Dynamic Arterial Elastance as a Predictor of Intraoperative Fluid Responsiveness in Elderly Patient over 70 Years of Age Undergoing Spine Surgery in the Prone Position Under General Anesthesia: A Validation Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Ethics
2.2. Anesthetic Management and Prone Positioning
2.3. Arterial Pressure Monitoring and Arterial Tone Parameters
2.4. Responders Versus Non-Responders
2.5. Statistical Analysis
3. Results
3.1. Hemodynamic Changes
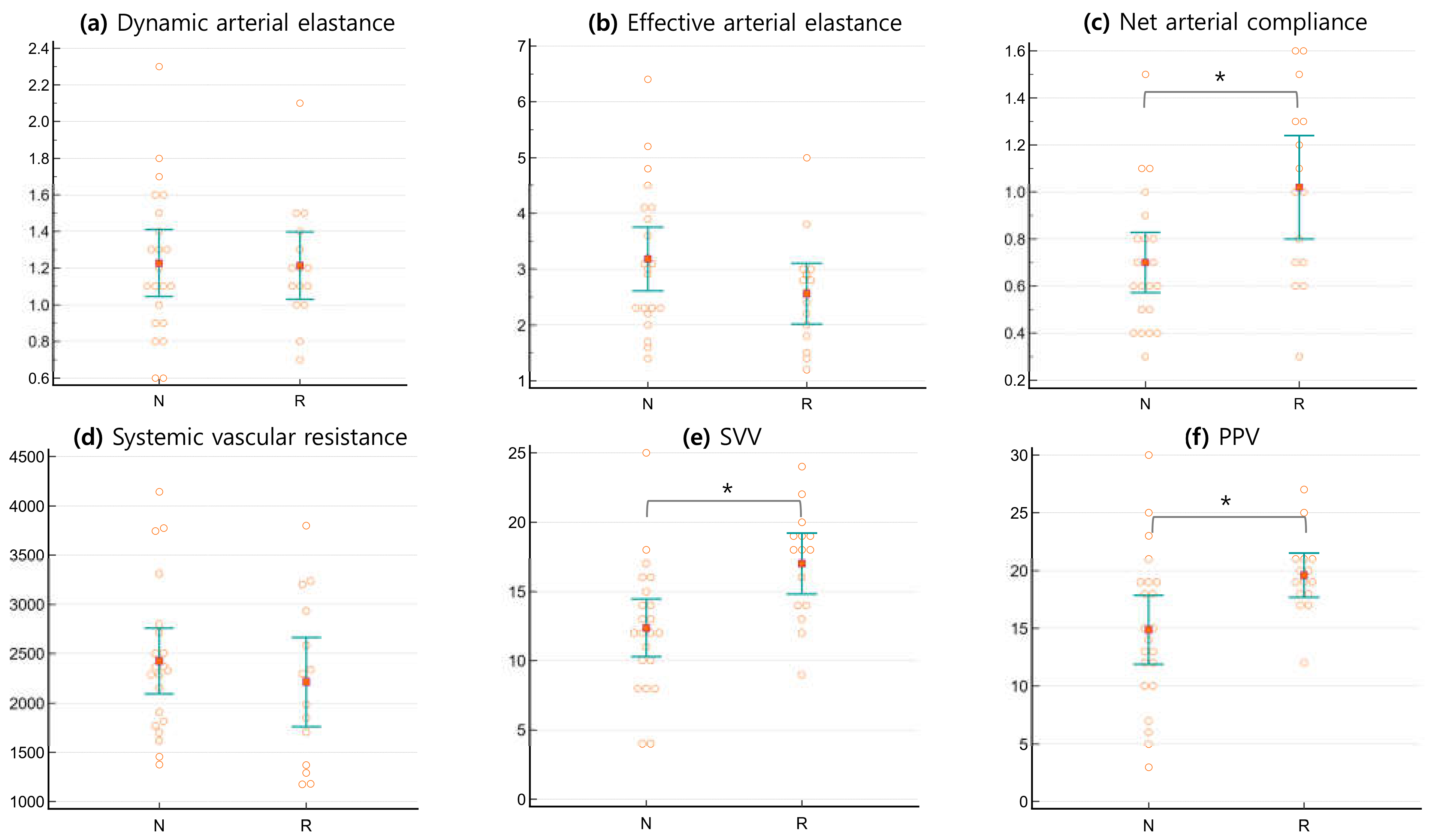
3.2. Arterial Tone Parameters
3.3. Receiver Operating Characteristic (ROC) Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| ASA | American Society of Anesthesiologists |
| BIS | Bispectral Index |
| BMI | Body Mass Index |
| C | Net Arterial Compliance |
| CO | Cardiac Output |
| Ea | Effective Arterial Elastance |
| Eadyn | Dynamic Arterial Elastance (ratio of PPV to SVV) |
| HR | Heart Rate |
| KBSMC | Kangbuk Samsung Medical Center |
| LVEF | Left Ventricular Ejection Fraction |
| MAP | Mean Arterial Pressure |
| PPV | Pulse Pressure Variation |
| ROC | Receiver Operating Characteristic |
| SAP | Systolic Arterial Pressure |
| SV | Stroke Volume |
| SVI | Stroke Volume Index |
| SVR | Systemic Vascular Resistance |
| SVV | Stroke Volume Variation |
| TOF | Train-of-Four |
References
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008, 34, 17–60. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef] [PubMed]
- Pearse, R.M.; Harrison, D.A.; MacDonald, N.; Gillies, M.A.; Blunt, M.; Ackland, G.; Grocott, M.P.; Ahern, A.; Griggs, K.; Scott, R.; et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: A randomized clinical trial and systematic review. Jama 2014, 311, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Le Manach, Y.; Hofer, C.K.; Lehot, J.J.; Vallet, B.; Goarin, J.P.; Tavernier, B.; Cannesson, M. Can changes in arterial pressure be used to detect changes in cardiac output during volume expansion in the perioperative period? Anesthesiology 2012, 117, 1165–1174. [Google Scholar] [CrossRef]
- García, M.I.; Romero, M.G.; Cano, A.G.; Aya, H.D.; Rhodes, A.; Grounds, R.M.; Cecconi, M. Dynamic arterial elastance as a predictor of arterial pressure response to fluid administration: A validation study. Crit. Care 2014, 18, 626. [Google Scholar] [CrossRef]
- Monge García, M.I.; Gil Cano, A.; Gracia Romero, M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit. Care 2011, 15, R15. [Google Scholar] [CrossRef]
- Biais, M.; Bernard, O.; Ha, J.C.; Degryse, C.; Sztark, F. Abilities of pulse pressure variations and stroke volume variations to predict fluid responsiveness in prone position during scoliosis surgery. Br. J. Anaesth. 2010, 104, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Abdullah, T.; Sabanci, P.A.; Dogan, L.; Orhan-Sungur, M.; Akinci, I.O. Comparison of ability of pulse pressure variation to predict fluid responsiveness in prone and supine position: An observational study. J. Clin. Monit. Comput. 2019, 33, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Mesquida, J.; Kim, H.K.; Pinsky, M.R. Effect of tidal volume, intrathoracic pressure, and cardiac contractility on variations in pulse pressure, stroke volume, and intrathoracic blood volume. Intensive Care Med. 2011, 37, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Kamenskiy, A.V.; Pipinos, I.I.; Carson, J.S.; MacTaggart, J.N.; Baxter, B.T. Age and disease-related geometric and structural remodeling of the carotid artery. J. Vasc. Surg. 2015, 62, 1521–1528. [Google Scholar] [CrossRef]
- Herbert, J.A.; Valentine, M.S.; Saravanan, N.; Schneck, M.B.; Pidaparti, R.; Fowler, A.A., 3rd; Reynolds, A.M.; Heise, R.L. Conservative fluid management prevents age-associated ventilator induced mortality. Exp. Gerontol. 2016, 81, 101–109. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, J.; Shim, J.G.; Lee, S.H.; Ryu, K.H.; Jeong, T.; Cho, E.A. Dynamic Arterial Elastance as a Predictor of Supine-to-Prone Hypotension (SuProne Study): An Observational Study. Medicina 2023, 59, 2049. [Google Scholar] [CrossRef] [PubMed]
- Chemla, D.; Hébert, J.L.; Coirault, C.; Zamani, K.; Suard, I.; Colin, P.; Lecarpentier, Y. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am. J. Physiol. 1998, 274, H500–H505. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Rietzschel, E.R.; Shiva-Kumar, P.; De Buyzere, M.L.; Zamani, P.; Claessens, T.; Geraci, S.; Konda, P.; De Bacquer, D.; Akers, S.R.; et al. Effective arterial elastance is insensitive to pulsatile arterial load. Hypertension 2014, 64, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Asanoi, H.; Sasayama, S.; Kameyama, T. Ventriculoarterial coupling in normal and failing heart in humans. Circ. Res. 1989, 65, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, K.; Maughan, W.L.; Sagawa, K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ. Res. 1985, 56, 586–595. [Google Scholar] [CrossRef]
- Monge García, M.I.; Guijo González, P.; Gracia Romero, M.; Gil Cano, A.; Rhodes, A.; Grounds, R.M.; Cecconi, M. Effects of arterial load variations on dynamic arterial elastance: An experimental study. Br. J. Anaesth. 2017, 118, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Kong, Y.G.; Jin, S.J.; Chin, J.H.; Kim, H.Y.; Lee, Y.K.; Hwang, J.H.; Kim, Y.K. Dynamic Arterial Elastance in Predicting Arterial Pressure Increase After Fluid Challenge During Robot-Assisted Laparoscopic Prostatectomy: A Prospective Observational Study. Medicine 2015, 94, e1794. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, S.; Zhu, Q. Reliability of stroke volume or pulse pressure variation as dynamic predictors of fluid responsiveness in laparoscopic surgery: A systematic review. J. Clin. Monit. Comput. 2023, 37, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Dai, H.W.; Yan, M.L.; Gong, S.J.; Cai, G.L.; Zhang, Z.C.; Chen, J.; Yan, J. An evaluation of stroke volume variation as a predictor of fluid responsiveness in mechanically ventilated elderly patients with severe sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2009, 21, 463–465. [Google Scholar] [PubMed]
- Zhang, Z.; Lu, B.; Sheng, X.; Jin, N. Accuracy of stroke volume variation in predicting fluid responsiveness: A systematic review and meta-analysis. J. Anesth. 2011, 25, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Dharmavaram, S.; Jellish, W.S.; Nockels, R.P.; Shea, J.; Mehmood, R.; Ghanayem, A.; Kleinman, B.; Jacobs, W. Effect of prone positioning systems on hemodynamic and cardiac function during lumbar spine surgery: An echocardiographic study. Spine 2006, 31, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Shim, J.K.; Song, Y.; Seo, S.J.; Kwak, Y.L. Validation of pulse pressure variation and corrected flow time as predictors of fluid responsiveness in patients in the prone position. Br. J. Anaesth. 2013, 110, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Cunha, P.; Vitorino, P.V.O.; Souza, A.L.L.; Deus, G.D.; Feitosa, A.; Barbosa, E.C.D.; Gomes, M.M.; Jardim, P.; Barroso, W.K.S. Vascular Aging and Arterial Stiffness. Arq. Bras. Cardiol. 2022, 119, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Briet, M.; Boutouyrie, P.; Laurent, S.; London, G.M. Arterial stiffness and pulse pressure in CKD and ESRD. Kidney Int. 2012, 82, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Michard, F.; Teboul, J.L. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit. Care 2000, 4, 282–289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siebenmann, C.; Hug, M.; Keiser, S.; Müller, A.; van Lieshout, J.; Rasmussen, P.; Lundby, C. Hypovolemia explains the reduced stroke volume at altitude. Physiol. Rep. 2013, 1, e00094. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.C.; Levenson, J.; Bouthier, J.; Safar, M.E.; Avolio, A.P. Evidence of early degenerative changes in large arteries in human essential hypertension. Hypertension 1985, 7, 675–680. [Google Scholar] [CrossRef] [PubMed]


| Responders (n = 15) | Non-Responders (n = 22) | |
|---|---|---|
| Gender (Female/Male) | 8/7 | 16/6 |
| Age, years | 77 ± 6 | 76 ± 4 |
| Weight, kg | 59 ± 15 | 58 ± 10 |
| Ideal body weight, kg | 52 ± 9 | 48 ± 10 |
| Height, cm | 157 ± 9 | 153 ± 9 |
| BMI | 24 ± 5 | 25 ± 3 |
| ASA PS (II/III) | 5/10 | 7/15 |
| Ventilator settings | ||
| Tidal volume, mL/kg ideal body weight | 427 ± 75 | 406 ± 68 |
| Total PEEP, cmH2O | 15 ± 2 | 16 ± 2 |
| FiO2, % | 60 | 60 |
| SpO2, % | 99 [98–100] | 99 [98–100] |
| Operation type, n | ||
| Discectomy | 7 | 14 |
| Laminectomy | 6 | 2 |
| Posterior fusion | 2 | 6 |
| Pre-Loading | Post-Loading | p-Value | Mean Diff. | |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | ||||
| Responder | 70 ± 9 a | 85 ± 10 ab | <0.0001 | 14.53 |
| Non-responder | 80 ± 12 | 76 ± 12 | 0.0512 | −4.27 |
| Intergroup P | 0.0092 | 0.0301 | ||
| Heart rate, beats/min | ||||
| Responder | 72 ± 10 | 64 ± 6 b | 0.0023 | −7.93 |
| Non-responder | 76 ± 12 | 66 ± 10 b | <0.0001 | −10.22 |
| Intergroup P | 0.359 | 0.6381 | ||
| Pulse pressure, mmHg | ||||
| Responder | 41 ± 18 | 51 ± 13 b | 0.0088 | 10.07 |
| Non-responder | 60 ± 16 | 51 ± 12 b | 0.0021 | −8.82 |
| Intergroup P | 0.655 | 0.979 | ||
| Cardiac output, L/min | ||||
| Responder | 2.6 ± 0.8 | 3.1 ± 0.6 b | 0.0089 | 0.46 |
| Non-responder | 2.9 ± 0.6 | 3.2 ± 0.6 b | 0.0030 | 0.34 |
| Intergroup P | 0.359 | 0.6381 | ||
| Stroke volume (mL) | ||||
| Responder | 37 ± 9 | 49 ± 9 b | <0.0001 | 10.95 |
| Non-responder | 39 ± 11 | 50 ± 12 b | <0.0001 | 12.00 |
| Intergroup P | 0.5629 | 0.7839 | ||
| Stroke volume Index (mL/m2) | ||||
| Responder | 22 ± 5 | 30 ± 5 b | <0.0001 | 8.00 |
| Non-responder | 24 ± 5 | 31 ± 7 b | <0.0001 | 8.00 |
| Intergroup P | 0.2794 | 0.5428 | ||
| PPV (%) | ||||
| Responder | 20 ± 3 a | 11 ± 4 b | <0.0001 | −8.80 |
| Non-responder | 15 ± 7 | 9 ± 5 b | 0.0002 | −5.59 |
| Intergroup P | 0.0187 | 0.3003 | ||
| SVV (%) | ||||
| Responder | 17 ± 4 a | 12 ± 5 b | 0.0023 | −4.87 |
| Non-responder | 12 ± 5 | 9 ± 4 b | 0.0002 | −2.95 |
| Intergroup P | 0.0030 | 0.0719 |
| Parameter | Pre-Loading | Post-Loading | p-Value |
|---|---|---|---|
| Eadyn | |||
| Responder | 1.2 ± 0.3 | 1.0 ± 0.4 | 0.1540 |
| Non-responder | 1.2 ± 0.4 | 1.0 ± 0.4 b | 0.0321 |
| Intergroup P | 0.9141 | 0.8707 | |
| Ea, mmHg/mL | |||
| Responder | 2.6 ± 1.0 | 2.2 ± 0.7 | 0.0650 |
| Non-responder | 3.2 ± 1.3 | 2.2 ± 0.8 b | <0.0001 |
| Intergroup P | 0.1222 | 0.9039 | |
| C, mL/mmHg | |||
| Responder | 1.02 ± 0.40 a | 1.01 ± 0.28 | 0.9115 |
| Non-responder | 0.70 ± 0.29 | 1.04 ± 0.38 b | <0.0001 |
| Intergroup P | 0.0076 | 0.8413 | |
| SVR, dyn·s·cm−5 | |||
| Responder | 2213 ± 818 | 2172 ± 587 | 0.7661 |
| Non-responder | 2426 ± 754 | 2032 ± 579 b | 0.0004 |
| Intergroup P | 0.4187 | 0.4777 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, E.J.; Cho, E.A.; Jun, J.; Lee, S.H.; Lee, S.; Ahn, J.H. Dynamic Arterial Elastance as a Predictor of Intraoperative Fluid Responsiveness in Elderly Patient over 70 Years of Age Undergoing Spine Surgery in the Prone Position Under General Anesthesia: A Validation Study. J. Clin. Med. 2025, 14, 1247. https://doi.org/10.3390/jcm14041247
Oh EJ, Cho EA, Jun J, Lee SH, Lee S, Ahn JH. Dynamic Arterial Elastance as a Predictor of Intraoperative Fluid Responsiveness in Elderly Patient over 70 Years of Age Undergoing Spine Surgery in the Prone Position Under General Anesthesia: A Validation Study. Journal of Clinical Medicine. 2025; 14(4):1247. https://doi.org/10.3390/jcm14041247
Chicago/Turabian StyleOh, Eun Jung, Eun Ah Cho, Joohyun Jun, Sung Hyun Lee, Seunghyeon Lee, and Jin Hee Ahn. 2025. "Dynamic Arterial Elastance as a Predictor of Intraoperative Fluid Responsiveness in Elderly Patient over 70 Years of Age Undergoing Spine Surgery in the Prone Position Under General Anesthesia: A Validation Study" Journal of Clinical Medicine 14, no. 4: 1247. https://doi.org/10.3390/jcm14041247
APA StyleOh, E. J., Cho, E. A., Jun, J., Lee, S. H., Lee, S., & Ahn, J. H. (2025). Dynamic Arterial Elastance as a Predictor of Intraoperative Fluid Responsiveness in Elderly Patient over 70 Years of Age Undergoing Spine Surgery in the Prone Position Under General Anesthesia: A Validation Study. Journal of Clinical Medicine, 14(4), 1247. https://doi.org/10.3390/jcm14041247






