Reflectance Confocal Microscopy Can Help Differentiate Adult Xanthogranulomatous Disease from Xanthelasma—A Case Report
Abstract
1. Introduction
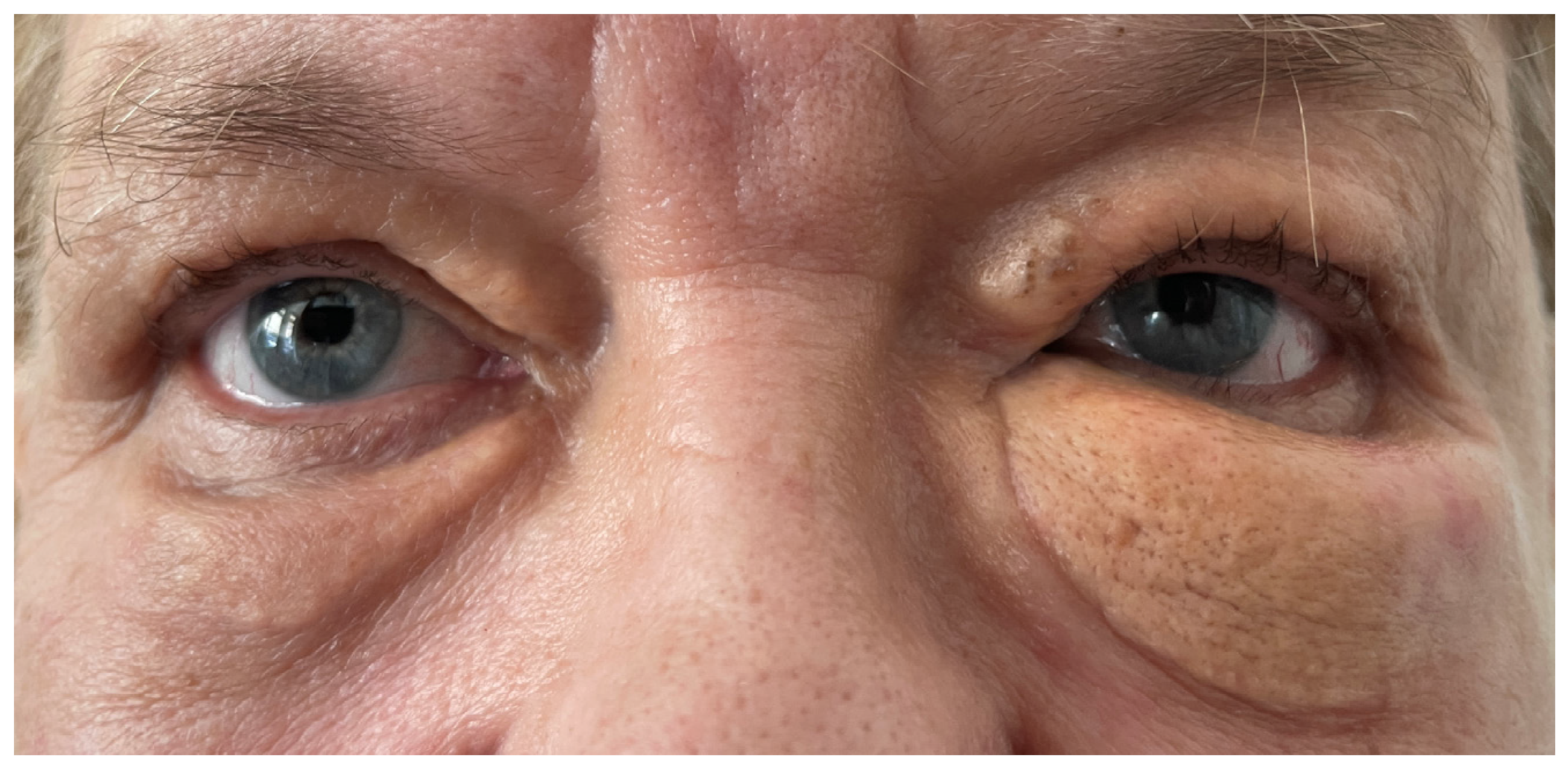
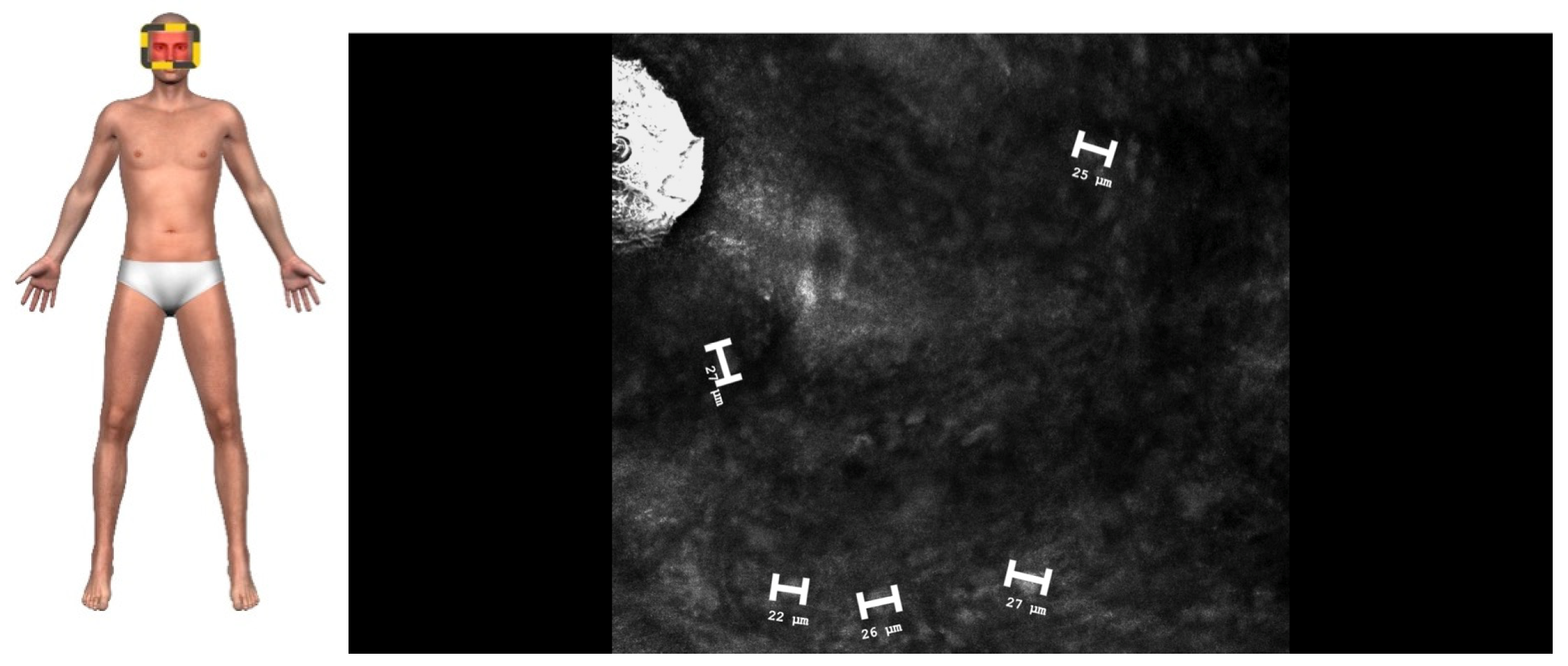
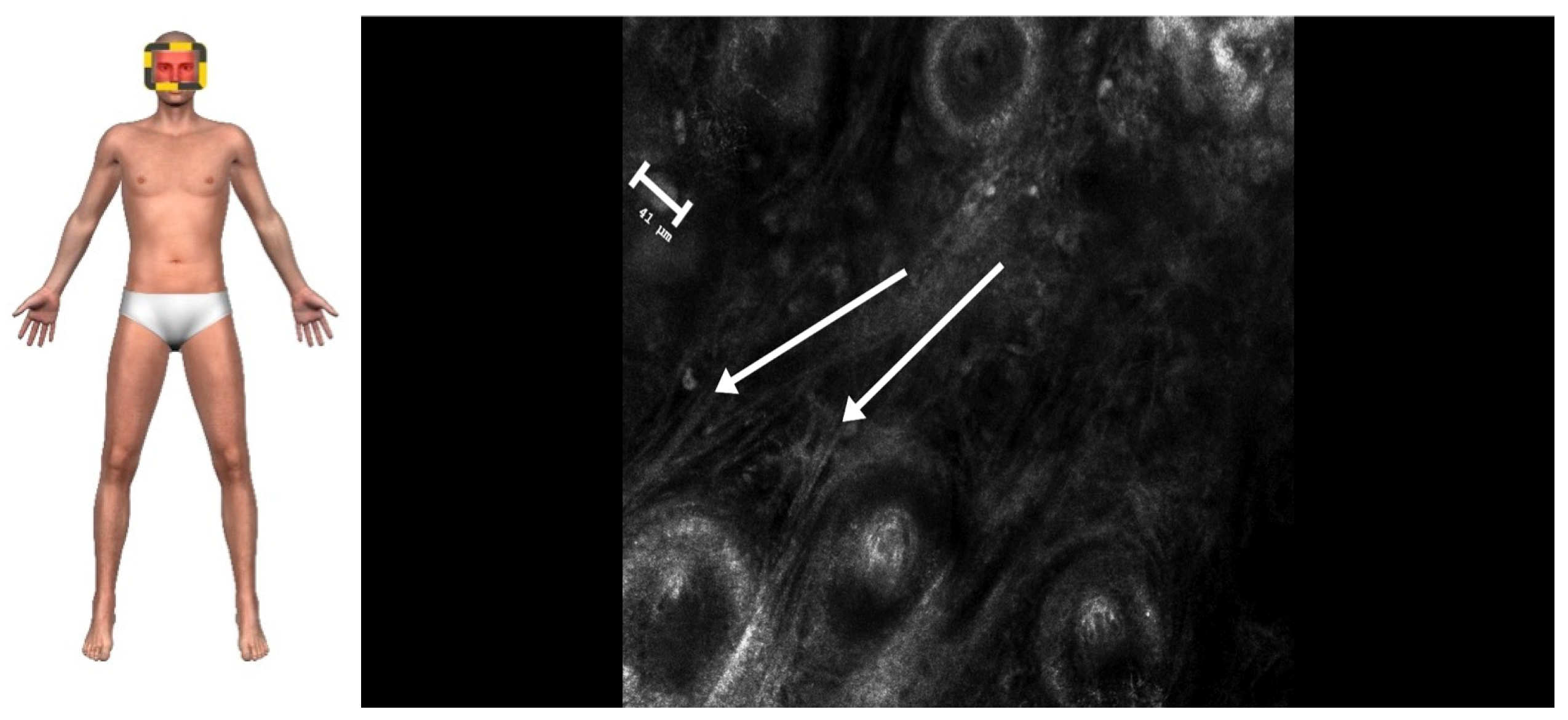
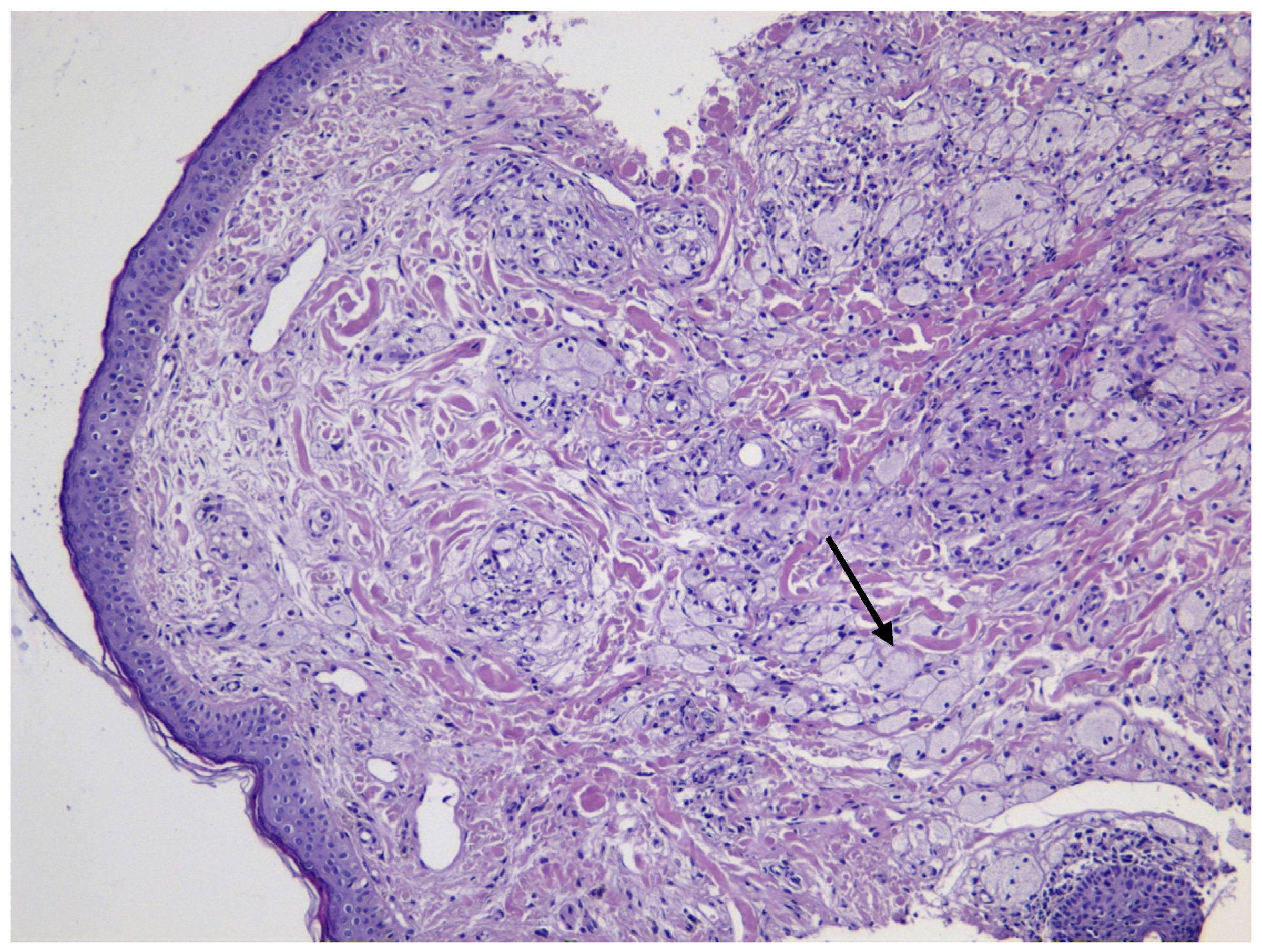
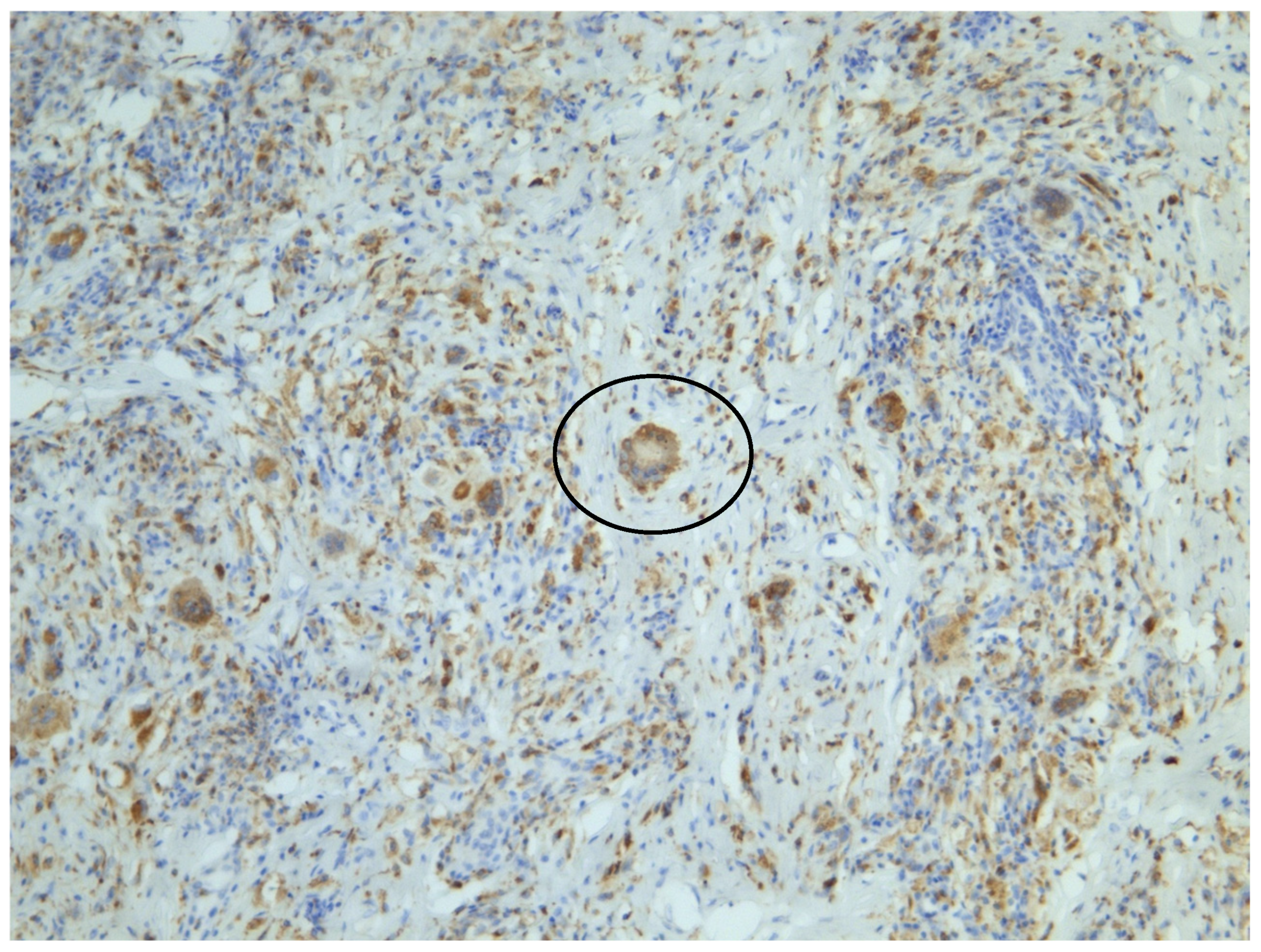
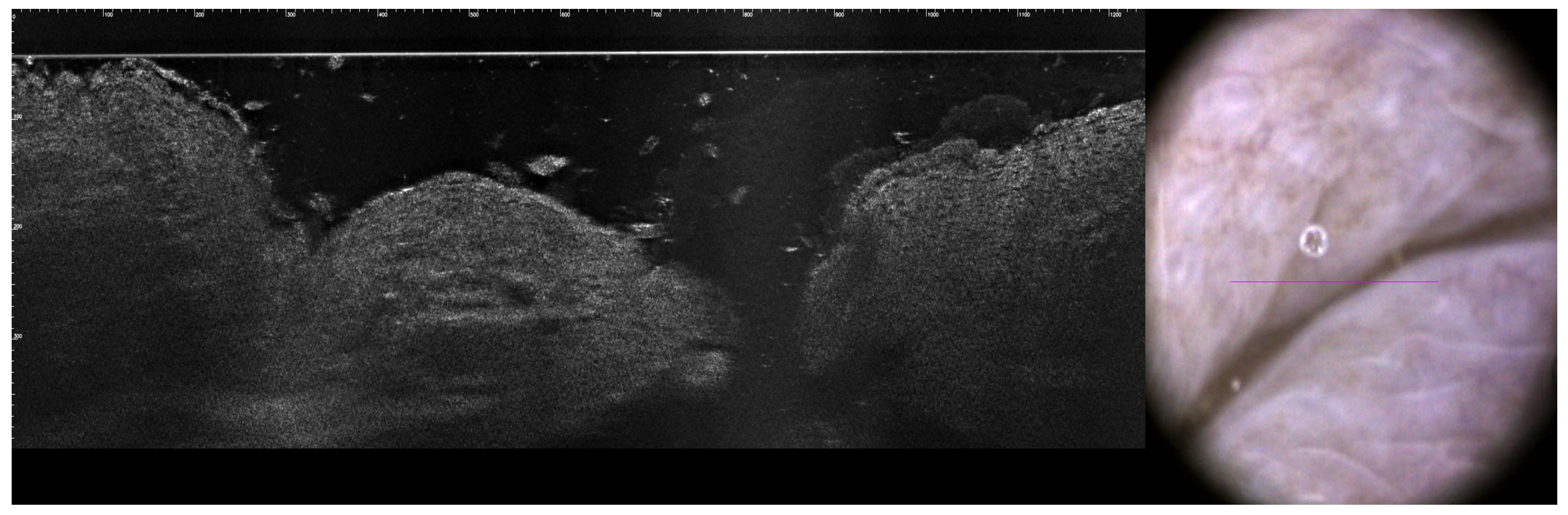
2. Case Presentation
3. Discussion
3.1. Histopathological Distinction
3.2. Advantages of RCM in Periocular Diagnosis
3.3. RCM as an Emerging Diagnostic Tool
3.4. Clinical Implications
3.5. Future Directions
3.6. Comparison of RCM with Other Modalities
3.6.1. Dermatoscopy
3.6.2. LC-OCT (Line-Scan Optical Coherence Tomography)
3.6.3. High-Frequency Ultrasound (HFUS)
3.7. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Go, R.S.; Jacobsen, E.; Baiocchi, R.; Buhtoiarov, I.; Butler, E.B.; Campbell, P.K.; Coulter, D.W.; Diamond, E.; Flagg, A.; Goodman, A.M.; et al. Histiocytic Neoplasms, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 1277–1303. [Google Scholar] [CrossRef] [PubMed]
- Ratzinger, G.; Zelger, B.W.H. Juvenile Xanthogranuloma and Other Non-Langerhans Cell Histiocytoses. In Harper’s Textbook of Pediatric Dermatology, 1st ed.; Hoeger, P., Kinsler, V., Yan, A., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 1078–1096. [Google Scholar] [CrossRef]
- Kulkarni, A.M.; Gayam, P.K.R.; Aranjani, J.M. Advances in Understanding and Management of Erdheim-Chester Disease. Life Sci. 2024, 348, 122692. [Google Scholar] [CrossRef] [PubMed]
- Geoloaica, L.G.; Pătraşcu, V.; Ciurea, R.N. Necrobiotic Xanthogranuloma—Case Report and Literature Review. Curr. Health Sci. J. 2021, 47, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, A.M.; Shah, S.S.; Blair, K.; Al Aboud, D.M. Xanthelasma Palpebrarum; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK531501/ (accessed on 15 December 2024).
- Sivak-Callcott, J.A.; Rootman, J.; Rasmussen, S.L.; Nugent, R.A.; White, V.A.; Paridaens, D.; Currie, Z.; Rose, G.; Clark, B.; McNab, A.A.; et al. Adult xanthogranulomatous disease of the orbit and ocular adnexa: New immunohistochemical findings and clinical review. Br. J. Ophthalmol. 2006, 90, 602–608. [Google Scholar] [CrossRef]
- Dey, A.; Aggarwal, R.; Dwivedi, S. Cardiovascular profile of xanthelasma palpebrarum. BioMed Res. Int. 2013, 2013, 932863. [Google Scholar] [CrossRef]
- Nair, P.A.; Singhal, R. Xanthelasma palpebrarum—A brief review. Clin. Cosmet. Investig. Dermatol. 2018, 11, 1–5. [Google Scholar] [CrossRef]
- Dosari, S.A. Periocular Adult-Onset Xanthogranuloma (AOX) Initially Misdiagnosed as Xanthelasma: A Case Report. J. Clin. Case Stud. 2016, 1. [Google Scholar] [CrossRef]
- Shahriari, N.; Grant-Kels, J.M.; Rabinovitz, H.; Oliviero, M.; Scope, A. Reflectance confocal microscopy. J. Am. Acad. Dermatol. 2021, 84, 1–14. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.; Longo, C.; Venturini, M.; Sala, R.; Pellacani, G. Reflectance confocal microscopy for in vivo skin imaging. Photochem. Photobiol. 2008, 84, 1421–1430. [Google Scholar] [CrossRef]
- Rajadhyaksha, M.; Grossman, M.; Esterowitz, D.; Webb, R.H.; Rox Anderson, R. In Vivo Confocal Scanning Laser Microscopy of Human Skin: Melanin Provides Strong Contrast. J. Investig. Dermatol. 1995, 104, 946–952. [Google Scholar] [CrossRef]
- González, S.; Gilaberte-Calzada, Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int. J. Cosmet. Sci. 2008, 30, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Burris, C.K.H.; Rodriguez, M.E.; Raven, M.L.; Burkat, C.N.; Albert, D.M. Adult-onset asthma and periocular xanthogranulomas associated with systemic IgG4-related disease. Am. J. Ophthalmol. Case Rep. 2016, 1, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Kozubowska, K.; Sławińska, M.; Sobjanek, M. The role of dermoscopy in diagnostics of dermatological conditions of the eyelid, eyelashes, and conjunctiv—A literature review. Int. J. Dermatol. 2021, 60, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Perino, F.; Suarez, R.; Perez-Anker, J.; Carrera, C.; Rezze, G.G.; Primiero, C.A.; Alos, L.L.; Diaz, A.; Barreiro, A.; Puig, S.; et al. Concordance of in vivo reflectance confocal microscopy and horizontal-sectioning histology in skin tumours. Acad. Dermatol. Venereol. 2024, 38, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, Y.; Marques, I.R.; Pera Calvi, I.; Cruz, S.A.; Godoi, A.; Lapenda, I.L.; de Morales-Souza, R.; Relvas, J.H.; Vilbert, M.; Nehal, K.S.; et al. Reflectance confocal microscopy for margin mapping of melanoma of the lentigo maligna type: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2024. [Google Scholar] [CrossRef]
- Haroon, A.; Shafi, S.; Rao, B. Adult xanthogranuloma diagnosed on reflectance confocal microscopy. J. Cutan. Pathol. 2017, 44, 809–810. [Google Scholar] [CrossRef]
- Lovato, L.; Salerni, G.; Puig, S.; Carrera, C.; Palou, J.; Malvehy, J. Adult xanthogranuloma mimicking basal cell carcinoma: Dermoscopy, reflectance confocal microscopy and pathological correlation. Dermatology 2010, 220, 66–70. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Verzì, A.E.; Barresi, S.; Micali, G. Multiple xanthogranulomas in an adult patient: Clinical, dermoscopic, reflectance confocal microscopy and histopathological features. BMJ Case Rep. 2019, 12, e229772. [Google Scholar] [CrossRef]
- Sun, H.; Wang, A. Analysis of the characteristics of reflectance confocal microscopy images of xanthogranuloma and xanthoma. Int. J. Dermatol. 2025, 64, 155–163. [Google Scholar] [CrossRef]
- Gambichler, T.; Regeniter, P.; Bechara, F.G.; Orlikov, A.; Vasa, R.; Moussa, G.; Stücker, M.; Altmeyer, P.; Hoffmann, K. Characterization of benign and malignant melanocytic skin lesions using optical coherence tomography in vivo. J. Am. Acad. Dermatol. 2007, 57, 629–637. [Google Scholar] [CrossRef]
- Dinnes, J.; Bamber, J.; Chuchu, N.; Bayliss, S.E.; Takwoingi, Y.; Davenport, C.; Godfrey, K.; O’Sullivan, C.; Matin, R.N.; Deeks, J.J.; et al. High-frequency ultrasound for diagnosing skin cancer in adults. Cochrane Database Syst. Rev. 2018, 2018, CD013188. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.; Whang, T.B.; Bard, R.L.; Marmur, E.S. Ultrasound in dermatology: Principles and applications. J. Am. Acad. Dermatol. 2012, 67, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Gualdi, G.; Zanca, A.; Lorenzi, L.; Pellacani, G.; Calzavara-Pinton, P.G. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br. J. Dermatol. 2016, 174, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Young, A.; Wong, A.; Stalling, S.; Wei, M.; Hadley, D. Precision Diagnosis of Melanoma and Other Skin Lesions From Digital Images. AMIA Jt. Summits Transl. Sci. Proc. 2017, 2017, 220–226. [Google Scholar]
- Dreyfuss, I.; Eckembrecher, F.J.; Eckembrecher, D.G.; Nouri, K. Reflectance Confocal Microscopy. In Telemedicine and Technological Advances in Dermatology; Nouri, K., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 253–258. [Google Scholar] [CrossRef]
- Edwards, S.J.; Osei-Assibey, G.; Patalay, R.; Wakefield, V.; Karner, C. Diagnostic accuracy of reflectance confocal microscopy using VivaScope for detecting and monitoring skin lesions: A systematic review. Clin. Exp. Dermatol. 2017, 42, 266–275. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska-Węglewicz, L.; Dźwigała, M.; Sobolewski, P.; Wasążnik-Jędras, A.; Walecka, I. Reflectance Confocal Microscopy Can Help Differentiate Adult Xanthogranulomatous Disease from Xanthelasma—A Case Report. J. Clin. Med. 2025, 14, 1359. https://doi.org/10.3390/jcm14041359
Krajewska-Węglewicz L, Dźwigała M, Sobolewski P, Wasążnik-Jędras A, Walecka I. Reflectance Confocal Microscopy Can Help Differentiate Adult Xanthogranulomatous Disease from Xanthelasma—A Case Report. Journal of Clinical Medicine. 2025; 14(4):1359. https://doi.org/10.3390/jcm14041359
Chicago/Turabian StyleKrajewska-Węglewicz, Larysa, Monika Dźwigała, Piotr Sobolewski, Anna Wasążnik-Jędras, and Irena Walecka. 2025. "Reflectance Confocal Microscopy Can Help Differentiate Adult Xanthogranulomatous Disease from Xanthelasma—A Case Report" Journal of Clinical Medicine 14, no. 4: 1359. https://doi.org/10.3390/jcm14041359
APA StyleKrajewska-Węglewicz, L., Dźwigała, M., Sobolewski, P., Wasążnik-Jędras, A., & Walecka, I. (2025). Reflectance Confocal Microscopy Can Help Differentiate Adult Xanthogranulomatous Disease from Xanthelasma—A Case Report. Journal of Clinical Medicine, 14(4), 1359. https://doi.org/10.3390/jcm14041359





