Non-Contrast Computed Tomography-Based Triage and Notification for Large Vessel Occlusion Stroke: A Before and After Study Utilizing Artificial Intelligence on Treatment Times and Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Heuron ELVO for Identifying Large Vessel Occlusion (Figure 1)
2.3. Study Population and Data Collection
2.3.1. Sample Size Calculation
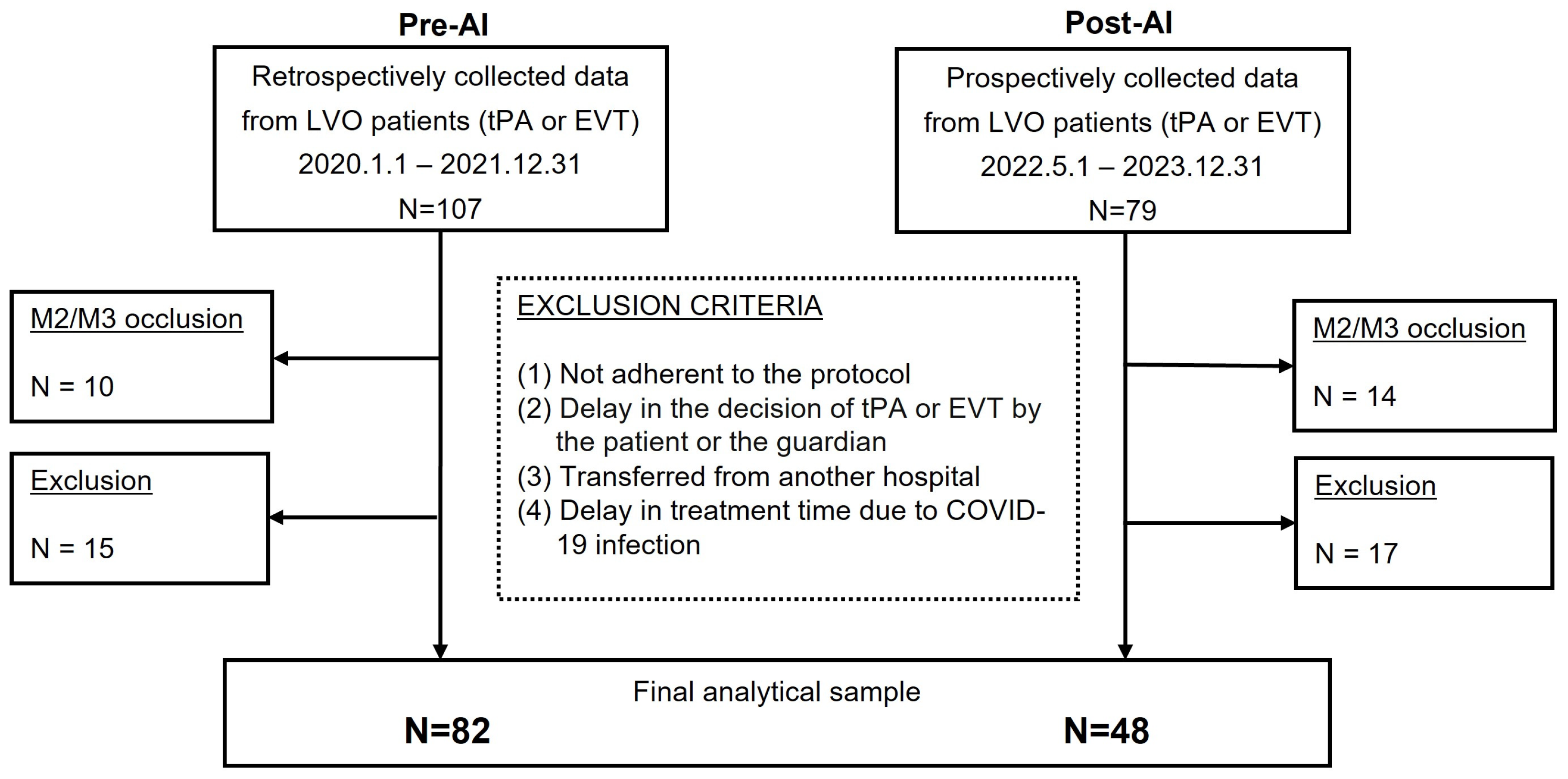
2.3.2. Inclusion and Exclusion Criteria
2.4. Outcomes Measurement
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, W.S.; Lev, M.H.; English, J.D.; Camargo, E.C.; Chou, M.; Johnston, S.C.; Gonzalez, G.; Schaefer, P.W.; Dillon, W.P.; Koroshetz, W.J.; et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009, 40, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Gornbein, J.; Saver, J.L. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke related dependence and death: A review. Front. Neurol. 2017, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; van der Lugt, A.; Menon, B.K.; Majoie, C.B.L.M.; Dippel, D.W.; Campbell, B.C.; Nogueira, R.G.; Demchuk, A.M.; Tomasello, A.; et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA 2016, 316, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.A.; Jahan, R.; Gralla, J.; Pereira, V.M.; Nogueira, R.G.; Levy, E.I.; Zaidat, O.O.; Saver, J.L. SWIFT-STAR Trialists. Time to endovascular reperfusion and degree of disability in acute stroke. Ann. Neurol. 2015, 78, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.; Demchuk, A.M. Computed tomography angiography in the assessment of patients with stroke/TIA. Neurohospitalist 2011, 1, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.G.; Warach, S. Imaging of acute stroke. Nat. Rev. Neurol. 2010, 6, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Almekhlafi, M.A.; Kunz, W.G.; Menon, B.K.; McTaggart, R.A.; Jayaraman, M.V.; Baxter, B.W.; Heck, D.; Frei, D.; Derdeyn, C.P.; Takagi, T.; et al. Imaging of patients with suspected large-vessel occlusion at primary stroke centers: Available modalities and a suggested approach. Am. J. Neuroradiol. 2019, 40, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.M.; Unberath, M.; Hager, G.D.; Hui, F.K. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: A systematic review. J. Neurointerv. Surg. 2020, 12, 156–164. [Google Scholar] [CrossRef]
- Soun, J.E.; Chow, D.S.; Nagamine, M.; Takhtawala, R.S.; Filippi, C.G.; Yu, W.; Chang, P.D. Artificial intelligence and acute stroke imaging. Am. J. Neuroradiol. 2021, 42, 2–11. [Google Scholar] [CrossRef]
- El Naamani, K.; Musmar, B.; Gupta, N.; Ikhdour, O.; Abdelrazeq, H.; Ghanem, M.; Wali, M.H.; El-Hajj, J.; Alhussein, A.; Alhussein, R.; et al. The artificial intelligence revolution in stroke care: A decade of scientific evidence in Review. World. Neurosurg. 2024, 184, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wen, Z.; Wang, Y.; Zhong, Y.; Wang, J.; Hu, Y.; Zhou, P.; Guo, S. Artificial intelligence in ischemic stroke images: Current applications and future directions. Front. Neurol. 2024, 15, 1418060. [Google Scholar] [CrossRef]
- Mair, G.; Boyd, E.V.; Chappell, F.M.; von Kummer, R.; Lindley, R.I.; Sandercock, P.; Wardlaw, J.M. IST-3 Collaborative Group. Sensitivity and specificity of the hyperdense artery sign for arterial obstruction in acute ischemic stroke. Stroke 2015, 46, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Magarik, J.A.; Froehler, M.T. The CT-defined hyperdense arterial sign as a marker for acute intracerebral large vessel occlusion. J. Neuroimaging. 2018, 28, 212–216. [Google Scholar] [CrossRef]
- Jodaitis, L.; Ligot, N.; Chapusette, R.; Bonnet, T.; Gaspard, N.; Naeije, G. The hyperdense middle cerebral artery sign in drip-and-ship models of acute stroke management. Cerebrovasc. Dis. Extra. 2020, 10, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Barber, P.A.; Demchuk, A.M.; Zhang, J.; Buchan, A.M. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group-Alberta Stroke Programme Early CT Score. Lancet 2000, 355, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Pexman, J.H.; Barber, P.A.; Hill, M.D.; Sevick, R.J.; Demchuk, A.M.; Hudon, M.E.; Hu, W.Y.; Buchan, A.M. Use of the alberta stroke program early CT score (ASPECTS) for assessing CT scans in patients with acute stroke. Am. J. Neuroradiol. 2001, 22, 1534–1542. [Google Scholar]
- McTaggart, R.A.; Jovin, T.G.; Lansberg, M.G.; Mlynash, M.; Jayaraman, M.V.; Choudhri, O.A.; Inoue, M.; Marks, M.P.; Albers, G.W.; DEFUSE 2 Investigators. Alberta stroke program early computed tomographic scoring performance in a series of patients undergoing computed tomography and MRI: Reader agreement, modality agreement, and outcome prediction. Stroke 2015, 46, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.E.; Schaefer, P.W.; Sorensen, A.G.; Halpern, E.F.; Ay, H.; He, J.; Koroshetz, W.J.; Gonzalez, R.G. CT and conventional and diffusion-weighted MR imaging in acute stroke: Study in 691 patients at presentation to the emergency department. Radiology 2002, 224, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Camargo, E.C.; Furie, K.L.; Singhal, A.B.; Roccatagliata, L.; Cunnane, M.E.; Halpern, E.F.; Harris, G.J.; Smith, W.S.; Gonzalez, R.G.; Koroshetz, W.J.; et al. Acute brain infarct: Detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology 2007, 244, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Fiebach, J.B.; Schellinger, P.D.; Jansen, O.; Meyer, M.; Wilde, P.; Bender, J.; Schramm, P.; Jüttler, E.; Oehler, J.; Hartmann, M.; et al. CT and diffusion-weighted MR imaging in randomized order: Diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke 2002, 33, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Yedavalli, V.; Heit, J.J.; Dehkharghani, S.; Haerian, H.; Mcmenamy, J.; Honce, J.; Timpone, V.M.; Harnain, C.; Kesselman, A.; Filly, A.; et al. Performance of RAPID noncontrast CT stroke platform in large vessel occlusion and intracranial hemorrhage detection. Front. Neurol. 2023, 14, 1324088. [Google Scholar] [CrossRef] [PubMed]
- Mijalski Sells, C.M.; Loube, D.; Phan, A.Q.; Albers, G.W. National Database demonstrates substantial delays between non-contrast CT and CT angiography in suspected stroke patients in 2023. Stroke 2023, 55 (Suppl. S1), AWP68. [Google Scholar] [CrossRef]
- Douglas, V.; Shamy, M.; Bhattacharya, P. Should CT angiography be a routine component of acute stroke imaging? Neurohospitalist 2015, 5, 97–98. [Google Scholar] [CrossRef] [PubMed]
- Olive-Gadea, M.; Crespo, C.; Granes, C.; Hernandez-Perez, M.; Pérez de la Ossa, N.; Laredo, C.; Urra, X.; Carlos Soler, J.; Soler, A.; Puyalto, P.; et al. Deep learning based software to identify large vessel occlusion on noncontrast computed tomography. Stroke 2020, 51, 2936–2944. [Google Scholar] [CrossRef]
- Aytaç, E.; Gönen, M.; Tatli, S.; Balgetir, F.; Dogan, S.; Tuncer, T. Large vessel occlusion detection by non-contrast CT using artificial intelligence. Neurol. Sci. 2024, 45, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Heuron, Inc. Available online: https://www.iheuron.com (accessed on 1 January 2025.).
- McCluskey, G.; Hunter, A.; Best, E.M.J.; McCarron, M.O.; McVerry, F. Radiological eye deviation as a predictor of large vessel occlusion in acute ischaemic stroke. J. Stroke. Cerebrovasc. Dis. 2019, 28, 2318–2323. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Morey, J.R.; Zhang, X.; Yaeger, K.A.; Fiano, E.; Marayati, N.F.; Kellner, C.P.; De Leacy, R.A.; Doshi, A.; Tuhrim, S.; Fifi, J.T. Real-World Experience with Artificial Intelligence-Based Triage in Transferred Large Vessel Occlusion Stroke Patients. Cerebrovasc. Dis. 2021, 50, 450–455. [Google Scholar] [CrossRef]
- Lum, C.; Ahmed, M.E.; Patro, S.; Thornhill, R.; Hogan, M.; Iancu, D.; Lesiuk, H.; Dos Santos, M.; Dowlatshahi, D.; Ottawa Stroke Research Group (OSRG). Computed tomographic angiography and cerebral blood volume can predict final infarct volume and outcome after recanalization. Stroke 2014, 45, 2683–2688. [Google Scholar] [CrossRef]
- Hassan, A.E.; Ringheanu, V.M.; Preston, L.; Tekle, W.G. Artificial intelligence–parallel stroke workflow tool improves reperfusion rates and door-in to puncture interval. Stroke. Vasc. Interv. Neurol. 2022, 2, e000224. [Google Scholar] [CrossRef]
- Karamchandani, R.R.; Helms, A.M.; Satyanarayana, S.; Yang, H.; Clemente, J.D.; Defilipp, G.; Strong, D.; Rhoten, J.B.; Asimos, A.W. Automated detection of intracranial large vessel occlusions using Viz.ai software: Experience in a large, integrated stroke network. Brain. Behav. 2022, 13, e2808. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, J.C.; Kim, Y.; Salazar-Marioni, S.; Tariq, M.B.; Abdelkhaleq, R.; Niktabe, A.; Ballekere, A.N.; Iyyangar, A.S.; Le, M.; Azeem, H.; et al. Automated large vessel occlusion detection software and thrombectomy treatment times: A cluster randomized clinical trial. JAMA. Neurol. 2023, 80, 1182–1190. [Google Scholar] [CrossRef]
- Al-Kawaz, M.; Primiani, C.; Urrutia, V.; Hui, F. Impact of RapidAI mobile application on treatment times in patients with large vessel occlusion. J. NeuroInterv. Surg. 2022, 14, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Adhya, J.; Li, C.; Eisenmenger, L.; Cerejo, R.; Tayal, A.; Goldberg, M.; Chang, W. Positive predictive value and stroke workflow outcomes using automated vessel density (RAPID-CTA) in stroke patients: One year experience. Neuroradiol. J. 2021, 34, 476–481. [Google Scholar] [CrossRef]
- Soun, J.E.; Zolyan, A.; McLouth, J.; Elstrott, S.; Nagamine, M.; Liang, C.; Dehkordi-Vakil, F.H.; Chu, E.; Floriolli, D.; Kuoy, E.; et al. Impact of an automated large vessel occlusion detection tool on clinical workflow and patient outcomes. Front. Neurol. 2023, 14, 1179250. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Ha, S.Y.; Kang, Y.R.; Hong, H.; Kim, D.; Lee, M.; Sunwoo, L.; Ryu, W.S.; Kim, J.T. Automated detection of large vessel occlusion using deep learning: A pivotal multicenter study and reader performance study. J. Neurointerv. Surg. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- BRAINOMIX, Inc. Available online: https://www.brainomix.com/stroke/e-aspects/ (accessed on 1 January 2025.).
- Rapid AI. iSchemaView, Inc. Available online: https://www.rapidai.com/trials (accessed on 1 January 2025.).
- Herweh, C.; Ringleb, P.A.; Rauch, G.; Gerry, S.; Behrens, L.; Möhlenbruch, M.; Gottorf, R.; Richter, D.; Schieber, S.; Nagel, S. Performance of e-ASPECTS software in comparison to that of stroke physicians on assessing CT scans of acute ischemic stroke patients. Int. J. Stroke 2016, 11, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.; Sinha, D.; Day, D.; Reith, W.; Chapot, R.; Papanagiotou, P.; Warburton, E.A.; Guyler, P.; Tysoe, S.; Fassbender, K.; et al. e-ASPECTS software is non-inferior to neuroradiologists in applying the ASPECT score to computed tomography scans of acute ischemic stroke patients. Int. J. Stroke 2017, 12, 615–622. [Google Scholar] [CrossRef]
- Albers, G.W.; Wald, M.J.; Mlynash, M.; Endres, J.; Bammer, R.; Straka, M.; Maier, A.; Hinson, H.E.; Sheth, K.N.; Taylor Kimberly, W.; et al. Automated Calculation of Alberta Stroke Program Early CT Score: Validation in Patients with Large Hemispheric Infarct. Stroke 2019, 50, 3277–3279. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.; Najm, M.; Chakraborty, D.; Maraj, N.; Sohn, S.I.; Goyal, M.; Hill, M.D.; Demchuk, A.M.; Menon, B.K.; Qiu, W. Automated ASPECTS on noncontrast CT scans in patients with acute ischemic stroke using machine learning. Am. J. Neuroradiol. 2019, 40, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, G.; Kim, D.; Jung, S.; Song, S.; Hong, J.M.; Shin, D.H.; Lee, J.S. Clinical evaluation of a deep-learning model for automatic scoring of the alberta stroke program early CT score on non-contrast CT. J. Neurointerv. Surg. 2023, 16, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Goebel, J.; Stenzel, E.; Guberina, N.; Wanke, I.; Koehrmann, M.; Kleinschnitz, C.; Umutlu, L.; Forsting, M.; Moenninghoff, C.; Radbruch, A. Automated aspect rating: Comparison between the frontier aspect score software and the brainomix software. Neuroradiology 2018, 60, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Weyland, C.S.; Papanagiotou, P.; Schmitt, N.; Joly, O.; Bellot, P.; Mokli, Y.; Ringleb, P.A.; Kastrup, A.; Möhlenbruch, M.A.; Bendszus, M.; et al. Hyperdense artery sign in patients with acute ischemic stroke–automated detection with artificial intelligence-driven software. Front. Neurol. 2022, 13, 807145. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Tsang, A.C.O.; Yu, P.L.H.; Tsui, E.L.H.; Woo, P.P.S.; Lui, C.S.M.; Leung, G.K.K. Automated hierarchy evaluation system of large vessel occlusion in acute ischemia stroke. Front. Neuroinform. 2020, 14, 13. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, D.H.; Jung, S.M.; Park, G.H.; Song, S.H.; Choi, D.H.; Shin, D.H.; Kim, M.J.; Lee, J.S. Clinical Evaluation of Deep-Learning Model for Classifying Stroke Patients of Emergent Large Vessel Occlusion on Non-Contrast CT; RSNA: Chicago, IL, USA, 2023; Volume 11, pp. 26–30, 2023-SP-11967-RSNA. [Google Scholar]
- Lee, S.J.; Kim, D.H.; Choi, D.H.; Lim, Y.S.; Park, G.H.; Jung, S.M.; Song, S.H.; Hong, J.M.; Shin, D.H.; Kim, M.J.; et al. Deep learning-based decision support system to predict emergent large vessel occlusion using non-contrast computed tomography. Sci. Rep. 2024. submitted. [Google Scholar]
- Kunz, W.G.; Hunink, M.G.; Almekhlafi, M.A.; Menon, B.K.; Saver, J.L.; Dippel, D.W.J.; Majoie, C.B.L.M.; Jovin, T.G.; Davalos, A.; Bracard, S.; et al. Public health and cost consequences of time delays to thrombectomy for acute ischemic stroke. Neurology 2020, 95, e2465–e2475. [Google Scholar] [CrossRef]
- Kunz, W.G.; Almekhlafi, M.A.; Menon, B.K.; Saver, J.; Hunink, M.G.; Dippel, D.W.J.; Majoie, C.B.L.M.; Liebeskind, D.S.; Jovin, T.G.; Davalos, A.; et al. Public Health and Cost Benefits of Successful Reperfusion After Thrombectomy for Stroke. Stroke 2020, 51, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.E. New Technology Add-On Payment (NTAP) for Viz LVO: A win for stroke care. J. Neurointerv. Surg. 2021, 13, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Westwood, M.; Ramaekers, B.; Grimm, S.; Armstrong, N.; Wijnen, B.; Ahmadu, C.; de Kock, S.; Noake, C.; Joore, M. Software with artificial intelligence-derived algorithms for analysing CT brain scans in people with a suspected acute stroke: A systematic review and cost-effectiveness analysis. Health Technol. Assess. 2024, 28, 1–204. [Google Scholar] [CrossRef] [PubMed]
- Cabitza, F.; Rasoini, R.; Gensini, G.F. Unintended consequences of machine learning in medicine. JAMA 2017, 318, 517–518. [Google Scholar] [CrossRef] [PubMed]
- Shlobin, N.A.; Baig, A.A.; Waqas, M.; Patel, T.R.; Dossani, R.H.; Wilson, M.; Cappuzzo, J.M.; Siddiqui, A.H.; Tutino, V.M.; Levy, E.I. Artificial intelligence for large-vessel occlusion stroke: A systematic review. World. Neurosurg. 2022, 159, 207–220.e1. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.A.R.; Qureshi, S.M.; Qureshi, H.A.; Shah, S.U.R.; Shiwlani, A.; Ahmad, A. Artificial Intelligence in Stroke Care: Enhancing Diagnostic Accuracy, Personalizing Treatment, and Addressing Implementation Challenges. Int. J. Appl. Res. Sustain. Sci. 2024, 2, 855–886. [Google Scholar] [CrossRef]


| Variables | Pre-AI (n = 82) | Post-AI (n = 48) | p-Value |
|---|---|---|---|
| Age (years) | 73 [57 to 81] | 71 [60 to 79] | 0.773 |
| Sex, Male (%) | 49 (60) | 32 (67) | 0.362 |
| Past medical history (%) | |||
| Hypertension | 52 (63) | 28 (58) | 0.164 |
| Diabetes | 27 (33) | 13 (27) | 0.101 |
| Chronic kidney disease | 2 (2) | 1 (2) | 1.000 |
| Atrial fibrillation | 26 (32) | 15 (31) | 0.989 |
| NIHSS at presentation (scores) | 13 [10 to 16] | 13 [9 to 16] | 0.973 |
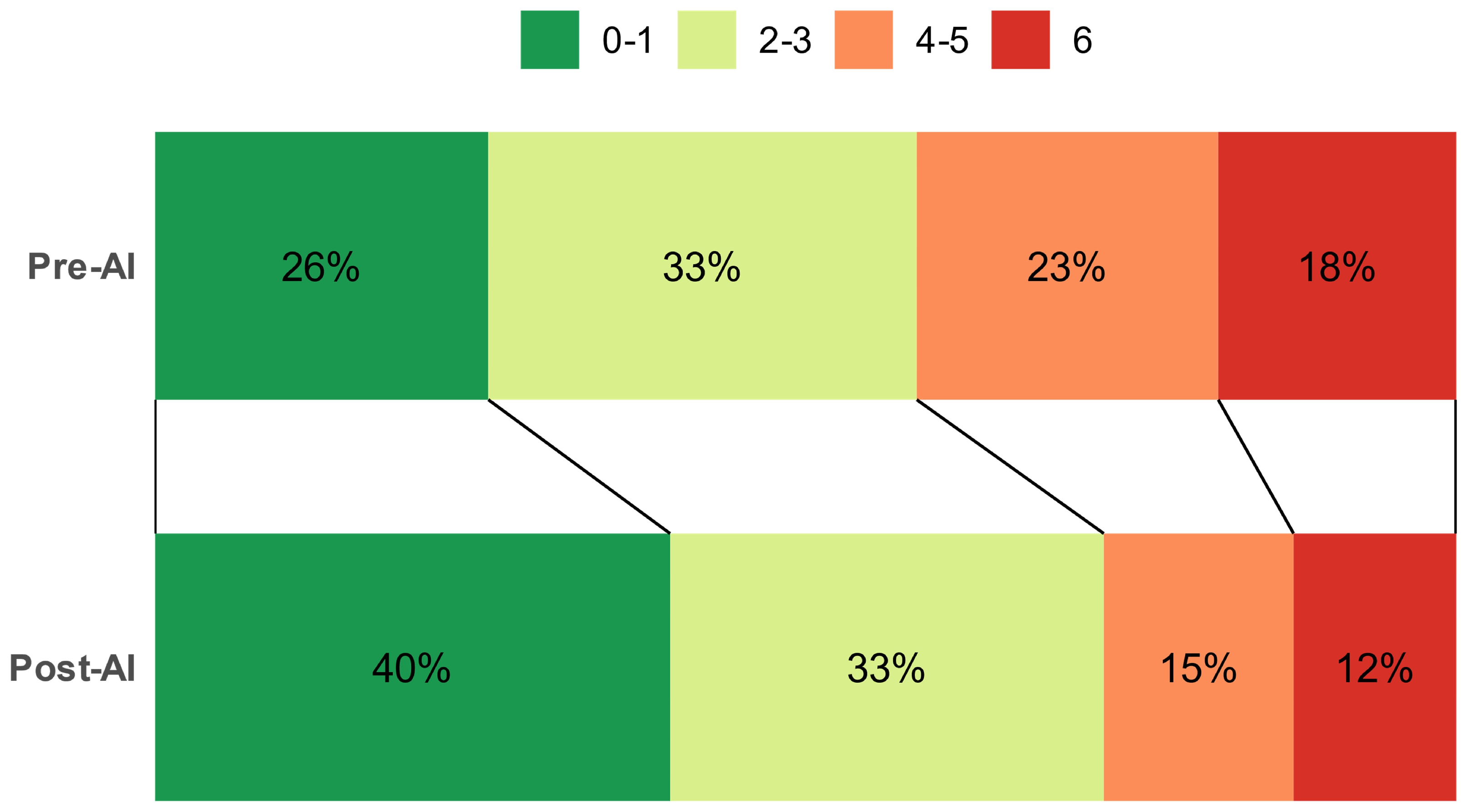
| mRS at presentation (scores) | 5 [4 to 5] | 4 [4 to 5] | <0.001 |
| Door to CT scan (minutes) | 17 [12 to 29] | 18 [13 to 21] | 0.434 |
| Clot location (%) | 0.029 | ||
| Internal carotid artery | 21 (26) | 10 (21) | |
| M1 middle cerebral artery | 61 (74) | 38 (79) | |
| Intervention (%) | 0.012 | ||
| IV tPA alone | 12 (15) | 13 (27) | |
| EVT alone | 26 (32) | 21 (44) | |
| IV tPA + EVT | 44 (54) | 14 (29) |
| Variables | Pre-AI (n = 82) | Post-AI (n = 48) | p-Value |
|---|---|---|---|
| Primary outcomes: | |||
| Door to intervention (minutes) | |||
| Door to IV tPA | 56 [47 to 60] | 52 [43 to 57] | 0.072 |
| Door to EVT | 154 [129 to 177] | 146 [127 to 164] | 0.137 |
| Secondary outcomes: | |||
| Detailed treatment time (minutes) | |||
| CT scan to NR | 23 [18 to 37] | 19 [14 to 23] | <0.001 |
| CT scan to intervention | |||
| CT scan to IV tPA | 37 [31 to 47] | 33 [26 to 42] | 0.104 |
| CT scan to EVT | 132 [112 to 160] | 126 [111 to 146] | 0.191 |
| Changes in scores from initial status (score) | |||
| ∆NIHSS (discharge—presentation) | −5 [−9 to −1] | −7 [−10 to −4] | 0.106 |
| ∆mRS (discharge—presentation) | −1 [−2 to 0] | −1 [−2 to 0] | 0.721 |
| ∆mRS (3-month follow-up—presentation) | −2 [−3 to 0] | −2 [−3 to −1] | 0.462 |
| Variables | Pre-AI (n = 82) | Post-AI (n = 48) | p-Value |
|---|---|---|---|
| Primary outcomes: | |||
| Door to intervention (minutes) | |||
| Door to IV tPA | 60.8 (±2.8) | 51.9 (±4.2) | 0.058 |
| Door to EVT | 169.1 (±8.7) | 138.9 (±11.9) | 0.025 |
| Secondary outcomes: | |||
| Detailed treatment time (minutes) | |||
| CT scan to NR | 31.2 (±3.9) | 14.8 (±5.1) | 0.005 |
| CT scan to intervention | |||
| CT scan to IV tPA | 40.1 (±2.1) | 33.8 (±3.2) | 0.072 |
| CT scan to EVT | 147.8 (±8.1) | 118.5 (±11.2) | 0.020 |
| Changes in scores from initial status (score) | |||
| ∆NIHSS (discharge—presentation) | −1.4 (±1.5) | −5.7 (±1.9) | 0.044 |
| ∆mRS (discharge—presentation) | −1.2 (±0.2) | −1.1 (±0.3) | 0.952 |
| ∆mRS (3-month follow-up—presentation) | −1.6 (±0.2) | −1.7 (±0.3) | 0.645 |
| Variables | Simple Regression | Multiple Regression | ||
|---|---|---|---|---|
| Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | |
| Primary outcomes: | ||||
| Door to intervention (minutes) | ||||
| Door to IV tPA | −9.2 (−17.8 to −0.5) | 0.041 | −8.9 (−18.0 to 0.2) | 0.058 |
| Door to EVT | −29.6 (−55.4 to −3.9) | 0.026 | −30.2 (−56.1 to −4.3) | 0.025 |
| Secondary outcomes: | ||||
| Detailed treatment time (minutes) | ||||
| CT scan to NR | −15.5 (−26.6 to −4.5) | 0.007 | −16.4 (−27.6 to −5.3) | 0.005 |
| CT scan to intervention | ||||
| CT scan to IV tPA | −6.1 (−12.6 to 0.5) | 0.074 | −6.4 (−13.2 to 0.5) | 0.072 |
| CT scan to EVT | −28.1 (−52.5 to −3.7) | 0.026 | −29.3 (−53.6 to −5.0) | 0.020 |
| Changes in scores from initial status (score) | ||||
| ∆NIHSS (discharge—presentation) | −4.8 (−9 to −0.6) | 0.027 | −4.3 (−8.3 to −0.2) | 0.044 |
| ∆mRS (discharge—presentation) | 0.0 (−0.6 to 0.6) | 0.916 | 0.0 (−0.6 to 0.6) | 0.952 |
| ∆mRS (3-month follow-up—presentation) | −0.2 (−0.9 to 0.5) | 0.513 | −0.2 (−0.9 to 0.5) | 0.645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.S.; Kim, E.; Choi, W.S.; Yang, H.J.; Moon, J.Y.; Jang, J.H.; Cho, J.; Choi, J.; Woo, J.-H. Non-Contrast Computed Tomography-Based Triage and Notification for Large Vessel Occlusion Stroke: A Before and After Study Utilizing Artificial Intelligence on Treatment Times and Outcomes. J. Clin. Med. 2025, 14, 1281. https://doi.org/10.3390/jcm14041281
Lim YS, Kim E, Choi WS, Yang HJ, Moon JY, Jang JH, Cho J, Choi J, Woo J-H. Non-Contrast Computed Tomography-Based Triage and Notification for Large Vessel Occlusion Stroke: A Before and After Study Utilizing Artificial Intelligence on Treatment Times and Outcomes. Journal of Clinical Medicine. 2025; 14(4):1281. https://doi.org/10.3390/jcm14041281
Chicago/Turabian StyleLim, Yong Su, Eunji Kim, Woo Sung Choi, Hyuk Jun Yang, Jong Youn Moon, Jae Ho Jang, Jinseong Cho, Jeayeon Choi, and Jae-Hyug Woo. 2025. "Non-Contrast Computed Tomography-Based Triage and Notification for Large Vessel Occlusion Stroke: A Before and After Study Utilizing Artificial Intelligence on Treatment Times and Outcomes" Journal of Clinical Medicine 14, no. 4: 1281. https://doi.org/10.3390/jcm14041281
APA StyleLim, Y. S., Kim, E., Choi, W. S., Yang, H. J., Moon, J. Y., Jang, J. H., Cho, J., Choi, J., & Woo, J.-H. (2025). Non-Contrast Computed Tomography-Based Triage and Notification for Large Vessel Occlusion Stroke: A Before and After Study Utilizing Artificial Intelligence on Treatment Times and Outcomes. Journal of Clinical Medicine, 14(4), 1281. https://doi.org/10.3390/jcm14041281






