Patient with Vulnerable Coronary Plaque and Treatment with Evolocumab: A Clinical Case
Abstract
1. Introduction
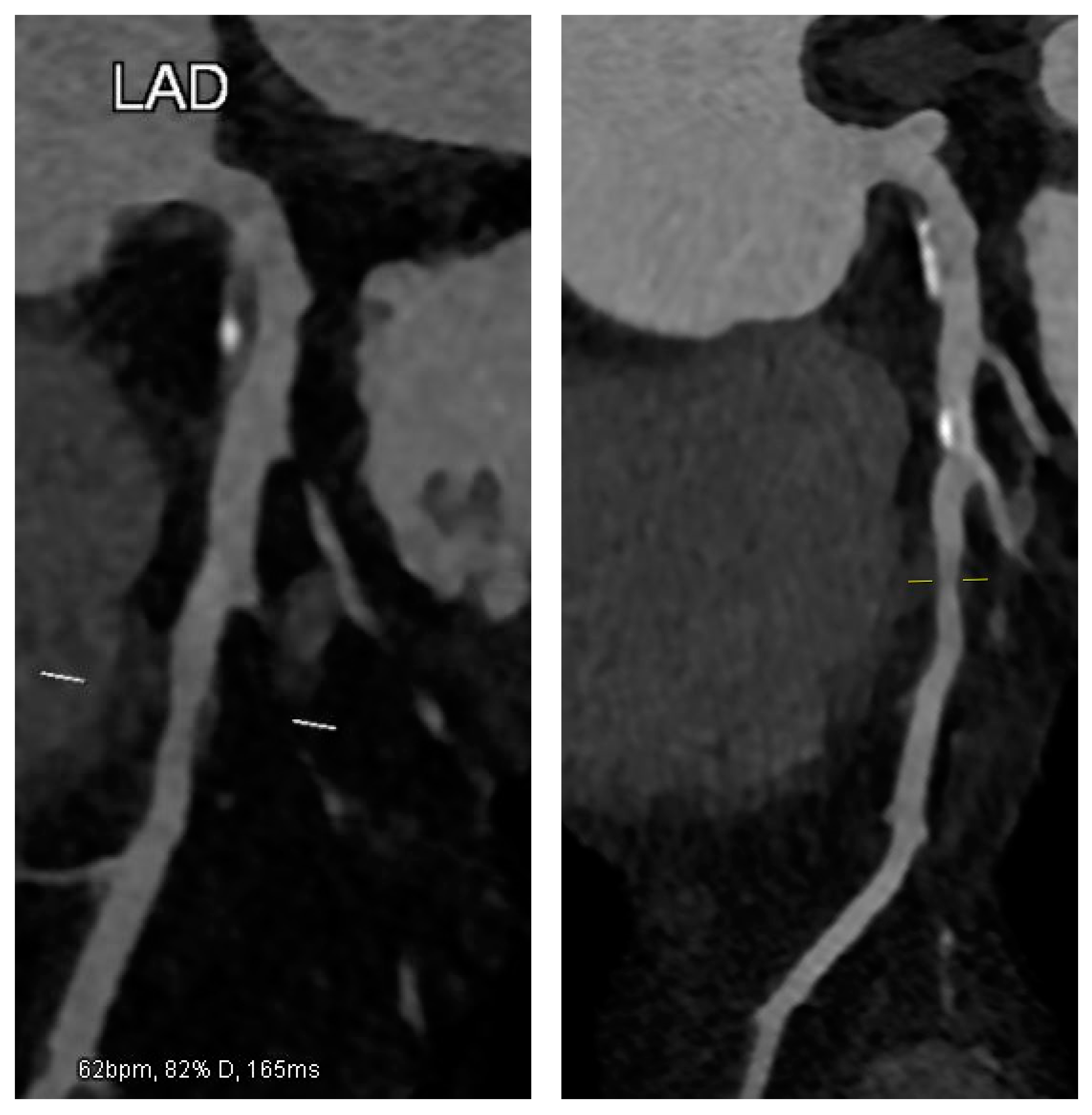
2. Case Presentation
3. Discussion
4. Conclusions
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAD | Coronary artery disease |
| CCTA | Coronary CT angiography |
| Cx | Circumflex artery |
| LAD | Left anterior descending artery |
| MI | Myocardial infarction |
| PCI | Percutaneous coronary intervention |
References
- Gao, Y.; Lou, Y.; Liu, Y.; Wu, S.; Xi, Z.; Wang, X.; Zhou, Y.; Liu, W. The relationship between residual cholesterol risk and plaque characteristics in patients with acute coronary syndrome: Insights from an optical coherence tomography study. Atherosclerosis 2021, 317, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Fang, C.; Xu, X.; Xing, L.; Sun, S.; Peng, C.; Yin, Y.; Lei, F.; Wang, Y.; Li, L.; et al. Identification of High-Risk Coronary Lesions by 3-Vessel Optical Coherence Tomography. J. Am. Coll. Cardiol. 2023, 81, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Sarai, M.; Harigaya, H.; Anno, H.; Inoue, K.; Hara, T.; Naruse, H.; Ishii, J.; Hishida, H.; Wong, N.D.; et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J. Am. Coll. Cardiol. 2009, 54, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Pasterkamp, G. Requiem for the ‘vulnerable plaque’. Eur. Heart J. 2015, 36, 2984–2987. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Kataoka, Y.; Nissen, S.E.; Prati, F.; Windecker, S.; Puri, R.; Hucko, T.; Aradi, D.; Herrman, J.-P.R.; Hermanides, R.S.; et al. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc. Imaging 2022, 15, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Emami, H.; Takx, R.A.; Mayrhofer, T.; Janjua, S.; Park, J.; Pursnani, A.; Tawakol, A.; Lu, M.T.; Ferencik, M.; Hoffmann, U. Nonobstructive Coronary Artery Disease by Coronary CT Angiography Improves Risk Stratification and Allocation of Statin Therapy. JACC Cardiovasc. Imaging 2017, 10, 1031–1038. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadamitzky, M.; Achenbach, S.; Al-Mallah, M.; Berman, D.; Budoff, M.; Cademartiri, F.; Callister, T.; Chang, H.-J.; Cheng, V.; Chinnaiyan, K.; et al. Optimized prognostic score for coronary computed tomographic angiography: Results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). J. Am. Coll. Cardiol. 2013, 62, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, H.; Han, Y.; Yuan, X.; Jiang, M.; Wang, W.; Gao, L. Plaque Stabilization and Regression, from Mechanisms to Surveillance and Clinical Strategies. Rev. Cardiovasc. Med. 2024, 25, 459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, Z.; Yu, L.; Zhang, Y.; Xu, Q.; Li, C.; Zhao, S.; Xi, X.; Tian, Y.; Wang, Z.; Tian, J.; et al. Coronary artery calcification and plaque stability: An optical coherence tomography study. Heliyon 2023, 9, e23191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-Risk Plaque Detected on Coronary CT Angiography Predicts Acute Coronary Syndromes Independent of Significant Stenosis in Acute Chest Pain: Results From the ROMICAT-II Trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, S.; Kondo, T.; Sarai, M.; Sugiura, A.; Harigaya, H.; Sato, T.; Inoue, K.; Okumura, M.; Ishii, J.; Anno, H.; et al. Multislice computed tomography characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 2007, 50, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef]
- Katsuki, S.; Jha, P.K.; Aikawa, E.; Aikawa, M. The role of proprotein convertase subtilisin/kexin 9 (PCSK9) in macrophage activation: A focus on its LDL receptor-independent mechanisms. Front. Cardiovasc. Med. 2024, 11, 1431398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, D.; Kim, S.; Lee, H.; Lee, H.-C.; Lee, J.; Park, H.-W.; Fukai, M.; Choi, E.; Choi, S.; Koo, B.-J.; et al. PCSK9 stimulates Syk, PKCδ, and NF-κB, leading to atherosclerosis progression independently of LDL receptor. Nat. Commun. 2024, 15, 2789. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC Guideline on the Management of Blood Cholesterol. Circulation 2019, 139, e1143. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.J.; Puri, R.; Brennan, D.M.; Anderson, T.J.; Cho, L.; Ballantyne, C.M.; Kastelein, J.J.; Koenig, W.; Kassahun, H.; Somaratne, R.M.; et al. Erratum to “C-Reactive Protein Levels and Plaque Regression with Evolocumab: Insights From GLAGOV” [American Journal of Preventive Cardiology 3C (2020) 100091]. Am. J. Prev. Cardiol. 2021, 6, 100153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaw, L.J.; Min, J.K.; Nasir, K.; Xie, J.X.; Berman, D.S.; Miedema, M.D.; Whelton, S.P.; A Dardari, Z.; Rozanski, A.; Rumberger, J.; et al. Sex differences in calcified plaque and long-term cardiovascular mortality: Observations from the CAC Consortium. Eur. Heart J. 2018, 39, 3727–3735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, T.; Wang, Y.; Wang, X.; Hu, W.; Li, A.; Li, S.; Xu, X.; Cao, R.; Fan, L.; Cao, F. Effect of long-term intensive cholesterol control on the plaque progression in elderly based on CTA cohort study. Eur. Radiol. 2022, 32, 4374–4383. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.-M.; Kang, D.-Y.; Lee, P.H.; Ahn, Y.-K.; Kim, W.-J.; Nam, C.-W.; Jeong, J.-O.; Chae, I.-H.; Shiomi, H.; Kao, P.H.L.; et al. Preventive PCI or medical therapy alone for vulnerable atherosclerotic coronary plaque: Rationale and design of the randomized, controlled PREVENT trial. Am. Heart J. 2023, 264, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Venuti, G.; Castellana, C.; Piedimonte, G.; Ferrarotto, L.; Tamburino, C.; La Manna, A. Trattamento della malattia coronarica calcifica: Stato dell’arte. G. Ital. Cardiol. 2020, 21 (Suppl. 1), 48S–57S. [Google Scholar] [CrossRef]
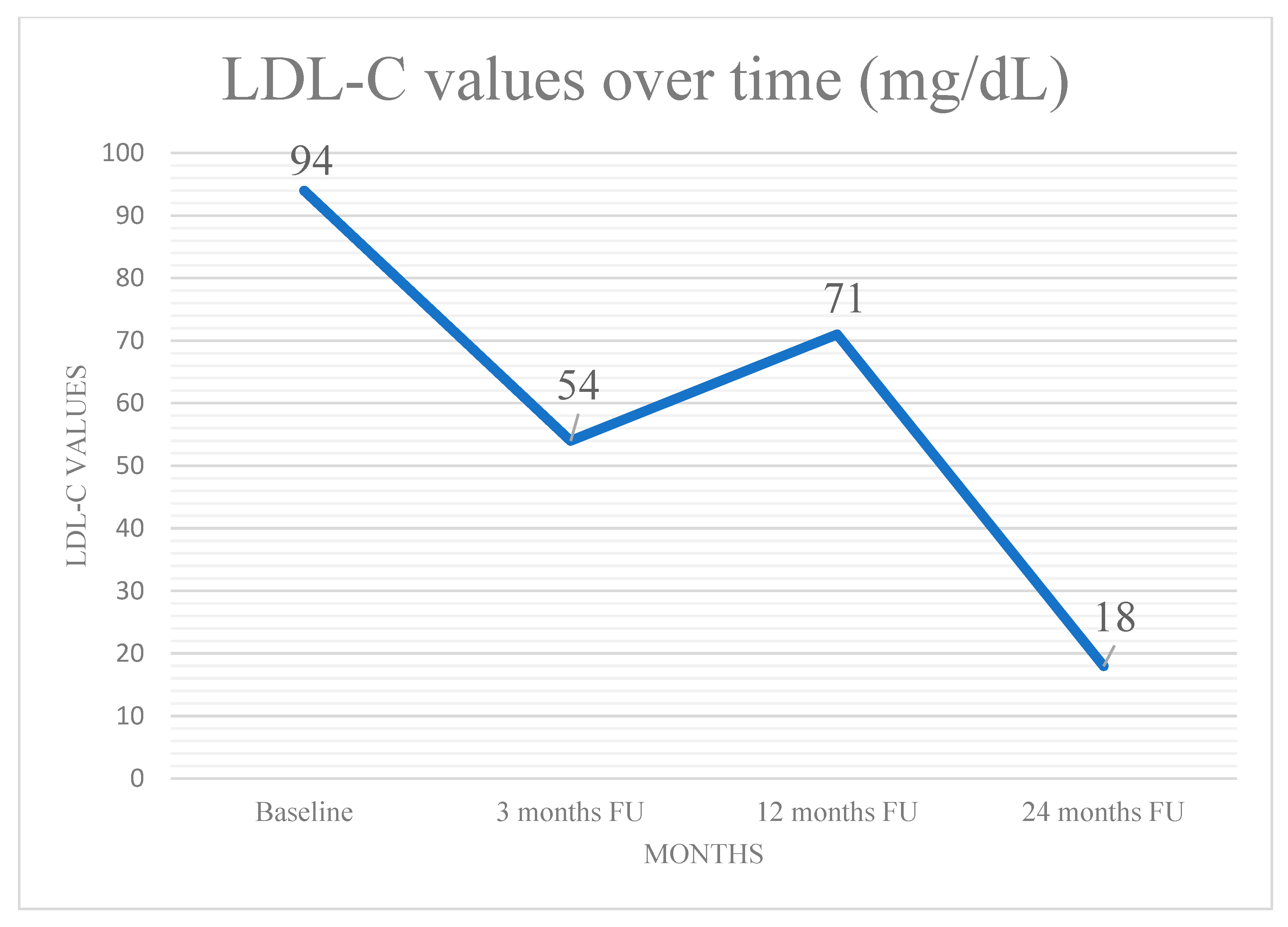
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addeo, L.; Guarini, P.; Campana, P.; Argenziano, L.; Nardi, S.; Tedeschi, C.; Scatteia, A.; Silvestre, M.; Rapacciuolo, A.; Esposito, G.; et al. Patient with Vulnerable Coronary Plaque and Treatment with Evolocumab: A Clinical Case. J. Clin. Med. 2025, 14, 1257. https://doi.org/10.3390/jcm14041257
Addeo L, Guarini P, Campana P, Argenziano L, Nardi S, Tedeschi C, Scatteia A, Silvestre M, Rapacciuolo A, Esposito G, et al. Patient with Vulnerable Coronary Plaque and Treatment with Evolocumab: A Clinical Case. Journal of Clinical Medicine. 2025; 14(4):1257. https://doi.org/10.3390/jcm14041257
Chicago/Turabian StyleAddeo, Lucio, Pasquale Guarini, Pasquale Campana, Luigi Argenziano, Stefano Nardi, Carlo Tedeschi, Alessandra Scatteia, Mattia Silvestre, Antonio Rapacciuolo, Giovanni Esposito, and et al. 2025. "Patient with Vulnerable Coronary Plaque and Treatment with Evolocumab: A Clinical Case" Journal of Clinical Medicine 14, no. 4: 1257. https://doi.org/10.3390/jcm14041257
APA StyleAddeo, L., Guarini, P., Campana, P., Argenziano, L., Nardi, S., Tedeschi, C., Scatteia, A., Silvestre, M., Rapacciuolo, A., Esposito, G., Giordano, S., Dalla Vecchia, L. A., & Donatelli, F. (2025). Patient with Vulnerable Coronary Plaque and Treatment with Evolocumab: A Clinical Case. Journal of Clinical Medicine, 14(4), 1257. https://doi.org/10.3390/jcm14041257







