Cardioprotective Effects of Dapagliflozin and Trimetazidine on Doxorubicin-Induced Cardiotoxicity in Streptozotocin-Induced Type 1 Diabetic Rats via Endoplasmic Reticulum Stress
Abstract
1. Introduction
2. Materials and Methods
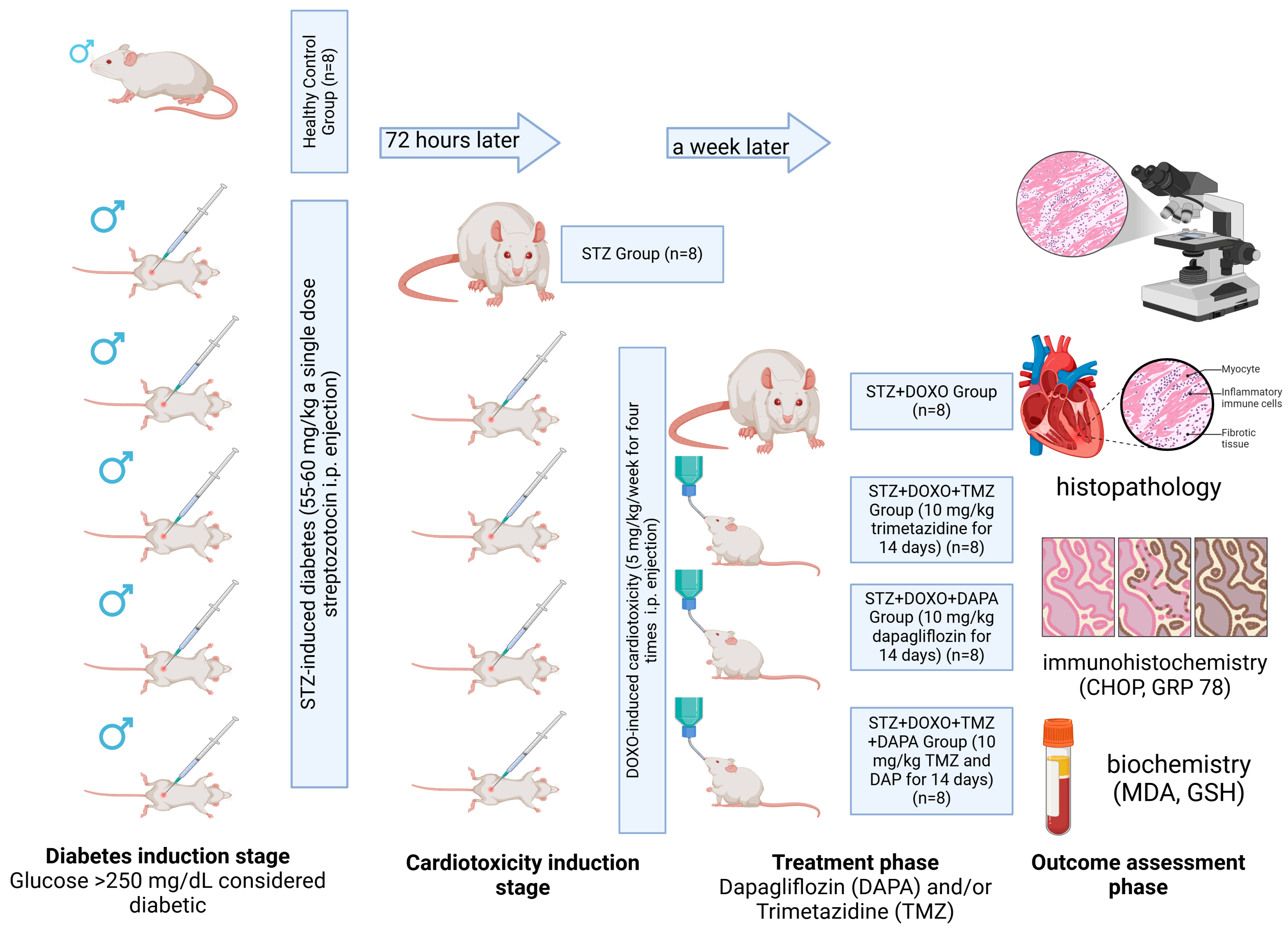
2.1. Experimental Study Design
2.2. Outcomes
- Histopathological outcomes: Cardiomyocyte degeneration, vascular congestion, and edema (evaluated using histopathological cardiac damage scoring, HCDS).
- Immunohistochemical outcomes: CHOP and GRP 78 positivity in cardiomyocytes (markers of ER stress).
- Biochemical outcomes: Malondialdehyde (TBARS assay as MDA, marker of oxidative stress) and Glutathione (Total thiol, GSH, antioxidant marker).
- Mediator Variables (Mechanistic Pathways Tested):
- ER Stress Pathway: Evaluated using CHOP and GRP 78 immunohistochemistry.
- Oxidative Stress Pathway: Assessed via MDA and GSH levels biochemically.
2.3. Biochemical Analysis
2.3.1. Homogenization of Heart Tissues
2.3.2. Thiobarbituric Acid Reactive Substances (TBARS) Assay
2.3.3. Determination of Total Thiol (TT) Groups
2.4. Histopathological Analysis
2.5. Immunohistochemical Analysis Procedure
2.6. Semi-Quantitative Analysis
2.7. Statistical Analysis
3. Results
3.1. Biochemical Analysis
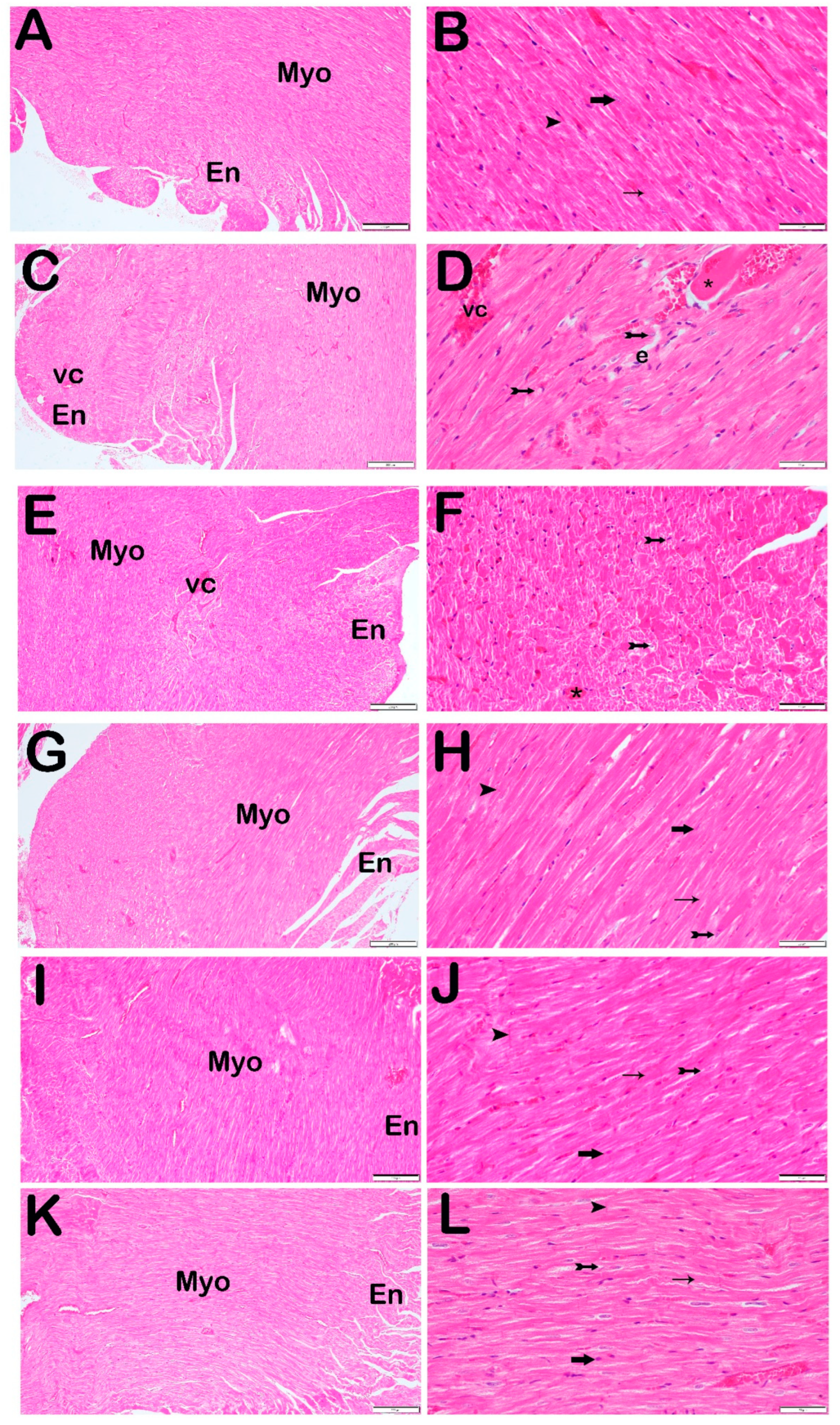
3.2. Histopathological Analysis
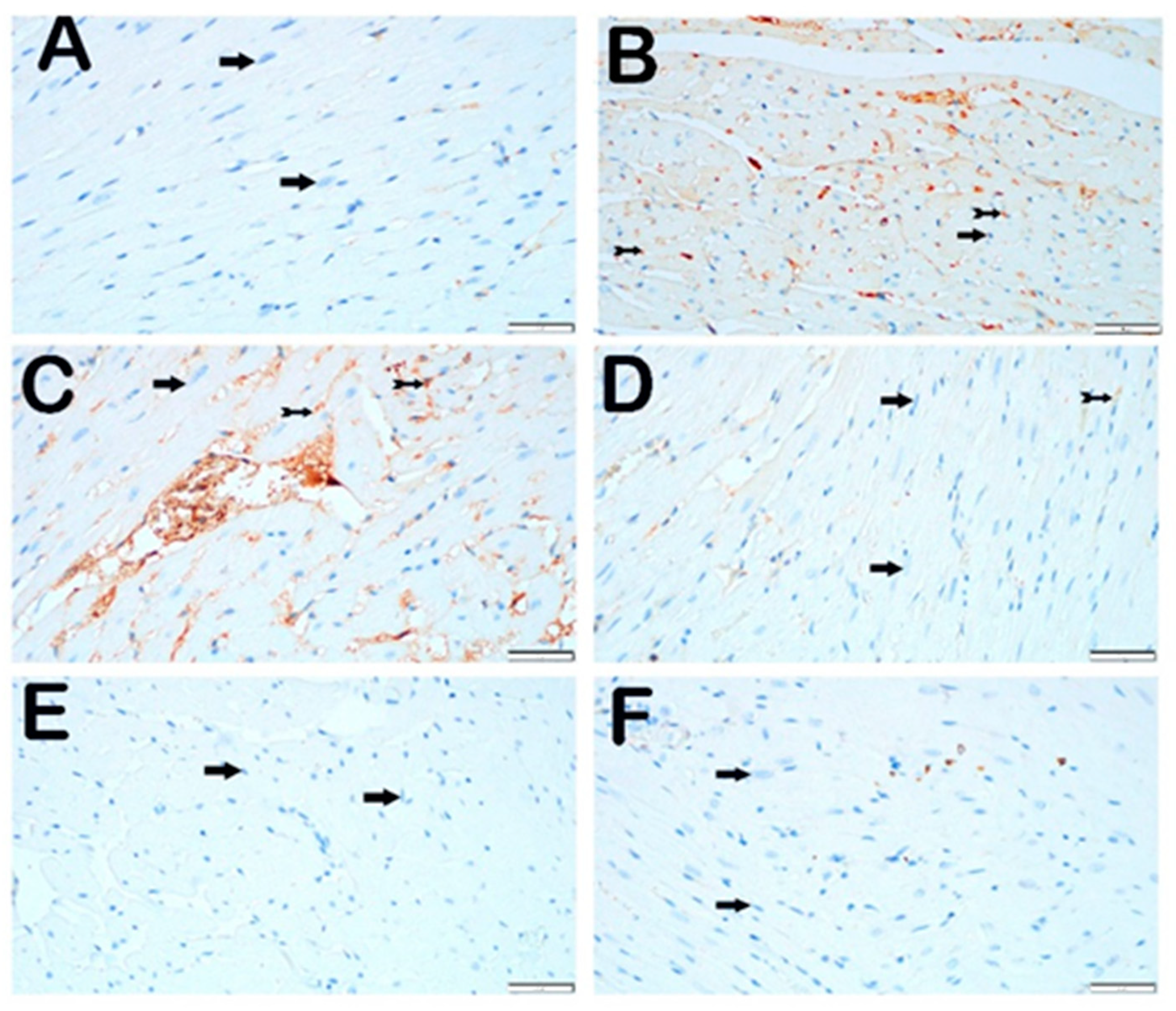
3.3. Immunohistochemical Analysis
3.3.1. CHOP Primary Antibody
3.3.2. GRP 78 Primary Antibody
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Yu, P.; Yu, H.; Qian, B.; Li, Y.; Sun, K.; Shi, B.; Zhang, N.; Xu, G. Therapeutic Effects on the Development of Heart Failure with Preserved Ejection Fraction by the Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin in Type 2 Diabetes. Diabetol. Metab. Syndr. 2023, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jin, X.; Lam, C.W.K.; Yan, S.K. Oxidative Stress and Diabetes Mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Maiorino, M.I.; Bellastella, G.; Giugliano, D.; Esposito, K. Cooling down Inflammation in Type 2 Diabetes: How Strong Is the Evidence for Cardiometabolic Benefit? Endocrine 2017, 55, 360–365. [Google Scholar] [CrossRef]
- Lorenzo-almorós, A.; Cepeda-rodrigo, J.M.; Lorenzo, Ó. Diabetic Cardiomyopathy. Rev. Clín. Esp. (Engl. Ed.) 2022, 222, 100–111. [Google Scholar] [CrossRef]
- Brunvand, L.; Fugelseth, D.; Stensaeth, K.H.; Dahl-Jørgensen, K.; Margeirsdottir, H.D. Early Reduced Myocardial Diastolic Function in Children and Adolescents with Type 1 Diabetes Mellitus a Population-Based Study. BMC Cardiovasc. Disord. 2016, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin Alleviates Cardiac Fibrosis through Suppressing EndMT and Fibroblast Activation via AMPKα/TGF-β/Smad Signalling in Type 2 Diabetic Rats. J. Cell. Mol. Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyoshi, T.; Yoshida, M.; Akagi, S.; Saito, Y.; Ejiri, K.; Matsuo, N.; Ichikawa, K.; Iwasaki, K.; Naito, T.; et al. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 3587. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Chwalba, A.; Basek, A.; Cieślar, G.; Pawlas, N. Glycated Hemoglobin and Cardiovascular Disease in Patients Without Diabetes. J. Clin. Med. 2025, 14, 53. [Google Scholar] [CrossRef]
- Murtaza, G.; Virk, H.U.H.; Khalid, M.; Lavie, C.J.; Ventura, H.; Mukherjee, D.; Ramu, V.; Bhogal, S.; Kumar, G.; Shanmugasundaram, M.; et al. Diabetic Cardiomyopathy—A Comprehensive Updated Review. Prog. Cardiovasc. Dis. 2019, 62, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in Diabetes: Diabetic Kidney Disease versus Nondiabetic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef]
- Raz, I.; Mosenzon, O.; Bonaca, M.P.; Cahn, A.; Kato, E.T.; Silverman, M.G.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; et al. DECLARE-TIMI 58: Participants’ Baseline Characteristics. Diabetes Obes. Metab. 2018, 20, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Hallow, K.M.; Helmlinger, G.; Greasley, P.J.; McMurray, J.J.V.; Boulton, D.W. Why Do SGLT2 Inhibitors Reduce Heart Failure Hospitalization? A Differential Volume Regulation Hypothesis. Diabetes Obes. Metab. 2018, 20, 479–487. [Google Scholar] [CrossRef]
- Zhang, J.; Huan, Y.; Leibensperger, M.; Seo, B.; Song, Y. Comparative Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Serum Electrolyte Levels in Patients with Type 2 Diabetes: A Pairwise and Network Meta-Analysis of Randomized Controlled Trials. Kidney360 2022, 3, 477–487. [Google Scholar] [CrossRef]
- Grubić Rotkvić, P.; Cigrovski Berković, M.; Bulj, N.; Rotkvić, L. Minireview: Are SGLT2 Inhibitors Heart Savers in Diabetes? Heart Fail. Rev. 2020, 25, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Brottier, L.; Barat, J.L.; Combe, C.; Boussens, B.; Bonnet, J.; Bricaud, H.; Bricaud, J. Therapeutic Value of a Cardioprotective Agent in Patients with Severe Ischaemic Cardiomyopathy. Eur. Heart J. 1990, 11, 207–212. [Google Scholar] [CrossRef]
- Eid, B.G.; El-Shitany, N.A.E.A.; Neamatallah, T. Trimetazidine Improved Adriamycin-Induced Cardiomyopathy by Downregulating TNF-α, BAX, and VEGF Immunoexpression via an Antioxidant Mechanism. Environ. Toxicol. 2021, 36, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, A.; Hawkes, E.; Sehn, L.H.; Smith, S.M. Diffuse Large B-Cell Lymphoma. Hematol. Oncol. 2023, 384, 842–858. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-Induced Cardiotoxicity: An Update on the Molecular Mechanism and Novel Therapeutic Strategies for Effective Management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhao, M.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Ye, D.; Li, D.; Wan, J. Resolvin D1 Attenuates Doxorubicin-Induced Cardiotoxicity by Inhibiting Inflammation, Oxidative and Endoplasmic Reticulum Stress. Front. Pharmacol. 2022, 12, 749899. [Google Scholar] [CrossRef]
- Bar-Or, D.; Bar-Or, R.; Rael, L.T.; Brody, E.N. Oxidative Stress in Severe Acute Illness. Redox Biol. 2015, 4, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Hazelhoff, M.H.; Bulacio, R.P.; Torres, A.M. Trimetazidine Protects from Mercury-Induced Kidney Injury. Pharmacology 2021, 106, 332–340. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Tanday, N.; Irwin, N.; Moffett, R.C.; Flatt, P.R.; O’Harte, F.P.M. Beneficial Actions of a Long-Acting Apelin Analogue in Diabetes Are Related to Positive Effects on Islet Cell Turnover and Transdifferentiation. Diabetes Obes. Metab. 2020, 22, 2468–2478. [Google Scholar] [CrossRef]
- Chang, W.T.; Lin, Y.W.; Ho, C.H.; Chen, Z.C.; Liu, P.Y.; Shih, J.Y. Dapagliflozin Suppresses ER Stress and Protects Doxorubicin-Induced Cardiotoxicity in Breast Cancer Patients. Arch. Toxicol. 2021, 95, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Bagchi, A.K.; Jassal, D.S.; Singal, P.K. Doxorubicin-induced Cardiomyopathy Is Mitigated by Empagliflozin via the Modulation of Endoplasmic Reticulum Stress Pathways. Mol. Med. Rep. 2024, 29, 74. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Bagchi, A.K.; Jassal, D.S.; Singal, P.K. Interleukin-10 Mitigates Doxorubicin-Induced Endoplasmic Reticulum Stress as Well as Cardiomyopathy. Biomedicines 2022, 10, 890. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, J.; Sun, Y.; Huang, W.; Fan, J.; Ye, M.; Hu, B.; Sun, X. Influence of Trimetazidine on Myocardial Injury in Mice with Diabetic Cardiomyopathy. J. Diabetes Complicat. 2024, 38, 108744. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Kitakaze, M. ER Stress in Cardiovascular Disease. J. Mol. Cell. Cardiol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef]
- Thangaraj, A.; Sil, S.; Tripathi, A.; Chivero, E.T.; Periyasamy, P.; Buch, S. Targeting endoplasmic reticulum stress and autophagy as therapeutic approaches for neurological diseases. Int. Rev. Cell Mol. Biol. 2020, 350, 285–325. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Kim, S.W.; Ahn, B.Y.; Tran, T.T.V.; Pyun, J.H.; Kang, J.S.; Leem, Y.E. PRMT1 Suppresses Doxorubicin-Induced Cardiotoxicity by Inhibiting Endoplasmic Reticulum Stress. Cell. Signal. 2022, 98, 110412. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, I.H.; Lee, A.H.; Kim, H.J.; Lim, Y.H.; Shin, J.H. Sacubitril/Valsartan Reduces Endoplasmic Reticulum Stress in a Rat Model of Doxorubicin-Induced Cardiotoxicity. Arch. Toxicol. 2022, 96, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Han, L. Upregulation of Serum and Glucocorticoid-Regulated Kinase 1 (SGK1) Ameliorates Doxorubicin-Induced Cardiotoxic Injury, Apoptosis, Inflammation and Oxidative Stress by Suppressing Glucose Regulated Protein 78 (GRP78)-Mediated Endoplasmic Reticulum Stress. Bioengineered 2022, 13, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Boncompagni, E.; Reiter, R.J.; Bejaoui, M.; Freitas, I.; Pantazi, E.; Folch-Puy, E.; Abdennebi, H.B.; Garcia-Gil, F.A.; Roselló-Catafau, J. AMPK Involvement in Endoplasmic Reticulum Stress and Autophagy Modulation after Fatty Liver Graft Preservation: A Role for Melatonin and Trimetazidine Cocktail. J. Pineal Res. 2013, 55, 65–78. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, 1769–1777. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to Calculate Sample Size in Animal Studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, J.; Tan, X.; Li, D.; Yao, D.; Xu, B.; Lei, Y. Dapagliflozin Protects against Dilated Cardiomyopathy Progression by Targeting NLRP3 Inflammasome Activation. Naunyn. Schmiedebergs Arch. Pharmacol. 2023, 396, 1461–1470. [Google Scholar] [CrossRef]
- Sikandar, A.; Farhat, K.; Afzal, A.; Ajmal, K.; Laeeq, M.; Khokhar, A. Protective Effects of Trimetazidine Against Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity in Mice. J. Ayub Med. Abbottabad 2020, 32, 304–309. [Google Scholar]
- El-Sawy, W.S.M.; El-Bahrawy, A.H.; Messiha, B.A.S.; Hemeida, R.A.M.; Khalaf, M.M. The Impact of PPAR-γ/Nrf-2/HO-1, NF-ΚB/IL-6/ Keap-1, and Bcl-2/Caspase-3/ATG-5 Pathways in Mitigation of DOX-Induced Cardiotoxicity in an Animal Model: The Potential Cardioprotective Role of Oxyresveratrol and/or Dapagliflozin. Food Chem. Toxicol. 2024, 191, 114863. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Takemura, G.; Fujiwara, H. Doxorubicin-Induced Cardiomyopathy. From the Cardiotoxic Mechanisms to Management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Shetty, P.; Sainath, P.; Bharati, S.; Ahmed, A.Z.; Singh, V.K.; Ashwal, A.J. Metformin and Dapagliflozin Attenuate Doxorubicin-Induced Acute Cardiotoxicity in Wistar Rats: An Electrocardiographic, Biochemical, and Histopathological Approach. Cardiovasc. Toxicol. 2023, 23, 107–119. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Li, L.; Liang, H.; Ye, H.; Kang, P.; Li, Z.; Yu, Y.; Gao, Q. Effect of NLRP3 Gene Knockdown on Pyroptosis and Ferroptosis in Diabetic Cardiomyopathy Injury. BMC Cardiovasc. Disord. 2024, 24, 351. [Google Scholar] [CrossRef]
- Maleki, M.H.; Vakili, O.; Tavakoli, R.; Nadimi, E.; Noori, Z.; Taghizadeh, M.; Dehghanian, A.; Tayebi, L.; Shafiee, S.M. Protective and Curative Effects of Unconjugated Bilirubin on Gene Expression of LOX-1 and INOS in the Heart of Rats Receiving High-Fat Diet and Low Dose Streptozotocin: A Histomorphometric Approach. J. Inflamm. 2024, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, G.; Shi, Y.; Liu, X.; Liu, S.; Chen, W.; Ning, Y.; Cao, Y.; Zhao, Y.; Li, M. Growth Differentiation Factor 11 Regulates High Glucose-Induced Cardiomyocyte Pyroptosis and Diabetic Cardiomyopathy by Inhibiting Inflammasome Activation. Cardiovasc. Diabetol. 2024, 23, 160. [Google Scholar] [CrossRef]
- Scheen, A.J.; Marre, M.; Thivolet, C. Prognostic Factors in Patients with Diabetes Hospitalized for COVID-19: Findings from the CORONADO Study and Other Recent Reports. Diabetes Metab. 2020, 46, 265–271. [Google Scholar] [CrossRef]
- Baskal, S.; Tsikas, S.A.; Begou, O.; Bollenbach, A.; Lenzen, S.; Jörns, A.; Tsikas, D. Advanced Glycation End-Products (AGEs) of Lysine and Effects of Anti-TCR/Anti-TNF-α Antibody-Based Therapy in the LEW.1AR1-Iddm Rat, an Animal Model of Human Type 1 Diabetes. Int. J. Mol. Sci. 2022, 23, 1541. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, W.H.; Diego, S.; Jolla, L.U.S. Department of Veterans Affairs Diabetic Cardiomyopathy: What Is It and Can It Be Fixed? Circ. Res. 2020, 124, 1160–1162. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, M.E.; Bode, C.; Bugger, H. Diabetic Cardiomyopathy: Does the Type of Diabetes Matter? Int. J. Mol. Sci. 2016, 17, 2136. [Google Scholar] [CrossRef]
- Tang, S.G.; Liu, X.Y.; Wang, S.P.; Wang, H.H.; Jovanović, A.; Tan, W. Trimetazidine Prevents Diabetic Cardiomyopathy by Inhibiting Nox2/TRPC3-Induced Oxidative Stress. J. Pharmacol. Sci. 2019, 139, 311–318. [Google Scholar] [CrossRef]
- Radu, F.; Potcovaru, C.G.; Salmen, T.; Filip, P.V.; Pop, C.; Fierbințeanu-Braticievici, C. The Link between NAFLD and Metabolic Syndrome. Diagnostics 2023, 13, 614. [Google Scholar] [CrossRef]
- Genc, S.; Evren, B.; Yigit, O.S.; Sahin, I.; Dayanan, R.; Klisic, A.; Erturk, A.; Mercantepe, F. Evolving Clinical Features of Diabetic Ketoacidosis: The Impact of SGLT2 Inhibitors. Pharmaceuticals 2024, 17, 1553. [Google Scholar] [CrossRef] [PubMed]
- Salouege, I.; Ali, R.; Saïd, D.; Elkadri, N.; Kourda, N.; Lakhal, M.; Klouz, A. Means of Evaluation and Protection from Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity in Rats. J. Cancer Res. Ther. 2014, 10, 274–278. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Jhund, P.S.; Docherty, K.F.; Diez, M.; Petrie, M.C.; Verma, S.; Nicolau, J.C.; Merkely, B.; Kitakaze, M.; Demets, D.L.; et al. Effects of Dapagliflozin on Symptoms, Function, and Quality of Life in Patients With Heart Failure and Reduced Ejection Fraction: Results From the DAPA-HF Trial. Circulation 2020, 141, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Canale, M.L.; Bisceglia, I.; Iovine, M.; Paccone, A.; Maurea, C.; Scherillo, M.; Merola, A.; Giordano, V.; Palma, G.; et al. Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Ejection Fraction Reduction, Reduces Myocardial and Renal NF-ΚB Expression and Systemic pro-Inflammatory Biomarkers in Models of Short-Term Doxorubicin Cardiotoxicity. Front. Cardiovasc. Med. 2024, 11, 1289663. [Google Scholar] [CrossRef]
- Zaouali, M.A.; Ben Mosbah, I.; Boncompagni, E.; Ben Abdennebi, H.; Mitjavila, M.T.; Bartrons, R.; Freitas, I.; Rimola, A.; Roselló-Catafau, J. Hypoxia Inducible Factor-1α Accumulation in Steatotic Liver Preservation: Role of Nitric Oxide. World J. Gastroenterol. 2010, 16, 3499–3509. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Vellayappan, B.A.; Wong, L.C.; Chiang, C.L.; Chan, S.K.; Wan, E.Y.F.; Wong, I.C.K.; Lambert, P.C.; Rachet, B.; Ng, A.K.; et al. Cardiovascular Diseases among Diffuse Large B-Cell Lymphoma Long-Term Survivors in Asia: A Multistate Model Study. ESMO Open 2022, 7, 100363. [Google Scholar] [CrossRef] [PubMed]
- Kantor, P.F.; Lucien, A.; Kozak, R.; Lopaschuk, G.D. The Antianginal Drug Trimetazidine Shifts Cardiac Energy Metabolism from Fatty Acid Oxidation to Glucose Oxidation by Inhibiting Mitochondrial Long-Chain 3-Ketoacyl Coenzyme A Thiolase. Circ. Res. 2000, 86, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Li, G.; Liu, B.; Guo, H.; Wang, D.; Jie, Q.; Che, W.; Hou, L.; Wei, Y. The Protective Effect of Lacidipine on Myocardial Remodeling Is Mediated by the Suppression in Expression of GPR78 and CHOP in Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 945076. [Google Scholar] [CrossRef]
- Huang, K.; Luo, X.; Liao, B.; Li, G.; Feng, J. Insights into SGLT2 Inhibitor Treatment of Diabetic Cardiomyopathy: Focus on the Mechanisms. Cardiovasc. Diabetol. 2023, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Rezaee, R.; Haye, A.W.; Karimi, G. Endoplasmic Reticulum Stress in Doxorubicin-Induced Cardiotoxicity May Be Therapeutically Targeted by Natural and Chemical Compounds: A Review. Pharmacol. Res. 2021, 164, 105383. [Google Scholar] [CrossRef]
- Belen, E.; Canbolat, I.P.; Yiğittürk, G.; Cetinarslan, O.; Akdeniz, C.S.; Karaca, M.; Sönmez, M.; Erbaş, O. Cardio-Protective Effect of Dapagliflozin against Doxorubicin Induced Cardiomyopathy in Rats. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4403–4408. [Google Scholar] [CrossRef] [PubMed]
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Freimark, D.; Kornowski, R.; Aravot, D.; Hochhauser, E.; et al. Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Attenuates Diabetic Cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.; Eid, E.A.; Taha, M.; Elshazli, R.M.; Bedir, R.F.; Lashin, L.S. Comparative Study of the Effects of GLP1 Analog and SGLT2 Inhibitor against Diabetic Cardiomyopathy in Type 2 Diabetic Rats: Possible Underlying Mechanisms. Biomedicines 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]

| Score | Findings |
|---|---|
| Degenerative cardiomyocyte | |
| 0 | ≤5% |
| 1 | 6–25% |
| 2 | 26–50% |
| 3 | >50% |
| Vascular congestion | |
| 0 | ≤5% |
| 1 | 6–25% |
| 2 | 26–50% |
| 3 | >50% |
| Edematous area | |
| 0 | ≤5% |
| 1 | 6–25% |
| 2 | 26–50% |
| 3 | >50% |
| Score | Finding Cell Distribution That Shows IHC Positivity |
|---|---|
| 0 | ≤5% |
| 1 | 6–25% |
| 2 | 26–50% |
| 3 | ˃50% |
| Group | MDA (TBARS) (nmol/g Tissue) | GSH (TT) (mmol/g Tissue) |
|---|---|---|
| Control | 17.57 ± 1.11 | 8.93 ± 0.48 |
| STZ | 24.22 ± 4.12 | 8.95 ± 1.24 |
| STZ + DOXO | 18.82 ± 3.02 | 9.3 ± 1.66 |
| STZ + DOXO + TMZ | 17.58 ± 3.34 | 10.3 ± 1.88 |
| STZ + DOXO + DAPA | 19.52 ± 4.00 | 8.68 ± 1.41 |
| STZ + DOXO + TMZ + DAPA | 19.68 ± 4.98 | 9.28 ± 1.27 |
| * p-value > 0.05 | ||
| Group | Degenerative Cardiomyocyte | Vascular Congestion | Edema | HCDS |
|---|---|---|---|---|
| Control | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) |
| STZ | 2 (2–2) a | 2 (2–2) a | 1 (1–1) a | 5 (5–5) a |
| STZ + DOXO | 3 (2–3) a,b,d | 3 (2–2) a,b,d | 1 (1–1) a | 6 (5–6) a,e |
| STZ + DOXO + TMZ | 1 (1–1) a,b,c | 1 (1–1) a,b,c | 1 (1–1) a | 3 (3–4) a,e |
| STZ + DOXO + DAPA | 1 (1–1) a,b,c | 1 (1–1) a,b,c | 1 (1–1) a | 3 (3–3) a,e |
| STZ + DOXO + TMZ + DAPA | 1 (1–1) a,b,c | 1 (1–1) a,b,c | 1 (0–1) a,e | 3 (3–3) a,e |
| (median (25th–75th percentiles)). Kruskal–Wallis and Tamhane’s T2 test | ||||
| Group | CHOP Positivity Score | GRP 78 Positivity Score |
|---|---|---|
| Control | 0 (0–1) | 0 (0–0) |
| STZ | 1 (1–2) a | 1 (1–2) a |
| STZ + DOXO | 2 (2–2) a,b,d | 1 (1–2) a |
| STZ + DOXO + TMZ | 1 (1–1) a,c | 0 (0–1) e,c |
| STZ + DOXO + DAPA | 1 (1–1) a,c | 1 (0–1) f,c |
| STZ + DOXO + TMZ + DAPA | 1 (1–1) a,c | 0 (0–1) b,c |
| (median (25th–75th percentiles)). Kruskal–Wallis and Tamhane’s T2 test | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogutveren, M.M.; Satiroglu, O.; Ozden, Z.; Akyildiz, K.; Yilmaz, A.; Mercantepe, F.; Yilmaz, A.S.; Koc, H.; Mercantepe, T. Cardioprotective Effects of Dapagliflozin and Trimetazidine on Doxorubicin-Induced Cardiotoxicity in Streptozotocin-Induced Type 1 Diabetic Rats via Endoplasmic Reticulum Stress. J. Clin. Med. 2025, 14, 1315. https://doi.org/10.3390/jcm14041315
Ogutveren MM, Satiroglu O, Ozden Z, Akyildiz K, Yilmaz A, Mercantepe F, Yilmaz AS, Koc H, Mercantepe T. Cardioprotective Effects of Dapagliflozin and Trimetazidine on Doxorubicin-Induced Cardiotoxicity in Streptozotocin-Induced Type 1 Diabetic Rats via Endoplasmic Reticulum Stress. Journal of Clinical Medicine. 2025; 14(4):1315. https://doi.org/10.3390/jcm14041315
Chicago/Turabian StyleOgutveren, Muhammed Mursel, Omer Satiroglu, Zulkar Ozden, Kerimali Akyildiz, Adnan Yilmaz, Filiz Mercantepe, Ahmet Seyda Yilmaz, Haldun Koc, and Tolga Mercantepe. 2025. "Cardioprotective Effects of Dapagliflozin and Trimetazidine on Doxorubicin-Induced Cardiotoxicity in Streptozotocin-Induced Type 1 Diabetic Rats via Endoplasmic Reticulum Stress" Journal of Clinical Medicine 14, no. 4: 1315. https://doi.org/10.3390/jcm14041315
APA StyleOgutveren, M. M., Satiroglu, O., Ozden, Z., Akyildiz, K., Yilmaz, A., Mercantepe, F., Yilmaz, A. S., Koc, H., & Mercantepe, T. (2025). Cardioprotective Effects of Dapagliflozin and Trimetazidine on Doxorubicin-Induced Cardiotoxicity in Streptozotocin-Induced Type 1 Diabetic Rats via Endoplasmic Reticulum Stress. Journal of Clinical Medicine, 14(4), 1315. https://doi.org/10.3390/jcm14041315






