Cutaneous Infections Caused by Trichophyton indotineae: Case Series and Systematic Review
Abstract
1. Introduction
2. Relevant Sections
2.1. Case Reports
2.1.1. Patient 1
2.1.2. Patient 2
2.1.3. Patient 3
2.1.4. Patient 4
2.2. Identification of Isolates and Antifungal Susceptibility Testing
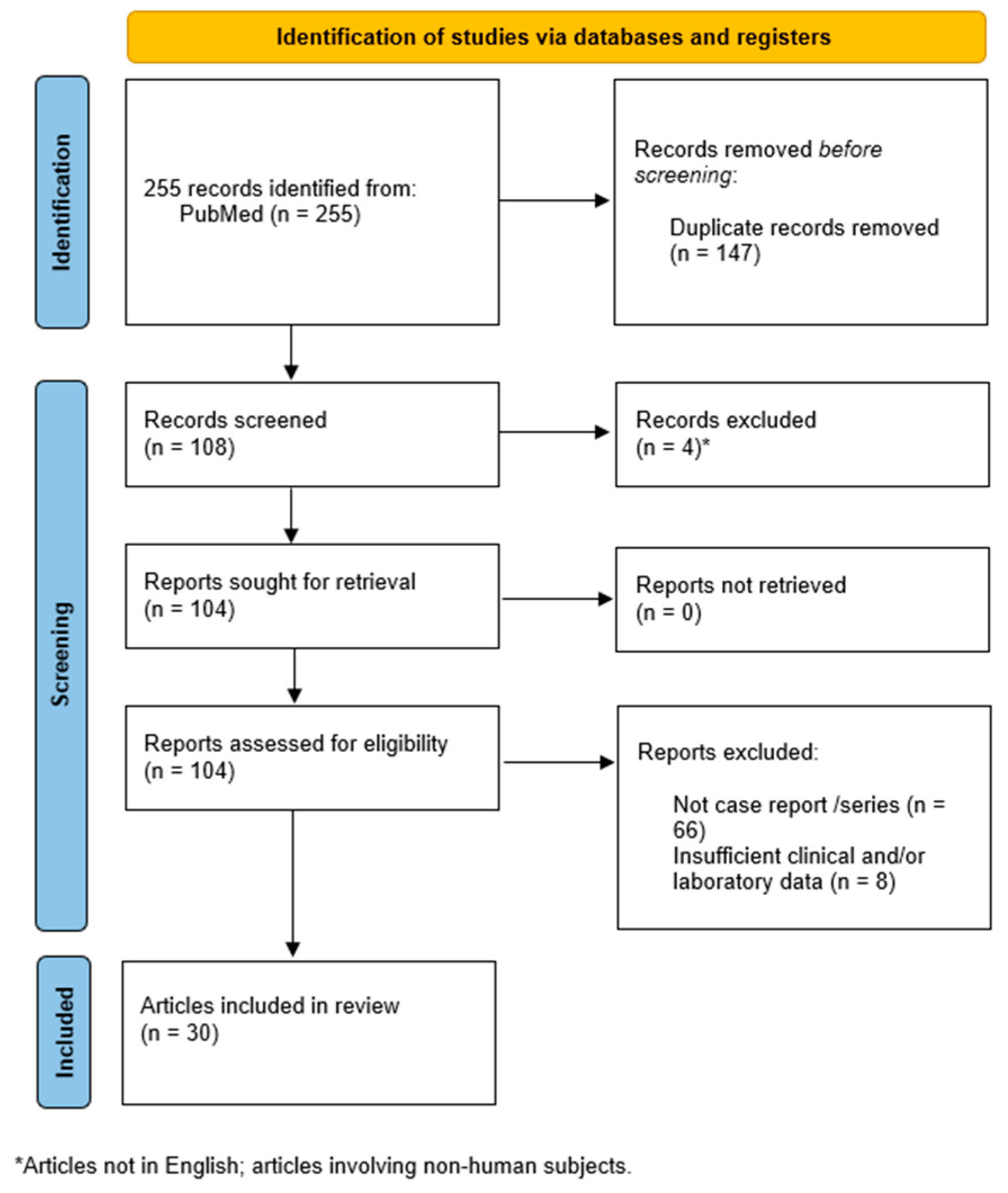
2.3. Systematic Review of Case Reports
2.3.1. Methods
2.3.2. Results
3. Discussion
4. Conclusions
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HTLV1 | Human T Leukocytes Virus |
| HIV | Human immunodeficiency virus |
| ITS | Internal transcribed spacer |
| WHO | World Health Organization |
References
- Ortiz, B.; Ballesteros-Monrreal, M.G.; Rosales-Tamashiro, J.; Bush, M.; Salmanton-García, J.; Fontecha, G. global insights and trends in research on dermatophytes and dermatophytosis: A bibliometric analysis. Mycoses 2024, 67, e13803. [Google Scholar] [CrossRef]
- Kruithoff, C.; Gamal, A.; McCormick, T.S.; Ghannoum, M.A. Dermatophyte infections worldwide: Increase in incidence and associated antifungal resistance. Life 2023, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.C.; Caplan, A.S.; Elewski, B.; Gold, J.A.W.; Lockhart, S.R.; Smith, D.J.; Lipner, S.R. Expert panel review of skin and hair dermatophytoses in an era of antifungal resistance. Am. J. Clin. Dermatol. 2024, 25, 359–389. [Google Scholar] [CrossRef]
- Gupta, A.K.; Venkataraman, M.; Hall, D.C.; Cooper, E.A.; Summerbell, R.C. The emergence of Trichophyton indotineae: Implications for clinical practice. Int. J. Dermatol. 2023, 62, 857–861. [Google Scholar] [CrossRef]
- Gupta, A.K.; Polla, R.S.; Wang, T.; Bakotic, W.L.; Shemer, A. Mapping the global spread of T. indotineae: An update on antifungal resistance, mutations, and strategies for effective management. Mycopathologia 2024, 189, 45. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Mann, A.; Polla Ravi, S.; Talukder, M.; Lincoln, S.A.; Foreman, H.C.; Kaplan, B.; Galili, E.; Piguet, V.; et al. Antifungal resistance in dermatophytes—Review of the epidemiology, diagnostic challenges and treatment strategies for managing Trichophyton indotineae infections. Expert. Rev. Anti Infect. Ther. 2024, 22, 739–751. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synthesis. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Dellière, S.; Joannard, B.; Benderdouche, M.; Mingui, A.; Gits-Muselli, M.; Hamane, S.; Alanio, A.; Petit, A.; Gabison, G.; Bagot, M.; et al. Emergence of difficult-to-treat tinea corporis caused by Trichophyton mentagrophytes complex isolates. Emerg. Infect. Dis. 2022, 28, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Gueneau, R.; Joannard, B.; Haddad, N.; Alby, F.; Jullien, V.; Schlatter, J.; Cotteret, C.; Bougnoux, M.E.; Lanternier, F.; Laroche, L.; et al. Extensive dermatophytosis caused by terbinafine-resistant Trichophyton indotineae, successfully treated with topical voriconazole. Int. J. Antimicrob. Agents 2022, 60, 106677. [Google Scholar] [CrossRef]
- Ngo, T.M.C.; Ton Nu, P.A.; Le, C.C.; Ha, T.N.T.; Do, T.B.T.; Tran Thi, G. First detection of Trichophyton indotineae causing tinea corporis in Central Vietnam. Med. Mycol. Case Rep. 2022, 36, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Posso-De Los Rios, C.J.; Tadros, E.; Summerbell, R.C.; Scott, J.A. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in Canadian patients. J. Cutan. Med. Surg. 2022, 26, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.S.; Chaturvedi, S.; Zhu, Y.; Todd, G.C.; Yin, L.; Lopez, A.; Travis, L.; Smith, D.J.; Chiller, T.; Lockhart, S.R.; et al. Notes from the field: First reported U.S. cases of tinea caused by Trichophyton indotineae—New York City; December 2021–March 2023. MMWR Morb. Mortal Wkly Rep. 2023, 72, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Crotti, S.; Cruciani, D.; Spina, S.; Piscioneri, V.; Natalini, Y.; Pezzotti, G.; Sabbatucci, M.; Papini, M. A terbinafine sensitive Trichophyton indotineae strain in Italy: The first clinical case of tinea corporis and onychomycosis. J. Fungi 2023, 9, 865. [Google Scholar] [CrossRef]
- Dashti, Y.; Alobaid, K.; Al-Rashidi, S.; Dashti, M.; AbdulMoneim, M.H.; Al-Enezi, M.; Abou-Chakra, N.; Jørgensen, K.M. Autochthonous case of Trichophyton indotineae in Kuwait. J. Mycol. Med. 2023, 33, 101432. [Google Scholar] [CrossRef]
- Durdu, M.; Kandemir, H.; Karakoyun, A.S.; Ilkit, M.; Tang, C.; de Hoog, S. First terbinafine-resistant Trichophyton indotineae isolates with phe397leu and/or thr414his mutations in Turkey. Mycopathologia 2023, 188, 2. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Long, X.; Hu, W.; Zhu, J.; Jiang, Y.; Ahmed, S.; de Hoog, G.S.; Liu, W.; Jiang, Y. The epidemic of the multiresistant dermatophyte Trichophyton indotineae has reached China. Front. Immunol. 2023, 13, 1113065. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Song, G.; Mei, H.; Zheng, H.; Tang, C.; de Hoog, S.; Li, X.; She, X.; Liu, W.; Liang, G. The domestic isolation of terbinafine- and itraconazole-resistant Trichophyton indotineae in Chinese Mainland. Mycopathologia 2023, 188, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.; Santiso, G.; Romero, M.; Bonifaz, A.; Fernandez, M.; Marin, E. First case report of tinea corporis caused by Trichophyton indotineae in Latin America. Med. Mycol. Case Rep. 2023, 41, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Toutous Trellu, L.; Fontao, L.; Ninet, B. Towards an early clinical and biological resistance detection in dermatophytosis: About 2 cases of Trichophyton indotineae. J. Fungi 2023, 9, 733. [Google Scholar] [CrossRef]
- Thakur, R.; Kushwaha, P.; Kalsi, A.S. Tinea universalis due to Trichophyton indotineae in an adult male. Indian. J. Med. Microbiol. 2023, 46, 100476. [Google Scholar] [CrossRef] [PubMed]
- Villa-Gonzalez, J.M.; Pascual Ares, M.; López-Soria, L.M.; Gonzalez-Hermosa, M.R.; Gardeazabal García, J.; Lasa Elgezua, O. Extensive tinea corporis caused by Trichophyton indotineae: Report of a case in Spain. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasouli, A.; Borman, A.M.; Johnson, E.M.; Hay, R.J.; Arias, M. Terbinafine-resistant Trichophyton indotineae causing extensive dermatophytosis in a returning traveller. Clin. Exp. Dermatol. 2024, 49, 635–637. [Google Scholar] [CrossRef]
- Bui, T.S.; Chan, J.B.; Katz, K.A. Extensive multidrug-resistant dermatophytosis from Trichophyton indotineae. Cutis 2024, 113, 20–23. [Google Scholar] [CrossRef]
- Caplan, A.S.; Todd, G.C.; Zhu, Y.; Sikora, M.; Akoh, C.C.; Jakus, J.; Lipner, S.R.; Graber, K.B.; Acker, K.P.; Morales, A.E.; et al. Clinical course; antifungal susceptibility; and genomic sequencing of Trichophyton indotineae. JAMA Dermatol. 2024, 160, 701–709. [Google Scholar] [CrossRef]
- Caplan, A.S.; Chaturvedi, S.; Todd, G.; Sikora, M.; Ugwu-Dike, P.O.; Pahalyants, V.; Taiwo, D.; Gold, J.A.W. Response to “Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae”; focus on griseofulvin. J. Am. Acad. Dermatol. 2024, 92, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Carroll, E.; Leahy, M.; Stanciu, M.; Laing, M. Trichophyton indotineae: First case in Ireland and response to topical griseofulvin. Clin. Exp. Dermatol. 2024, 49, 1707–1708. [Google Scholar] [CrossRef]
- Chua, K.Y.; Halliday, C.L.; Chen, S.C.; Koning, S.; Pawlikowski, J.; du Cros, P.; Korman, T.M. Treatment-resistant tinea caused by Trichophyton indotineae in Australia. Med. J. Aust. 2024, 221, 192–194. [Google Scholar] [CrossRef]
- Clemente Hernández, B.; Muelas Rives, I.; Aldea Manrique, B.; Hernández Aragües, I.; López Gómez, C.; Gracia Cazaña, T. Comment on: Report of terbinafine-resistant Trichophyton indotineae in a pregnant patient—A diagnostic and therapeutic challenge. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gonzalez, P.; García-Donoso, C.; Garcia-Mouronte, E.; Alejo Fernández-Baillo, J.L.; De Nicolas-Ruanes, B.; Berna-Rico, E.; Perez-Ayala, A.; Gomez-Garcia de la Pedrosa, E. Trichophyton indotineae extensive tinea: Successful posaconazole treatment in four cases from Spain. Clin. Exp. Dermatol. 2024, 50, llae417. [Google Scholar] [CrossRef] [PubMed]
- Fukada, N.; Kobayashi, H.; Nakazono, M.; Ohyachi, K.; Takeda, A.; Yaguchi, T.; Okada, M.; Sato, T. A case of tinea faciei due to Trichophyton indotineae with steroid rosacea related to topical over-the-counter drugs purchased outside of Japan. Med. Mycol. J. 2024, 65, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Galili, E.; Lubitz, I.; Shemer, A.; Astman, N.; Pevzner, K.; Gazit, Z.; Segal, O.; Lyakhovitsky, A.; Halevi, S.; Baum, S.; et al. First reported cases of terbinafine-resistant Trichophyton indotineae isolates in Israel: Epidemiology, clinical characteristics and response to treatment. Mycoses 2024, 67, 13812. [Google Scholar] [CrossRef]
- Mochizuki, T.; Anzawa, K.; Bernales-Mendoza, A.M.; Shimizu, A. Case of tinea corporis caused by a terbinafine-sensitive Trichophyton indotineae strain in a Vietnamese worker in Japan. J. Dermatol. 2025, 52, 163–166. [Google Scholar] [CrossRef]
- Smith, A.; Wong-O'Brien, B.; Lieberman, J.A.; Cookson, B.T.; Grinager, E.; Truong, T.T. The brief case: A case of tinea corporis caused by drug-resistant Trichophyton indotineae identified by broad-range fungal DNA sequencing. J. Clin. Microbiol. 2024, 62, 0023424. [Google Scholar] [CrossRef] [PubMed]
- Spivack, S.; Gold, J.A.W.; Lockhart, S.R.; Anand, P.; Quilter, L.A.S.; Smith, D.J.; Bowen, B.; Gould, J.M.; Eltokhy, A.; Gamal, A.; et al. Potential sexual transmission of antifungal-resistant Trichophyton indotineae. Emerg. Infect. Dis. 2024, 30, 807–809. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ishikura, Y.; Takahara, M.; Kano, R.; Nakahara, T. The first Japanese case of intractable tinea corporis caused by Trichophyton indotineae. J. Dermatol. 2024, in press. [Google Scholar] [CrossRef]
- Tan, T.Y.; Wang, Y.S.; Wong, X.Y.A.; Rajandran, P.; Tan, M.G.; Tan, A.L.; Tan, Y.E. First reported case of Trichophyton indotineae dermatophytosis in Singapore. Pathology 2024, 56, 909–913. [Google Scholar] [CrossRef]
- Xu, Z.; Caplan, A.S. Extensive tinea corporis and tinea cruris from Trichophyton indotineae. N. Engl. J. Med. 2024, 391, 1837. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chowdhary, A.; Singh, A.; Kaur, A.; Khurana, A. The emergence and worldwide spread of the species Trichophyton indotineae causing difficult-to-treat dermatophytosis: A new challenge in the management of dermatophytosis. PLoS Pathog. 2022, 18, 1010795. [Google Scholar] [CrossRef]
- Ferreira, C.B.; Lisboa, C. A systematic review on the emergence of terbinafine-resistant Trichophyton indotineae in Europe: Time to act? J. Eur. Acad. Dermatol. Venereol. 2025, 39, 364–376. [Google Scholar] [CrossRef]
- Khurana, A.; Sharath, S.; Sardana, K.; Chowdhary, A. Clinico-mycological and therapeutic updates on cutaneous dermatophytic infections in the era of Trichophyton indotineae. J. Am. Acad. Dermatol. 2024, 91, 315–323. [Google Scholar] [CrossRef]
- Yamada, T.; Yaguchi, T.; Maeda, M.; Alshahni, M.M.; Salamin, K.; Guenova, E.; Feuermann, M.; Monod, M. Gene amplification of CYP51B: A new mechanism of resistance to azole compounds in Trichophyton indotineae. Antimicrob. Agents Chemother. 2022, 66, 0005922. [Google Scholar] [CrossRef]
- Yamada, T.; Maeda, M.; Nagai, H.; Salamin, K.; Chang, Y.-T.; Guenova, E.; Feuermann, M.; Monod, M. Two different types of tandem sequences mediate the overexpression of TinCYP51B in azole-resistant Trichophyton indotineae. Antimicrob. Agents Chemother. 2023, 67, 0093323. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Datta, D. Trichophyton: Changing nomenclature and practical implications. Indian. J. Dermatol. 2023, 68, 503–507. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae—An emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—A multidimensional perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef]
- Jabet, A.; Normand, A.C.; Brun, S.; Dannaoui, E.; Bachmeyer, C.; Piarroux, R.; Hennequin, C.; Moreno-Sabater, A. Trichophyton indotineae, from epidemiology to therapeutic. J. Mycol. Med. 2023, 33, 101383. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Jørgensen, K.M.; Guinea, J.; Lagrou, K.; Chryssanthou, E.; Hayette, M.P.; Barchiesi, F.; Lass-Flörl, C.; Hamal, P.; Dannaoui, E.; et al. Multicentre validation of a EUCAST method for the antifungal susceptibility testing of microconidia-forming dermatophytes. J. Antimicrob. Chemother. 2020, 75, 1807–1819. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Jørgensen, K.M.; Guinea, J.; Lagrou, K.; Chryssanthou, E.; Hayette, M.P.; Barchiesi, F.; Lass-Flörl, C.; Hamal, P.; Dannaoui, E.; et al. Comment on: Multicentre validation of a EUCAST method for the antifungal susceptibility testing of microconidia-forming dermatophytes. J. Antimicrob. Chemother. 2022, 77, 1212–1213. [Google Scholar] [CrossRef] [PubMed]
- Normand, A.C.; Moreno-Sabater, A.; Jabet, A.; Hamane, S.; Cremer, G.; Foulet, F.; Blaize, M.; Dellière, S.; Bonnal, C.; Imbert, S.; et al. MALDI-TOF mass spectrometry online identification of Trichophyton indotineae using the MSI-2 application. J. Fungi 2022, 8, 1103. [Google Scholar] [CrossRef]
- De Paepe, R.; Normand, A.C.; Uhrlaß, S.; Nenoff, P.; Piarroux, R.; Packeu, A. Resistance profile; terbinafine resistance screening and MALDI-TOF MS identification of the emerging pathogen Trichophyton indotineae. Mycopathologia 2024, 189, 29. [Google Scholar] [CrossRef]
- Singh, A.; Singh, P.; Dingemans, G.; Meis, J.F.; Chowdhary, A. Evaluation of DermaGenius® resistance real-time polymerase chain reaction for rapid detection of terbinafine-resistant Trichophyton species. Mycoses 2021, 64, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.L.; Normand, A.C.; Jabet, A.; Brun, S.; Delliere, S.; Cremer, G.; Foulet, F.; Ayachi, A.; Imbert, S.; Hennequin, C.; et al. Reliability of a terbinafine agar containing method for the screening of dermatophyte resistance. Med. Mycol. 2023, 61, myad043. [Google Scholar] [CrossRef]
- Rouhi, F.; Aboutalebian, S.; Rezaei-Matehkolaei, A.; Jahanshiri, Z.; Shidfar, M.R.; Chadeganipour, A.S.; Shadzi, S.; Kharazi, M.; Erami, M.; Mirhendi, H. Development and evaluation of SYBR green real-time PCR for rapid and specific identification of Trichophyton indotineae. Mycoses 2025, 68, e70015. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Li, X.; Li, R.; Wen, H.; Gu, J.; Ran, Y.; Xi, L.; Li, F.; Zhang, Q.; Cao, C.; et al. Chinese expert consensus on management of antifungal-resistant dermatophytosis (2024 edition). Mycoses 2024, 67, e13785. [Google Scholar] [CrossRef]
- Sonego, B.; Corio, A.; Mazzoletti, V.; Zerbato, V.; Benini, A.; di Meo, N.; Zalaudek, I.; Stinco, G.; Errichetti, E.; Zelin, E. Trichophyton indotineae; an emerging drug-resistant dermatophyte: A review of the treatment options. J. Clin. Med. 2024, 13, 3558. [Google Scholar] [CrossRef]
- Song, G.; Kong, X.; Li, X.; Liu, W.; Liang, G. Prior selection of itraconazole in the treatment of recalcitrant Trichophyton indotineae infection: Real-world results from retrospective analysis. Mycoses 2024, 67, e13663. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, P.; Prigitano, A.; Sechi, A.; Boneschi, V.; Germiniasi, F.; Esposto, M.C.; Romanò, L.; Pavan, G.; Matinato, C.; Veraldi, S.; et al. Report of terbinafine resistant Trichophyton spp. in Italy: Clinical presentations; molecular identification; antifungal susceptibility testing and mutations in the squalene epoxidase gene. Mycoses 2023, 66, 680–687. [Google Scholar] [CrossRef]
- McCormick, T.S.; Ghannoum, M. Time to think antifungal resistance increased antifungal resistance exacerbates the burden of fungal infections including resistant dermatomycoses. Pathog. Immun. 2024, 8, 158–176. [Google Scholar] [CrossRef]

| Samples/Code Name | ITS Sequence Type | Nucleotide Identity (%) | Query Cover | Accession Number |
|---|---|---|---|---|
| Case 1/IG0a | ITSa | 100% | 100% | MN460830.1; MN460831.1.; MN460832.1; MN460833.1; OR416997.1 |
| Case 2/IG0b | ITSa | 100% | 100% | |
| Case 3/IG1 | ITSa | 100% | 100% | |
| Case 4/IG2 | ITSb | 99.84% | 99% |
| A long-lasting, relapsing, or recurrent itching dermatosis |
| Recent travel to a region with a high prevalence of T. indotineae infection or contact with a person who recently traveled to an endemic area (e.g., Asian countries, especially South Asia) |
| Evidence of diffuse skin lesions suggestive of tinea corporis (mainly when affecting the lower part of the body) and/or tinea cruris and/or (less commonly) tinea faciei |
| Prior culture identification of T. mentagrophytes or T. interdigitale in patients with compatible clinical findings and medical history |
| Recalcitrant dermatophytosis with inadequate response to topical antifungals and/or to oral antifungal medications (in particular oral terbinafine), used at standard doses and durations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Marco, A.; Liguori, G.; Cafarchia, C.; Triggiano, F.; Ciccarese, G.; Poli, M.A.; Ambrogio, F.; Bonamonte, D.; Cassano, N.; Vena, G.A.; et al. Cutaneous Infections Caused by Trichophyton indotineae: Case Series and Systematic Review. J. Clin. Med. 2025, 14, 1280. https://doi.org/10.3390/jcm14041280
De Marco A, Liguori G, Cafarchia C, Triggiano F, Ciccarese G, Poli MA, Ambrogio F, Bonamonte D, Cassano N, Vena GA, et al. Cutaneous Infections Caused by Trichophyton indotineae: Case Series and Systematic Review. Journal of Clinical Medicine. 2025; 14(4):1280. https://doi.org/10.3390/jcm14041280
Chicago/Turabian StyleDe Marco, Aurora, Giovanni Liguori, Claudia Cafarchia, Francesco Triggiano, Giulia Ciccarese, Melita Anna Poli, Francesca Ambrogio, Domenico Bonamonte, Nicoletta Cassano, Gino Antonio Vena, and et al. 2025. "Cutaneous Infections Caused by Trichophyton indotineae: Case Series and Systematic Review" Journal of Clinical Medicine 14, no. 4: 1280. https://doi.org/10.3390/jcm14041280
APA StyleDe Marco, A., Liguori, G., Cafarchia, C., Triggiano, F., Ciccarese, G., Poli, M. A., Ambrogio, F., Bonamonte, D., Cassano, N., Vena, G. A., Foti, C., & Caggiano, G. (2025). Cutaneous Infections Caused by Trichophyton indotineae: Case Series and Systematic Review. Journal of Clinical Medicine, 14(4), 1280. https://doi.org/10.3390/jcm14041280









