From Planning to Practice: Impact of Achieved Proximal Sealing Zone in Endovascular Aneurysm Repair (EVAR)
Abstract
1. Introduction
2. Materials and Methods
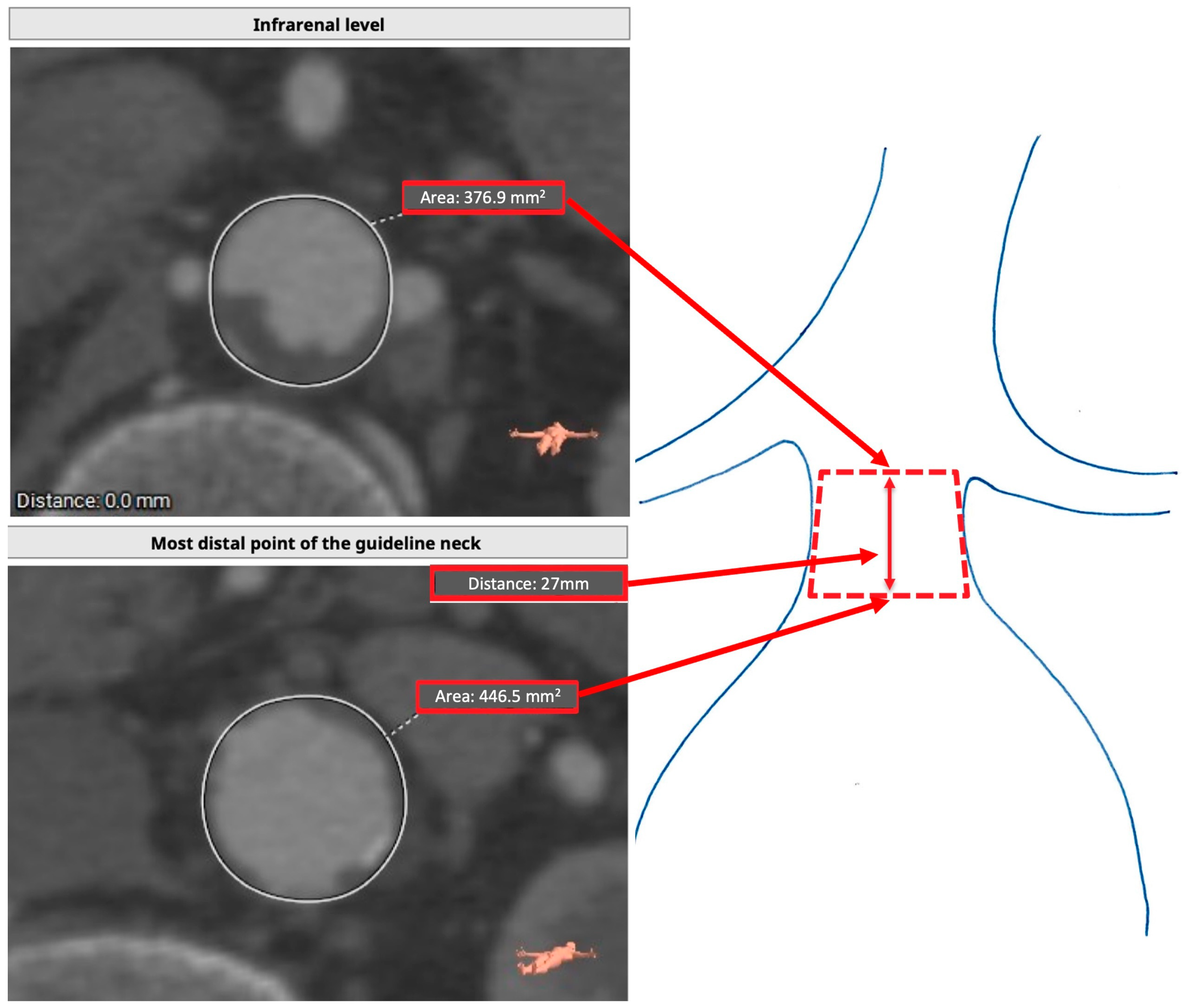
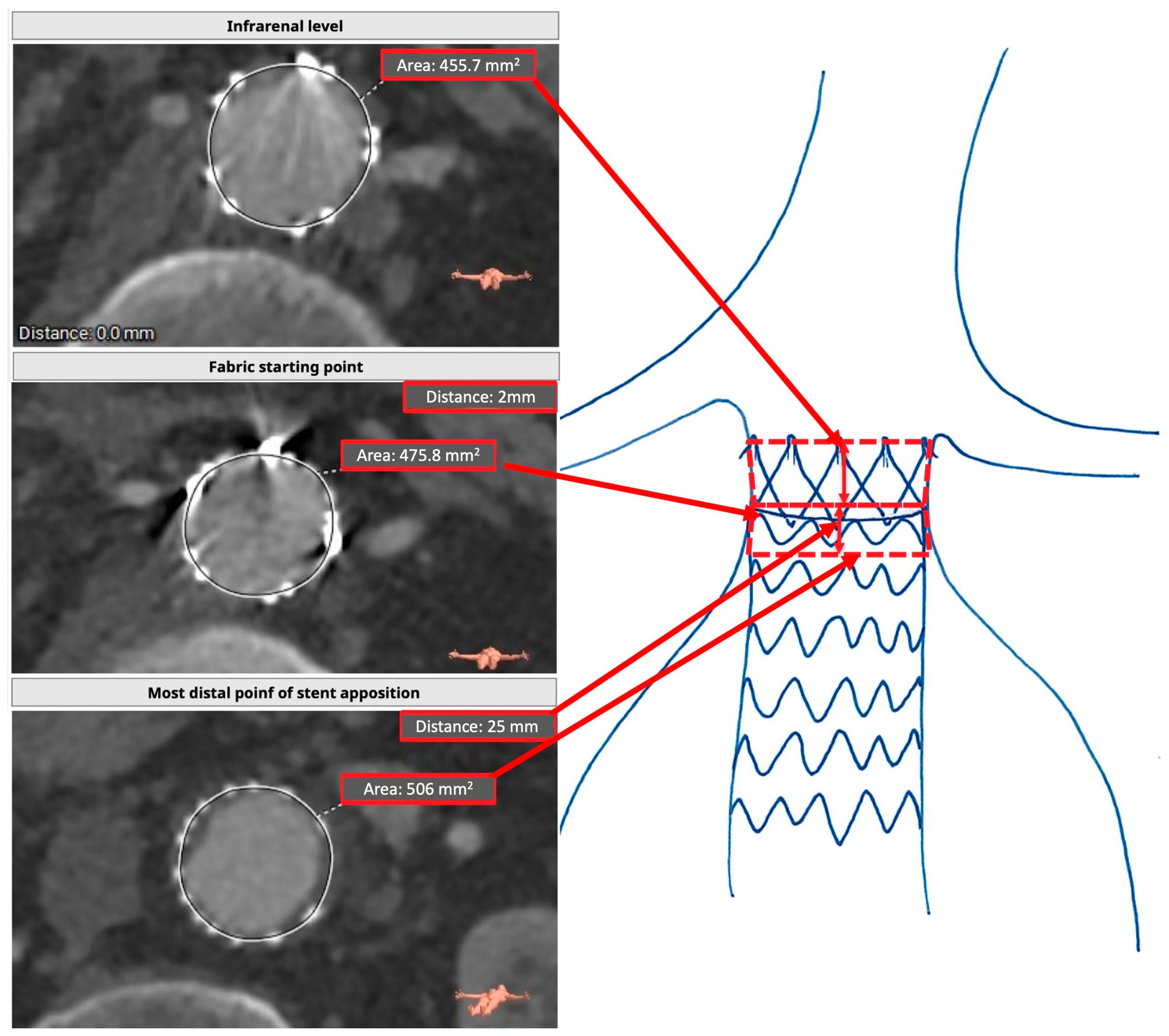
2.1. Proximal Sealing Zone Assessment
2.2. Measurement Protocol
2.3. Preoperative Assessment (TASZ)
2.4. Postoperative Assessment (RASZ)
2.5. Statistical Analysis
3. Results
4. Discussion
- -
- TASZ and RASZ were markedly different;
- -
- The primary factor contributing to the difference between TASZ and RASZ was the length of the sealing zone. This variation continued even after considering graft misplacement;
- -
- Oversizing did not affect RASZ length;
- -
- Short RASZ and TASZ values were effective predictors of type 1A endoleaks during follow-up.
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; Koelemay, M.J.; et al. Editor’s Choice-European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Siribumrungwong, B.; Kurita, J.; Ueda, T.; Yasui, D.; Takahashi, K.-i.; Sasaki, T.; Miyagi, Y.; Sakamoto, S.-i.; Ishii, Y.; Morota, T.; et al. Outcomes of Abdominal Aortic Aneurysm Repairs: Endovascular vs Open Surgical Repairs. Asian J. Surg. 2022, 45, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Sicard, G.A.; Zwolak, R.M.; Sidawy, A.N.; White, R.A.; Siami, F.S. Endovascular Abdominal Aortic Aneurysm Repair: Long-Term Outcome Measures in Patients at High-Risk for Open Surgery. J. Vasc. Surg. 2006, 44, 229–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zuidema, R.; van der Riet, C.; El Moumni, M.; Schuurmann, R.C.L.; Ünlü, Ç.; de Vries, J.P.P.M. Pre-Operative Aortic Neck Characteristics and Post-Operative Sealing Zone as Predictors of Type 1a Endoleak and Migration After Endovascular Aneurysm Repair: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 64, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Accarino, G.; Peluso, A.; Turchino, D.; Fornino, G.; Puca, A.E.; De Rosa, C.; Accarino, G.; Galasso, G.; Serra, R.; Bracale, U.M. Are Very Short Necks ESARpatients Safe from Type 1Aendoleak Risk? Ital. J. Vasc. Endovasc. Surg. 2024, 31, 10–18. [Google Scholar] [CrossRef]
- Rethinking the Concept of Aortic Neck Length—Endovascular Today. Available online: https://fyra.io (accessed on 10 October 2024).
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; Van Herwaarden, J.A.; Holt, P.J.E.; Van Keulen, J.W.; Rantner, B.; et al. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41, S1–S58. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.P.P.M.; Zuidema, R.; Bicknell, C.D.; Fisher, R.; Gargiulo, M.; Louis, N.; Oikonomou, K.; Pratesi, G.; Reijnen, M.M.P.J.; Valdivia, A.R.; et al. European Expert Opinion on Infrarenal Sealing Zone Definition and Management in Endovascular Aortic Repair Patients: A Delphi Consensus. J. Endovasc. Ther. 2023, 30, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Endurant TM II Endurant TM IIs Stent Graft System Instructions for Use. 2017. Available online: https://www.medtronic.com/en-us/healthcare-professionals/products/cardiovascular/aortic/aortic-stent-grafts/endurant-ii-stent-graft-system.html (accessed on 15 January 2025).
- Suckow, B.D.; Goodney, P.P.; Columbo, J.A.; Kang, R.; Stone, D.H.; Sedrakyan, A.; Cronenwett, J.L.; Fillinger, M.F. National Trends in Open Surgical, Endovascular and Branched/Fenestrated Endovascular Aortic Aneurysm Repair in Medicare Patients. J. Vasc. Surg. 2018, 67, 1690. [Google Scholar] [CrossRef] [PubMed]
- Aortic Aneurysm Market Size, Share & Trends Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/aortic-aneurysm-market (accessed on 16 February 2023).
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Endovascular Aneurysm Repair: State-of-Art Imaging Techniques for Preoperative Planning and Surveillance. J. Cardiovasc. Surg. 2009, 50, 423–438. Available online: https://www.minervamedica.it/en/journals/cardiovascular-surgery/article.php?cod=R37Y2009N04A0423 (accessed on 16 February 2023).
- Jordan, W.D.; Mehta, M.; Varnagy, D.; Moore, W.M.; Arko, F.R.; Joye, J.; Ouriel, K.; De Vries, J.P. Results of the ANCHOR Prospective, Multicenter Registry of EndoAnchors for Type Ia Endoleaks and Endograft Migration in Patients with Challenging Anatomy. J. Vasc. Surg. 2014, 60, 885–892.e2. [Google Scholar] [CrossRef] [PubMed]
- Leurs, L.J.; Kievit, J.; Dagnelie, P.C.; Nelemans, P.J.; Buth, J. Influence of Infrarenal Neck Length on Outcome of Endovascular Abdominal Aortic Aneurysm Repair. J. Endovasc. Ther. 2006, 13, 640–648. [Google Scholar] [CrossRef]
- Geraedts, A.C.M.; Zuidema, R.; Schuurmann, R.C.L.; Kwant, A.N.; Mulay, S.; Balm, R.; de Vries, J.P.P.M. Shortest Apposition Length at the First Postoperative Computed Tomography Angiography Identifies Patients at Risk for Developing a Late Type Ia Endoleak After Endovascular Aneurysm Repair. J. Endovasc. Ther. 2024, 31, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sandström, C.; Andersson, M.B.; Bogdanovic, M.; Fattahi, N.; Lundqvist, R.; Andersson, M.; Roy, J.; Hultgren, R.; Roos, H. Sealing Zone Failure Decreases the Long Term Durability of Endovascular Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 238–247. [Google Scholar] [CrossRef]
- Mwipatayi, B.P.; Faraj, J.; Oshin, O.; Fitridge, R.; Wong, J.; Schermerhorn, M.L.; Becquemin, J.P.; Boeckler, D.; Riambau, V.; Teijink, J.A.; et al. Endurant Stent Graft Demonstrates Promising Outcomes in Challenging Abdominal Aortic Aneurysm Anatomy. J. Vasc. Surg. 2021, 73, 69–80. [Google Scholar] [CrossRef]
- Accarino, G.; De Vuono, F.; Accarino, G.; Fornino, G.; Puca, A.E.; Fimiani, R.; Parrella, V.; Savarese, G.; Furgiuele, S.; Vecchione, C.; et al. Endurant Stent Graft for Treatment of Abdominal Aortic Aneurysm Inside and Outside of the Instructions for Use for the Proximal Neck: A 14-Year, Single-Center Experience. J. Clin. Med. 2024, 13, 2589. [Google Scholar] [CrossRef] [PubMed]
- Schuurmann, R.C.L.; van Noort, K.; Overeem, S.P.; van Veen, R.; Ouriel, K.; Jordan, W.D.; Muhs, B.E.; ‘t Mannetje, Y.W.; Reijnen, M.M.P.J.; Fioole, B.; et al. Determination of Endograft Apposition, Position, and Expansion in the Aortic Neck Predicts Type Ia Endoleak and Migration After Endovascular Aneurysm Repair. J. Endovasc. Ther. 2018, 25, 366–375. [Google Scholar] [CrossRef] [PubMed]
| Overall (275) | Group 1 (No Endoleak) | Group 2 (Any Endoleak) | p-Value (<0.05) | |
|---|---|---|---|---|
| Age (years, SD) | 72 ± 8 | 72 ± 8 | 73 ± 9 | 0.258 |
| Female sex | 21 (8.1%) | 17 (8.5%) | 4 (6.5%) | 0.590 |
| Diabetes | 43(18.4%) | 33 (18.4%) | 10 (18.1%) | 0.966 |
| Arterial hypertension | 225 (82%) | 191 (81.6%) | 46 (83%) | 0.659 |
| Dyslipidemia | 147 (62.8%) | 115 (64.2%) | 32 (58.2%) | 0.416 |
| Cardiac disease | 105 (44.8%) | 81 (45.2%) | 24 (43.6%) | 0.833 |
| Previous myocardial infarction | 69 (29.5%) | 53 (29.6) | 16 (29.1) | 0.941 |
| Chronic obstructive pulmonary disease | 125 (53.4%) | 96 (53.6%) | 29 (52.7%) | 0.906 |
| Chronic kidney disease | 48 (20.5%) | 35 (19.5%) | 13 (23.6%) | 0.512 |
| Emergency setting | 10 (4.1%) | 6 (3.2%) | 4 (6.9%) | 0.208 |
| Overall | Group 1 (No Endoleak) | Group 2 (Any Endoleak) | p-Value (<0.05) | |
|---|---|---|---|---|
| AAA diameter (mm) | 58 (51–67) | 57 (50–64) | 67 (56–78) | <0.001 * |
| Aortic bifurcation diameter (mm) | 28 (24–38) | 27 (23–35) | 33(27–48) | <0.001 * |
| Access common iliac artery diameter (mm) | 15 (13–21) | 14 (12–21) | 17 (13–22) | 0.081 |
| Access external iliac artery diameter (mm) | 9.5 ± 3.8 | 9 (8–10) | 10 (8.7–11) | 0.011 * |
| Access femoral artery diameter (mm) | 10.8 ± 4.2 | 10 ± 2.2 | 10.5 ± 2.2 | 0.085 |
| Lowest renal to aortic bifurcation length (mm) | 114 ± 19.8 | 112 (102–124) | 116 (100–130) | 0.623 |
| Number of patent lumbar arteries | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.256 |
| Infrarenal neck angulation | 28 ± 19 | 27 ± 20 | 33 ± 20 | 0.097 |
| Neck diameter (mm) | 24.6 ± 4 | 24 ± 3 | 25 ± 5 | 0.540 |
| Overall | Group 1 (No Endoleak) | Group 2 (Any Endoleak) | p-Value (<0.05) | |
|---|---|---|---|---|
| Length of stay (days) | 4 (3–5) | 4 (3–5) | 5 (3.5–6) | 0.882 |
| Procedure duration (hours) | 3 ± 1.6 | 3.2 ± 1.3 | 3.4 ± 2.3 | 0.228 |
| Sac embolization | 57 (22%) | 39 (15%) | 18 (6.9%) | 0.121 |
| Complications | 19 (7.3%) | 12 (4.6%) | 7 (11.3%) | 0.167 |
| Number of Endurant components | 3 (2–3) | 2 (2–3) | 3 (2–3) | 0.003 * |
| Bell-bottom component | 53 (19%) | 34 (17%) | 19 (9.3%) | 0.047 * |
| Proximal oversize | 1.14 (1.06–1.23) | 1.14 (1.06–1.24) | 1.12 (1.05–1.23) | 0.381 |
| Overall (275) | |
|---|---|
| Follow-up time (months) | 64 (31–106) |
| Any endoleak | 62 (22.6%) |
| -Type 1A | 24 (8.7%) |
| -Type 1B | 11 (4%) |
| -Type 2 | 27 (9.8%) |
| Graft thrombosis | 7 (2.6%) |
| Procedure-related reintervention | 34 (12.3%) |
| Aneurysm-related mortality | 5 (1.82%) |
| All-cause mortality | 71 (25.8%) |
| Sac regression at more than one year: | |
| ≥10 mm | 126 (45.7%) |
| ≥5 mm | 182 (66.4%) |
| <5 mm/stable | 51 (18.7%) |
| Growth > 5 mm | 36 (13.1%) |
| Preoperative Assessment | Postoperative Assessment | p-Value (<0.05) | |
|---|---|---|---|
| Sealing area (mm2) | 106 (50.0–210.1) | 78 (34–155) | <0.001 * |
| Infrarenal area (mm2) | 37.8 (31.5–47.4) | 38.1 (31.5–45.9) | 0.530 |
| Proximal sealing zone length corrected for graft misplacement (mm) | 15 (7–30) | 11 (4–22) | 0.001 * |
| Most distal point of neck/stent apposition area (mm2) | 43.1 (36.1–52.8) | 40.6 (33.4–51.9) | 0.374 |
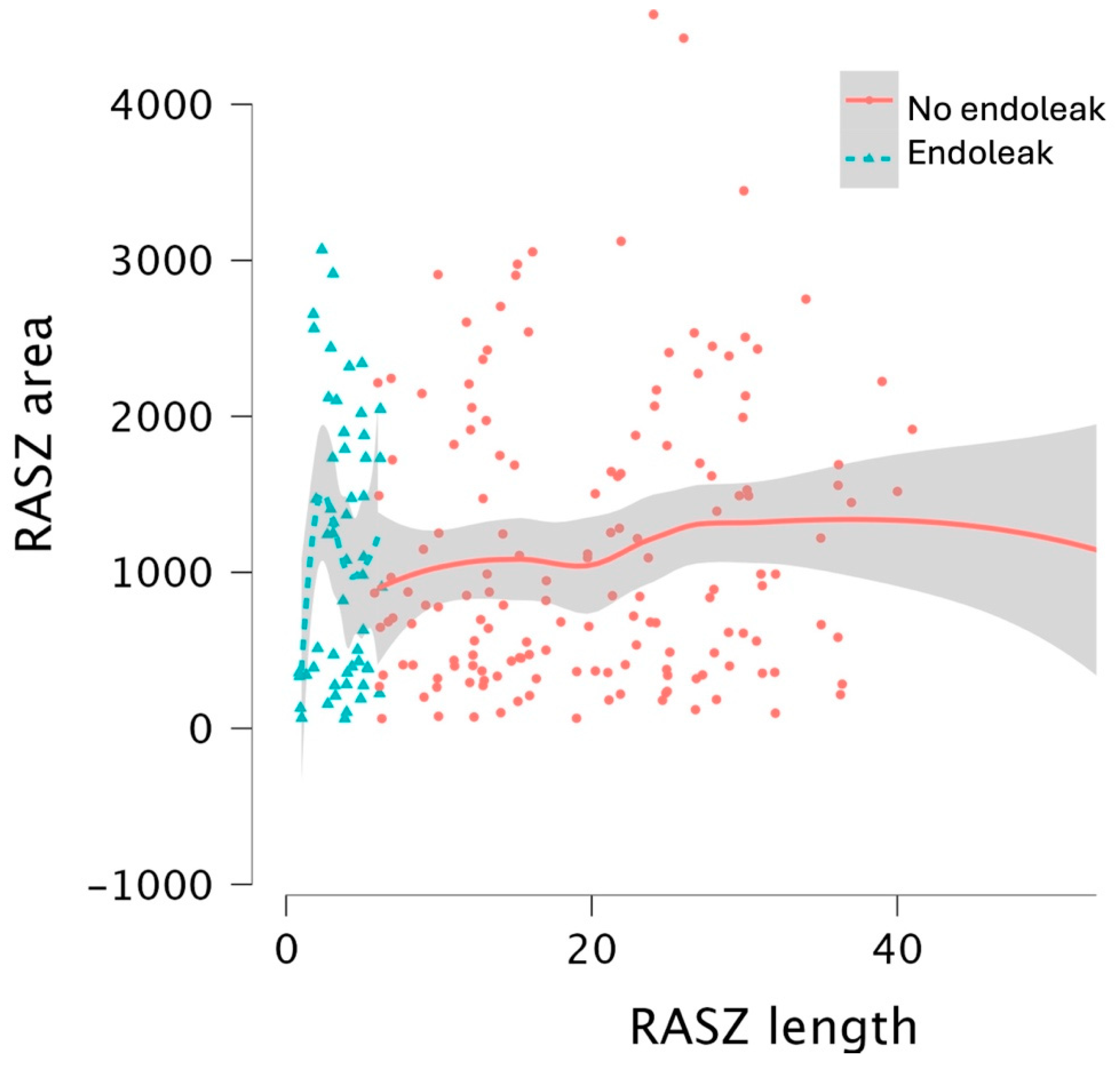
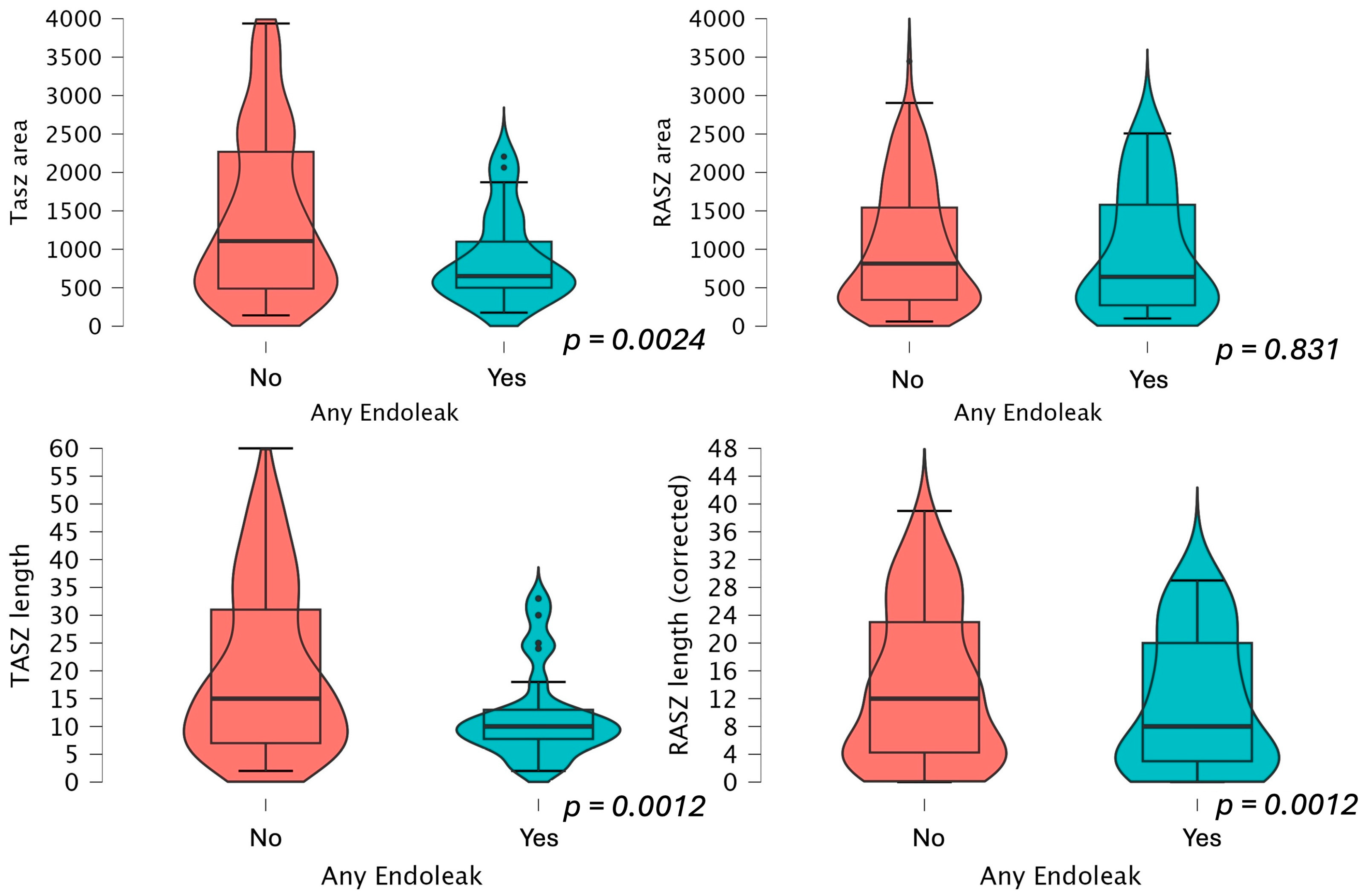
| Overall | Group 1 (No Endoleak) | Group 2 (Endoleak) | p-Value (<0.05) | |
|---|---|---|---|---|
| Target anticipated sealing zona area (mm2) | 106 (505–210) | 114.2 (50–248) | 65.0 (50.0–109.9) 60.0 (52.3–68.1) if type 1a splits groups | 0.0024 * 0.10 if type 1a splits groups |
| Real achieved sealing zone area (mm2) | 78.3 (34–155) | 81.4 (34–154) | 64.1 (27.2–158) 40.3 (23.6–71.7) if type 1a splits groups | 0.831 0.110 if type 1a splits groups |
| Infrarenal area (mm2) | 36.4 (30.8–54.9) | 36.4 (30.6–45.9) | 36.2 (32.3–43.4) | 0.651 |
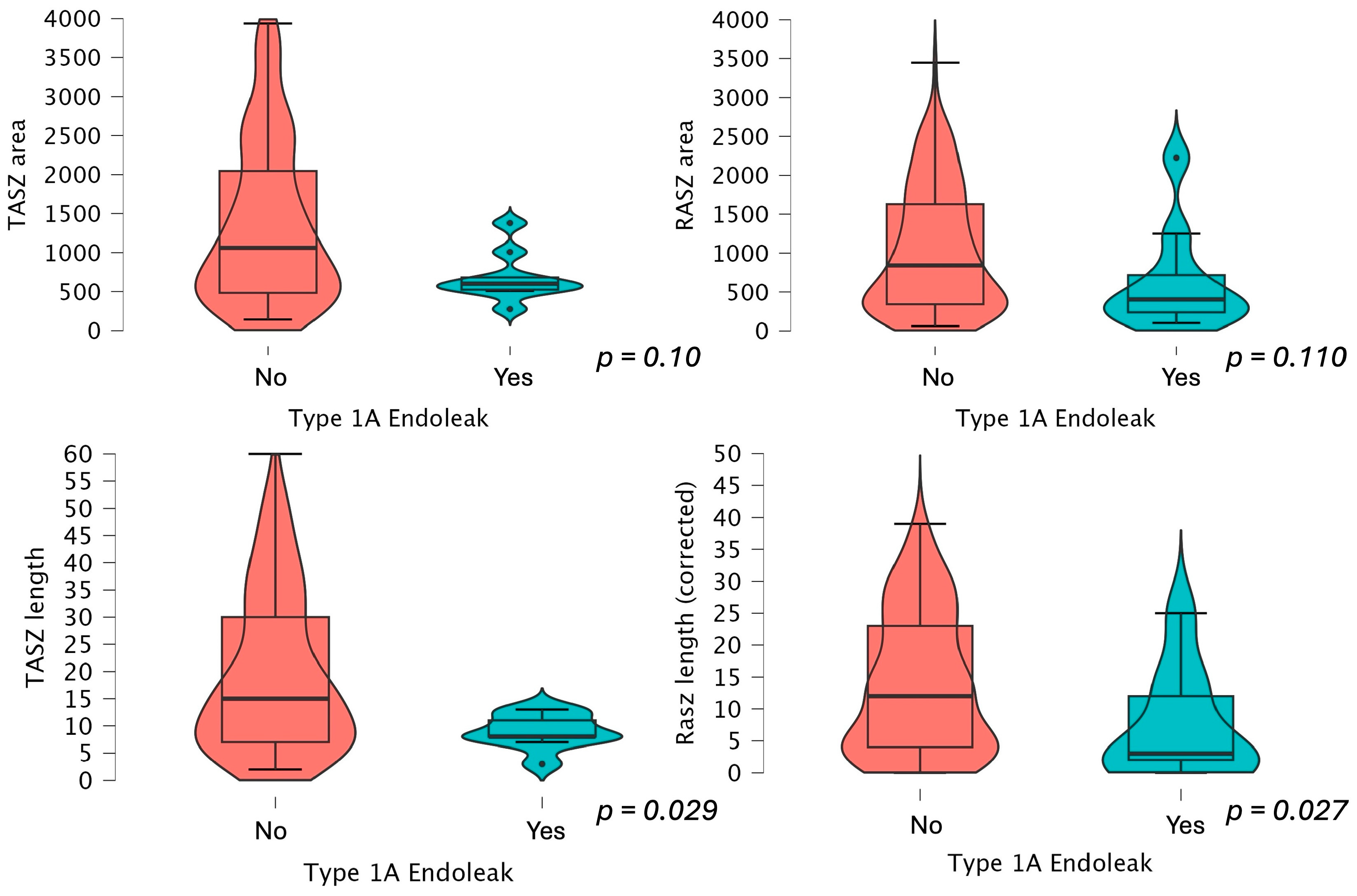
| Distance between infrarenal level and most distal point of guideline neck (mm) | 15 (7–30) | 15.5 (7–33) 15 (7–30) if type 1a splits groups | 10 (7–13) 8 (8–11) if type 1a splits groups | 0.012 * 0.029 * if type 1a splits groups |
| Most distal point of guideline neck area (mm2) | 43.1 (36.1–52.8) | 42.9 (35.8–51.9) 42.8 (35.7–52.5) if type 1a splits groups | 42.7 (36.0–54.2) 45.1 (38.1–61.4) if type 1a splits groups | 0.751 0.191 if type 1a splits groups |
| Distance between infrarenal level and most distal point of circumferential stent apposition (mm) | 13.5 (5.2–24) | 15 (7–33) 15 (7–30) if type 1a splits groups | 10 (7.7–13) 8 (8–11) if type 1a splits groups | 0.006 * 0.029 * if type 1a splits groups |
| Distance circumferentially covered by endograft (mm) | 11 (4–22) | 12 (4–22) 12 (4–22) if type 1a splits groups | 6 (3–20) 2.5 (0.5–10) if type 1a splits groups | 0.155 0.027 * if type 1a splits groups |
| Most distal point of circumferential stent apposition area (mm2) | 40.6 (33.4–52.0) | 39.9 (33.0–51.3) 40.9 (33.5–52.1) if type 1a splits groups | 46.2 (34.6–62.4) 36.6 (25.7–47.8) if type 1a splits groups | 0.855 0.191 if type 1a splits groups |
| Proximal ovesizing | 1.15 ± 0.12 | 1.15 ± 0.122 | 1.16 ± 0.12 | 0.186 |
| Low graft misplacement (mm) | 0 (0–2) | 0 (0–1) the same if type 1a splits groups | 0 (0–2) 2 (0–5) if type 1a splits groups | 0.245 0.026 * if type 1a splits groups |
| HR | 95% CI | p-Value (<0.05) | |
|---|---|---|---|
| TASZ area | 0.998 | 0.998–0.999 | 0.001 * |
| RASZ area | 0.999 | 0.999–1.000 | 0.028 * |
| TASZ length | 0.888 | 0.827–0.952 | <0.001 * |
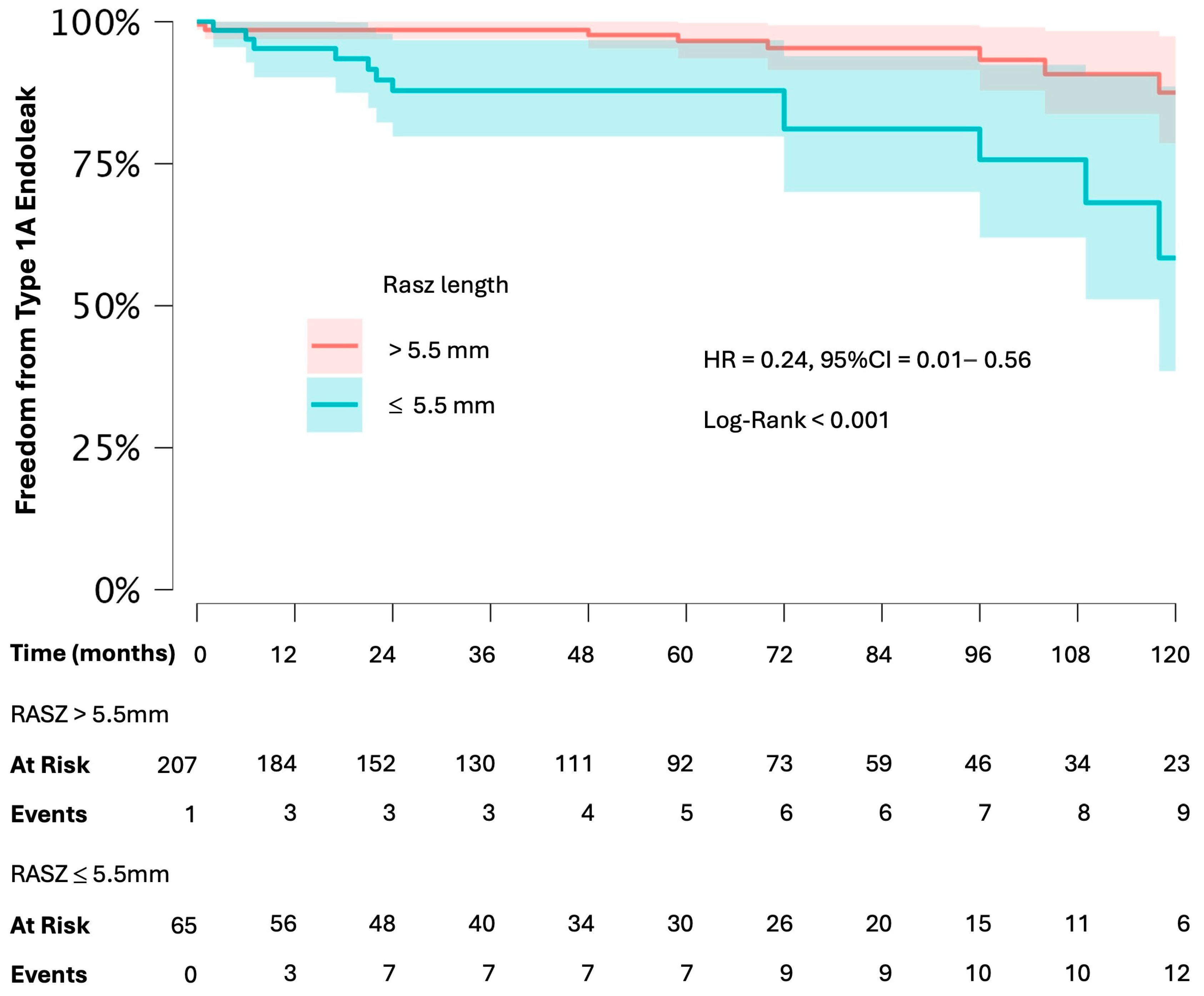
| RASZ length | 0.910 | 0.855–0.969 | 0.003 * |
| Lost RASZ length | 1.050 | 0.985–1.119 | 0.131 |
| AAA diameter | 1.021 | 1.001–1.042 | 0.041 * |
| Aortic bifurcation diameter | 1.015 | 0.986–1.044 | 0.317 |
| Infrarenal neck angulation | 1.022 | 1.001–1.043 | 0.043 * |
| HR | 95% CI | p-Value (<0.05) | |
|---|---|---|---|
| TASZ model | |||
| TASZ length | 0.877 | 0.804–0.955 | 0.003 * |
| AAA diameter | 1.001 | 0.972–1.032 | 0.924 |
| Infrarenal neck angulation | 1.028 | 0.972–1.032 | 0.062 |
| RASZ model | |||
| RASZ length | 0.921 | 0.860–0.987 | 0.020 * |
| AAA diameter | 1.019 | 0.991–1.048 | 0.186 |
| Infrarenal neck angulation | 1.024 | 1.000–1.049 | 0.054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accarino, G.; Silverio, A.; Bellino, M.; Furgiuele, S.; Fimiani, M.; Sica, M.; De Vuono, F.; Fornino, G.; Turchino, D.; Accarino, G.; et al. From Planning to Practice: Impact of Achieved Proximal Sealing Zone in Endovascular Aneurysm Repair (EVAR). J. Clin. Med. 2025, 14, 1309. https://doi.org/10.3390/jcm14041309
Accarino G, Silverio A, Bellino M, Furgiuele S, Fimiani M, Sica M, De Vuono F, Fornino G, Turchino D, Accarino G, et al. From Planning to Practice: Impact of Achieved Proximal Sealing Zone in Endovascular Aneurysm Repair (EVAR). Journal of Clinical Medicine. 2025; 14(4):1309. https://doi.org/10.3390/jcm14041309
Chicago/Turabian StyleAccarino, Giulio, Angelo Silverio, Michele Bellino, Sergio Furgiuele, Mario Fimiani, Mattia Sica, Francesco De Vuono, Giovanni Fornino, Davide Turchino, Giancarlo Accarino, and et al. 2025. "From Planning to Practice: Impact of Achieved Proximal Sealing Zone in Endovascular Aneurysm Repair (EVAR)" Journal of Clinical Medicine 14, no. 4: 1309. https://doi.org/10.3390/jcm14041309
APA StyleAccarino, G., Silverio, A., Bellino, M., Furgiuele, S., Fimiani, M., Sica, M., De Vuono, F., Fornino, G., Turchino, D., Accarino, G., Serra, R., Galasso, G., Vecchione, C., & Bracale, U. M. (2025). From Planning to Practice: Impact of Achieved Proximal Sealing Zone in Endovascular Aneurysm Repair (EVAR). Journal of Clinical Medicine, 14(4), 1309. https://doi.org/10.3390/jcm14041309








