA1c Level in Type 2 Diabetes Mellitus Patients. PLoS ONE 2018, 13, e0191134. [Google Scholar] [CrossRef]
- He, M.; Chen, H.; Wang, W. Refractive Errors, Ocular Biometry and Diabetic Retinopathy: A Comprehensive Review. Curr. Eye Res. 2021, 46, 151–158. [Google Scholar] [CrossRef]
- ETDRS Group Fundus Photographic Risk Factors for Progression of Diabetic Retinopathy. ETDRS Report Number 12. Early Treatment Diabetic Retinopathy Study Research Group—PubMed. Ophthalmology 1991, 98, 823–833. [Google Scholar]
- Armstrong, R.A. Statistical Guidelines for the Analysis of Data Obtained from One or Both Eyes. Ophthalmic Physiol. Opt. 2013, 33, 7–14. [Google Scholar] [CrossRef]
- Riendeau, R.P.; Sullivan, J.L.; Meterko, M.; Stolzmann, K.; Williamson, A.K.; Miller, C.J.; Kim, B.; Bauer, M.S. Factor Structure of the Q-LES-Q Short Form in an Enrolled Mental Health Clinic Population. Qual. Life Res. 2018, 27, 2953–2964. [Google Scholar] [CrossRef]
- Halfaker, D.A.; Akeson, S.T.; Hathcock, D.R.; Mattson, C.; Wunderlich, T.L. Psychological Aspects of Pain. Pain. Proced. Clin. Pract. 2011, 3, 13–22. [Google Scholar] [CrossRef]
- Mansour, A.M.; Koaik, M.; Lima, L.H.; Casella, A.M.B.; Uwaydat, S.H.; Shahin, M.; Tamim, H.; Sanchez-Ruiz, M.J.; Mansour, H.A.; Dodwell, D. Physiologic and Psychologic Risk Factors in Central Serous Chorioretinopathy. Ophthalmol. Retina 2017, 1, 497–507. [Google Scholar] [CrossRef]
- Zhou, X.; Fukuyama, H.; Okita, Y.; Kanda, H.; Yamamoto, Y.; Araki, T.; Gomi, F. Pupillary Responses Reveal Autonomic Regulation Impairments in Patients With Central Serous Chorioretinopathy. Invest. Ophthalmol. Vis. Sci. 2022, 63, 2. [Google Scholar] [CrossRef]
- Starkman, M.N.; Cameron, O.G.; Nesse, R.M.; Zelnik, T. Peripheral Catecholamine Levels and the Symptoms of Anxiety: Studies in Patients with and without Pheochromocytoma. Psychosom. Med. 1990, 52, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Kiel, M.; Grabitz, S.D.; Hopf, S.; Koeck, T.; Wild, P.S.; Schmidtmann, I.; Lackner, K.J.; Münzel, T.; Beutel, M.E.; Pfeiffer, N.; et al. Distribution of Pupil Size and Associated Factors: Results from the Population-Based Gutenberg Health Study. J. Ophthalmol. 2022, 2022, 9520512. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.; Seitz, D. Evidence of Ocular Side Effects of SSRIs and New Warnings. Evid. Based Ment. Health 2017, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Devan, S.; Jaisankar, D.; Swaminathan, G.; Pardhan, S.; Raman, R. Pupillary Abnormalities with Varying Severity of Diabetic Retinopathy. Sci. Rep. 2018, 8, 5636. [Google Scholar] [CrossRef]
- Gawęcki, M.; Grzybowski, A.; Święch, A. Biometric Risk Factors for Central Serous Chorioretinopathy. Ophthalmol. Ther. 2023, 12, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Oh, J.; Choi, Y.-M.; Kim, S.-W.; Huh, K. Anterior Chamber Depth in Eyes with Central Serous Chorioretinopathy. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2785. [Google Scholar]
- H Werry, C.A. Investigation in Patients with Central Serous Retinopathy with the MMPI Saarbrücken. Klin. Monbl. Augenheilkd. 1978, 172, 363–370. [Google Scholar]
- Conrad, R.; Bodeewes, I.; Schilling, G.; Geiser, F.; Imbierowicz, K.; Liedtke, R. Central Serous Chorioretinopathy and Psychological Stress. Ophthalmologe 2000, 97, 527–531. [Google Scholar] [CrossRef]
- Spahn, C.; Wiek, J.; Burger, T.; Hansen, L. Psychosomatic Aspects in Patients with Central Serous Chorioretinopathy. Br. J. Ophthalmol. 2003, 87, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.; Liu, Y.; Xue, D.; Zheng, Y.; Li, Y.; Zhang, B.; Dai, Y. The Role of Life Satisfaction and Living Arrangements in the Association between Chronic Disease and Depression: A National Cross-Sectional Survey. Front. Psychol. 2023, 14, 1266059. [Google Scholar] [CrossRef] [PubMed]
- Balkarli, A.; Erol, M.K.; Yucel, O.; Akar, Y. Frequency of Fibromyalgia Syndrome in Patients with Central Serous Chorioretinopathy. Arq. Bras. Oftalmol. 2017, 80, 4–8. [Google Scholar] [CrossRef] [PubMed]
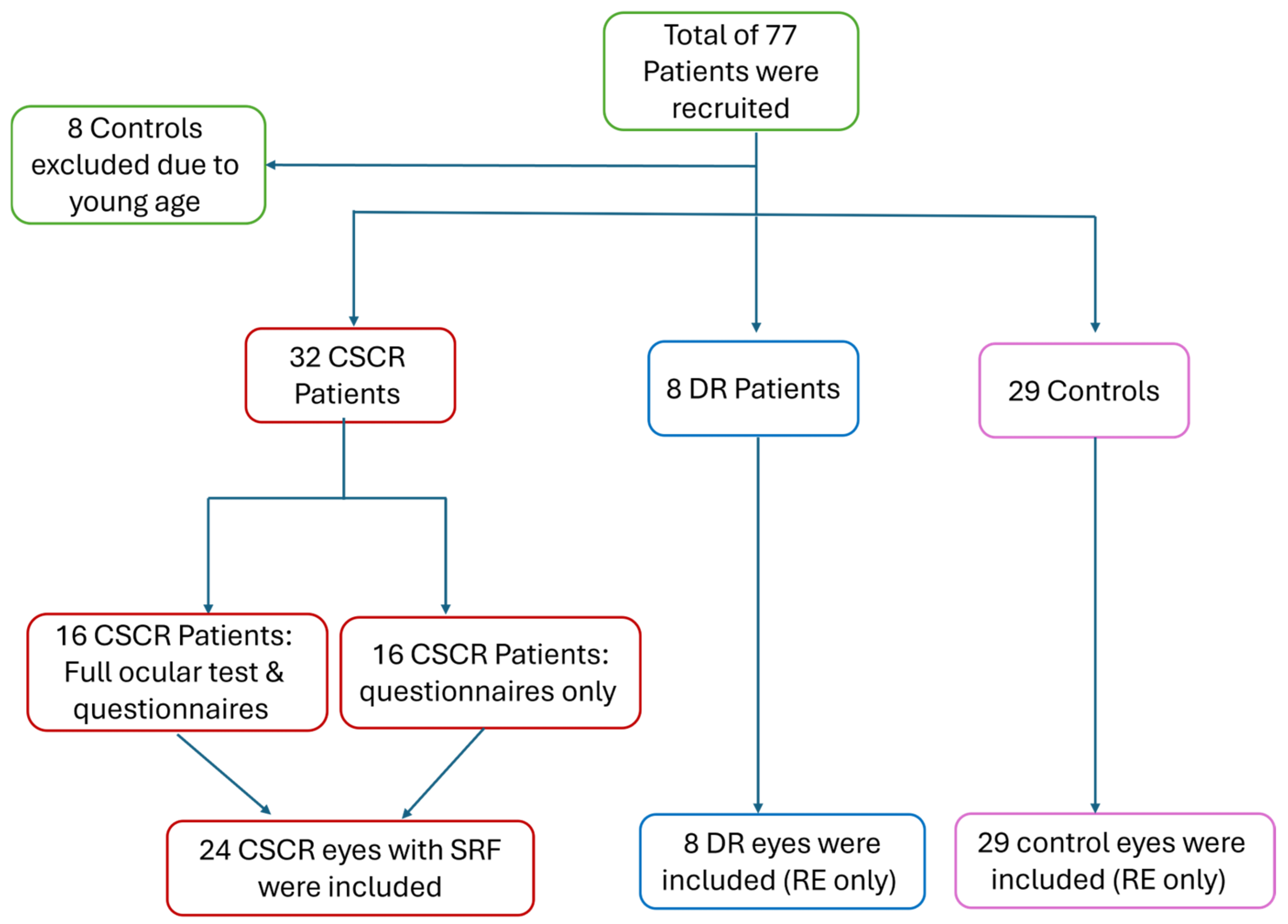


| CSCR | DR | Controls | p-Value | |
|---|---|---|---|---|
| N = 24 Eyes | N = 8 Eyes | N = 29 Eyes | ||
| Mean Age ± SD (years) | 43.8 ± 9.06 | 42.7 ± 9.96 | 37.06 ± 13.61 | 0.19 a |
| Age range (years) | 23–55 | 24–55 | 23–61 | N/A |
| Male gender n (%) | 14 (87.5%) | 4 (50%) | 10 (34.48%) | <0.01 b |
| BCVA (LogMAR) Mean ± SD | 0.19 ± 0.30 | 0.15 ± 0.13 | 0.01 ± 0.02 | <0.01 aPost Hoc: DR vs. Healthy 0.17 c DR vs. CSCR 0.87 c CSCR vs. Healthy <0.01 c |
| SPH (Diopter) Mean ± SD | 0.67 ± 1.08 | −0.13 ± 2.85 | −1.44 ± 2.76 | <0.01a Post Hoc: DR vs. Healthy 0.32 c DR vs. CSCR 0.66 c CSCR vs. Healthy <0.01c |
| Cyl (Diopter) Mean ± SD | −0.77 ± 0.43 | −1.31 ± 1.15 | −0.49 ± 0.62 | <0.01a Post Hoc: DR vs. Healthy <0.01 c DR vs. CSCR 0.11 c CSCR vs. Healthy 0.26 c |
| Spherical Equivalent (Diopter) Mean ± SD | 0.3 ± 1.14 | −1.78 ± 2.97 | −1.68 ± 2.68 | <0.01 a Post Hoc: DR vs. Healthy 0.99 c DR vs. CSCR 0.06 c CSCR vs. Healthy <0.01 c |
| CSCR | DR | Controls | p-Value | |
|---|---|---|---|---|
| N = 24 Eyes | N = 8 Eyes | N = 29 Eyes | ||
| CASIA Pupil Size (mm) Mean ± SD | 4.88 ± 0.89 | 4.84 ± 1.04 | 5.35 ± 1.02 | 0.16 a |
| ACC (Diopter) Mean ± SD | 6.04 ± 2.78 | 7.27 ± 3.68 | 6.09 ± 3.24 | 0.70 a |
| ACD (mm) Mean ± SD | 2.97 ± 0.34 | 2.83 ± 0.22 | 3.01 ± 0.36 | 0.31 a |
| IOP (mmHg) Mean ± SD | 13.16 ± 2.16 | 13.25 ± 2.55 | 14.16 ± 2.98 | 0.51 a |
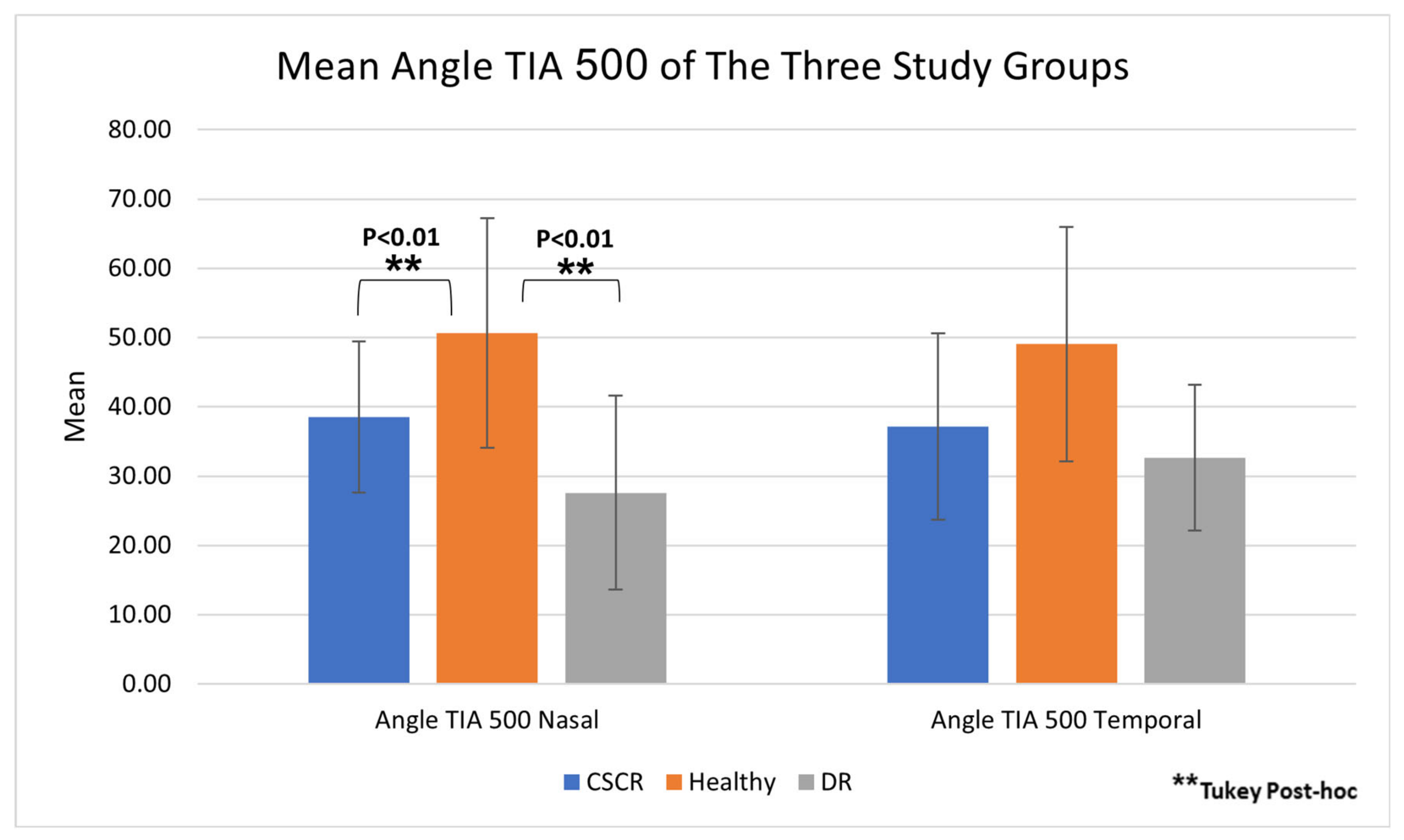
| Nasal Angle TIA 500 Mean ± SD | 38.52 ± 10.93 | 27.61 ± 14.02 | 50.65 ± 16.57 | 0.02 a Post Hoc: DR vs. Healthy <0.01 b DR vs. CSCR 0.15 b CSCR vs. Healthy <0.01 b |
| Temporal Angle TIA 500 Mean ± SD | 37.16 ± 13.44 | 32.65 ± 10.53 | 49.07 ± 16.92 | 0.30 a |
| CSCR N = 32 Patients | DR N = 8 Patients | Controls N = 29 Patients | p-Value | |
|---|---|---|---|---|
| Q-LES questionnaire Mean ± SD | 51.56 ± 9.17 | 53.75 ± 7.81 | 60.03 ± 7.32 | <0.01 a Post Hoc: DR vs. Healthy 0.14 b DR vs. CSCR 0.78 b CSCR vs. Healthy <0.01 b |
| BECK questionnaire Mean ± SD | 6.31 ± 8.50 | 6.25 ± 5.70 | 4.21 ± 6.99 | 0.19 a |
| BECK questionnaire (4, 5, 9, 14, 17) Mean ± SD | 2.12 ± 3.15 | 1.25 ± 2.05 | 1.10 ± 2.00 | 0.54 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Eli, H.; Asher, T.; Lender, R.; Mirsky, D.; Abu-Shkara, R.; Hamuda, M.; Aslee, N.; Marei, H.; Flug, R.; Eitan, R.; et al. Anterior Segment Characteristics and Quality of Life of Patients with Central Serous Chorioretinopathy. J. Clin. Med. 2025, 14, 1812. https://doi.org/10.3390/jcm14061812
Ben-Eli H, Asher T, Lender R, Mirsky D, Abu-Shkara R, Hamuda M, Aslee N, Marei H, Flug R, Eitan R, et al. Anterior Segment Characteristics and Quality of Life of Patients with Central Serous Chorioretinopathy. Journal of Clinical Medicine. 2025; 14(6):1812. https://doi.org/10.3390/jcm14061812
Chicago/Turabian StyleBen-Eli, Hadas, Tal Asher, Rivkah Lender, Devora Mirsky, Riad Abu-Shkara, Mahmud Hamuda, Nadin Aslee, Hadeel Marei, Reut Flug, Renana Eitan, and et al. 2025. "Anterior Segment Characteristics and Quality of Life of Patients with Central Serous Chorioretinopathy" Journal of Clinical Medicine 14, no. 6: 1812. https://doi.org/10.3390/jcm14061812
APA StyleBen-Eli, H., Asher, T., Lender, R., Mirsky, D., Abu-Shkara, R., Hamuda, M., Aslee, N., Marei, H., Flug, R., Eitan, R., & Khateb, S. (2025). Anterior Segment Characteristics and Quality of Life of Patients with Central Serous Chorioretinopathy. Journal of Clinical Medicine, 14(6), 1812. https://doi.org/10.3390/jcm14061812





