Evaluation of Refractive Predictive Accuracy in Intraocular Lens Power Calculations: A Comparative Study of Swept-Source Optical Coherence Tomography and Optical Low-Coherence Interferometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Ethics, and Informed Consent
2.2. Subjects and Inclusion/Exclusion Criteria
2.3. Procedure
2.4. Biometers
2.5. Refractive Prediction Calculations
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Comparison of Refractive Accuracy with SS-OCT-Based Studies
4.2. Comparison of Refractive Accuracy with OLCI/OLCR-Based Studies
4.3. Limitations
4.4. Future Lines of Research
4.5. Clinical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACD | Anterior Chamber Depth |
| AL | Axial Length |
| CDVA | Corrected Distance Visual Acuity |
| D | Diopters |
| IOL | Intraocular Lens |
| logMAR | Logarithm of the Minimum Angle of Resolution |
| MAE | Mean Absolute Error |
| MedAE | Median Absolute Error |
| ME | Mean Error |
| OLCI | Optical Low-Coherence Interferometry |
| SE | Spherical Equivalent |
| SS-OCT | Swept-Source Optical Coherence Tomography |
| UDVA | Uncorrected Distance Visual Acuity |
| WTW | White-to-White Corneal Diameter |
References
- Gjerdrum, B.; Gundersen, K.G.; Nilsen, C.; Gundersen, M.; Jensen, P. Refractive Predictability and Biometry Agreement of a Combined Swept Source Optical Coherence and Reflectometry Biometer Compared to an Optical Low Coherence Reflectometry Biometer and an Ss-Oct Biometer. Clin. Ophthalmol. 2023, 17, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vicent, A.; Venkataraman, A.P.; Dalin, A.; Brautaset, R.; Montés-Micó, R. Repeatability of a Fully Automated Swept-Source Optical Coherence Tomography Biometer and Agreement with a Low Coherence Reflectometry Biometer. Eye Vis. 2023, 10, 24. [Google Scholar] [CrossRef]
- Kanclerz, P.; Hecht, I.; Tuuminen, R. Technical Failure Rates for Biometry between Swept-Source and Older-Generation Optical Coherence Methods: A Review and Meta-Analysis. BMC Ophthalmol. 2023, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J.; Hoffmann, P.C.; Savini, G. Comparison of a New Optical Biometer Using Swept-Source Optical Coherence Tomography and a Biometer Using Optical Low-Coherence Reflectometry. J. Cataract Refract. Surg. 2016, 42, 1165–1172. [Google Scholar] [CrossRef]
- McAlinden, C.; Wang, Q.; Gao, R.; Zhao, W.; Yu, A.; Li, Y.; Guo, Y.; Huang, J. Axial Length Measurement Failure Rates With Biometers Using Swept-Source Optical Coherence Tomography Compared to Partial-Coherence Interferometry and Optical Low-Coherence Interferometry. Am. J. Ophthalmol. 2017, 173, 64–69. [Google Scholar] [CrossRef]
- Nemeth, G.; Modis, L.J. Ocular Measurements of a Swept-Source Biometer: Repeatability Data and Comparison with an Optical Low-Coherence Interferometry Biometer. J. Cataract Refract. Surg. 2019, 45, 789–797. [Google Scholar] [CrossRef]
- Kurian, M.; Negalur, N.; Das, S.; Puttaiah, N.K.; Haria, D.; Tejal, S.J.; Thakkar, M.M. Biometry with a New Swept-Source Optical Coherence Tomography Biometer: Repeatability and Agreement with an Optical Low-Coherence Reflectometry Device. J. Cataract Refract. Surg. 2016, 42, 577–581. [Google Scholar] [CrossRef]
- Calvo-Sanz, J.A.; Portero-Benito, A.; Arias-Puente, A. Efficiency and Measurements Agreement between Swept-Source OCT and Low-Coherence Interferometry Biometry Systems. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 559–566. [Google Scholar] [CrossRef]
- Shammas, H.J.; Ortiz, S.; Shammas, M.C.; Kim, S.H.; Chong, C. Biometry Measurements Using a New Large-Coherence-Length Swept-Source Optical Coherence Tomographer. J. Cataract Refract. Surg. 2016, 42, 50–61. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Li, Y.; Chen, Z.; Gao, R.; Yu, J.; Zhao, Y.; Lu, W.; McAlinden, C.; Wang, Q. Comprehensive Comparison of Axial Length Measurement With Three Swept-Source OCT-Based Biometers and Partial Coherence Interferometry. J. Refract. Surg. 2019, 35, 115–120. [Google Scholar] [CrossRef]
- Huang, J.; Savini, G.; Wu, F.; Yu, X.; Yang, J.; Yu, A.; Yu, Y.; Wang, Q. Repeatability and Reproducibility of Ocular Biometry Using a New Noncontact Optical Low-Coherence Interferometer. J. Cataract Refract. Surg. 2015, 41, 2233–2241. [Google Scholar] [CrossRef]
- Waring, G.O. Standard Graphs for Reporting Refractive Surgery. J. Refract. Surg. 2000, 16, 459–466. [Google Scholar] [PubMed]
- Reinstein, D.Z.; Waring, G.O. Graphic Reporting of Outcomes of Refractive Surgery. J. Refract. Surg. 2009, 25, 975–978. [Google Scholar] [CrossRef]
- Dupps, W.J.; Kohnen, T.; Mamalis, N.; Rosen, E.S.; Koch, D.D.; Obstbaum, S.A.; Waring, G.O.; Reinstein, D.Z.; Stulting, R.D. Standardized Graphs and Terms for Refractive Surgery Results. J. Cataract Refract. Surg. 2011, 37, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Stulting, R.D.; Dupps, W.J.; Kohnen, T.; Mamalis, N.; Rosen, E.S.; Koch, D.D.; Obstbaum, S.A.; Waring, G.O.; Reinstein, D.Z. Standardized Graphs and Terms for Refractive Surgery Results. Cornea 2011, 30, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Waring, G.O.; Reinstein, D.Z.; Dupps, W.J.; Kohnen, T.; Mamalis, N.; Rosen, E.S.; Koch, D.D.; Obstbaum, S.A.; Stulting, R.D. Standardized Graphs and Terms for Refractive Surgery Results. J. Refract. Surg. 2011, 27, 7–9. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Archer, T.J.; Randleman, J.B. JRS Standard for Reporting Astigmatism Outcomes of Refractive Surgery. J. Refract. Surg. 2014, 30, 654–659. [Google Scholar] [CrossRef]
- Cheng, S.M.; Li, X.; Zhang, J.S.; Mei, J.Q.; Shi, G.L.; Lin, J.; Li, T.T.; Yu, A.Y. Comparison of Refractive Prediction Accuracy With Three Optical Devices. J. Refract. Surg. 2023, 39, 48–55. [Google Scholar] [CrossRef]
- Kato, Y.; Kojima, T.; Tamaoki, A.; Ichikawa, K.; Tamura, K.; Ichikawa, K. Refractive Prediction Error in Cataract Surgery Using an Optical Biometer Equipped with Anterior Segment OCT. J. Cataract Refract. Surg. 2022, 48, 429–434. [Google Scholar] [CrossRef]
- Savini, G.; Hoffer, K.J.; Shammas, H.J.; Aramberri, J.; Huang, J.; Barboni, P. Accuracy of a New Swept-Source Optical Coherence Tomography Biometer for IOL Power Calculation and Comparison to IOLMaster. J. Refract. Surg. 2017, 33, 690–695. [Google Scholar] [CrossRef]
- Reitblat, O.; Levy, A.; Kleinmann, G.; Assia, E.I. Accuracy of Intraocular Lens Power Calculation Using Three Optical Biometry Measurement Devices: The OA-2000, Lenstar-LS900 and IOLMaster-500. Eye 2018, 32, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Noh, S.R.; Kim, K.Y. Refractive Prediction of Four Different Intraocular Lens Calculation Formulas Compared between New Swept Source Optical Coherence Tomography and Partial Coherence Interferometry. PLoS ONE 2021, 16, e0251152. [Google Scholar] [CrossRef] [PubMed]
| Variable (Units) | SS-OCT | OLCI | p-Value |
|---|---|---|---|
| Age (years) | 68.68 ± 11.28 (40–86) | 69.57 ± 9.24 (53–86) | 0.366 |
| Sphere (D) | 0.42 ± 3.13 (−12.00 to 8.00) | 0.66 ± 2.79 (−8.00 to 4.50) | 0.363 |
| Cylinder (D) | −0.10 ± 1.62 (−4.50 to 4.25) | −0.08 ± 1.10 (−2.50 to 1.25) | 0.481 |
| Axis (degrees) | 80.41 ± 60.72 (0–180) | 82.07 ± 60.29 (3–180) | 0.460 |
| SE (D) | 0.34 ± 3.34 (−13.13 to 8.63) | 0.45 ± 2.42 (−8.00 to 4.50) | 0.423 |
| CDVA (LogMAR) | 0.37 ± 0.31 (1.70 to 0.00) | 0.44 ± 0.32 (1.00 to 0.00) | 0.112 |
| CED (cells/mm2) | 2416.69 ± 304.43 (1442–3072) | 2355.00 ± 293.86 (1602–2872) | 0.140 |
| IOP (mmHg) | 16.06 ± 3.30 (10.0–27.5) | 15.64 ± 3.92 (10.0–27.0) | 0.258 |
| Flat keratometry (D) | 43.44 ± 1.63 (38.24–46.84) | 43.48 ± 1.70 (39.64–47.67) | 0.454 |
| Steep keratometry (D) | 44.49 ± 1.68 (39.68–48.16) | 44.29 ± 1.63 (40.73–48.61) | 0.267 |
| Axis (degrees) | 89.53 ± 57.25 (0–179) | 104.39 ± 48.47 (10–178) | 0.078 |
| J0 (D) | −0.04 ± 0.39 (−0.94 to 1.51) | 0.03 ± 0.28 (−0.58 to 1.18) | 0.429 |
| J45 (D) | −0.08 ± 0.44 (−1.55 to 1.65) | 0.02 ± 0.33 (−0.90 to 1.01) | 0.081 |
| Lens Thickness (mm) | 4.61 ± 0.42 (3.52–5.85) | 4.76 ± 0.31 (4.21–5.42) | 0.030 |
| Axial Length (mm) | 23.37 ± 1.23 (20.57–28.31) | 23.17 ± 0.82 (21.71–24.82) | 0174 |
| ACD (mm) | 3.23 ± 0.42 (2.01–4.02) | 3.04 ± 0.40 (2.50–4.20) | 0.009 |
| WTW (mm) | 11.93 ± 0.40 (10.94–12.96) | 12.03 ± 0.50 (11.27–14.12) | 0.117 |
| Variable (Units) | SS-OCT | OLCI | p-Value |
|---|---|---|---|
| IOL Spherical Power (D) | 21.83 ± 3.29 (10.50–33.00) | 22.08 ± 1.77 (19.00–26.00) | 0.332 |
| IOL Astigmatic Power (D) | 1.56 ± 0.98 (0.00–4.50) | 2.00 ± 1.27 (1.00–3.75) | 0.089 |
| IOL Axis (degrees) | 56.84 ± 66.94 (0–179) | 59.50 ± 75.62 (2–179) | 0.452 |
| Suggested IOL Spherical Power (D) | 21.68 ± 3.34 (10.00–33.00) | 22.12 ± 1.90 (18.50–26.00) | 0.221 |
| Suggested IOL Astigmatic Power (D) | 1.76 ± 0.83 (0.00–4.50) | 1.97 ± 0.97 (1.00–3.75) | 0.174 |
| Suggested IOL Axis (degrees) | 62.10 ± 67.45 (0–179) | 90.00 ± 80.18 (2–190) | 0.069 |
| Difference Implanted vs. Suggested Power (Spherical, D) | 0.15 ± 0.25 (−0.50 to 1.00) | −0.04 ± 0.61 (−2.50 to 0.50) | 0.002 |
| Difference Implanted vs. Suggested Power (Astigmatic, D) | −0.05 ± 0.35 (−3.00 to 1.00) | −0.27 ± 0.41 (−1.25 to 0.00) | 0.035 |
| Difference Implanted vs. Suggested Axis (degrees) | −3.98 ± 25.39 (−179 to 0) | −32.50 ± 76.59 (−180 to 15) | 0.136 |
| Expected Spherical Equivalent (D) | −0.16 ± 0.37 (−2.81 to 1.13) | −0.02 ± 0.36 (−0.47 to 1.25) | 0.020 |
| Mean Error 1-month vs. Expected SE (D) | 0.33 ± 0.48 (−1.08 to 1.46) | 0.24 ± 0.54 (−1.25 to 1.11) | 0.181 |
| Mean Error 6-months vs. Expected SE (D) | 0.34 ± 0.41 (−0.92 to 1.45) | 0.25 ± 0.49 (−1.25 to 1.21) | 0.156 |
| Mean Absolute Error 1-month vs. Expected SE (D) | 0.47 ± 0.34 (0.01 to 1.46) | 0.46 ± 0.35 (−0.92 to 1.45) | 0.474 |
| Mean Absolute Error 6-months vs. Expected SE (D) | 0.44 ± 0.31 (0.00 to 1.45) | 0.48 ± 0.26 (0.11 to 1.25) | 0.206 |
| Median Absolute Error 1-month vs. Expected SE (D) | 0.41 (0.53) (0.01 to 1.46) | 0.41 (0.65) (−0.92 to 1.45) | 0.860 |
| Median Absolute Error 6-months vs. Expected SE (D) | 0.37 (0.49) (0.00 to 1.45) | 0.49 (0.34) (0.11 to 1.25) | 0.317 |
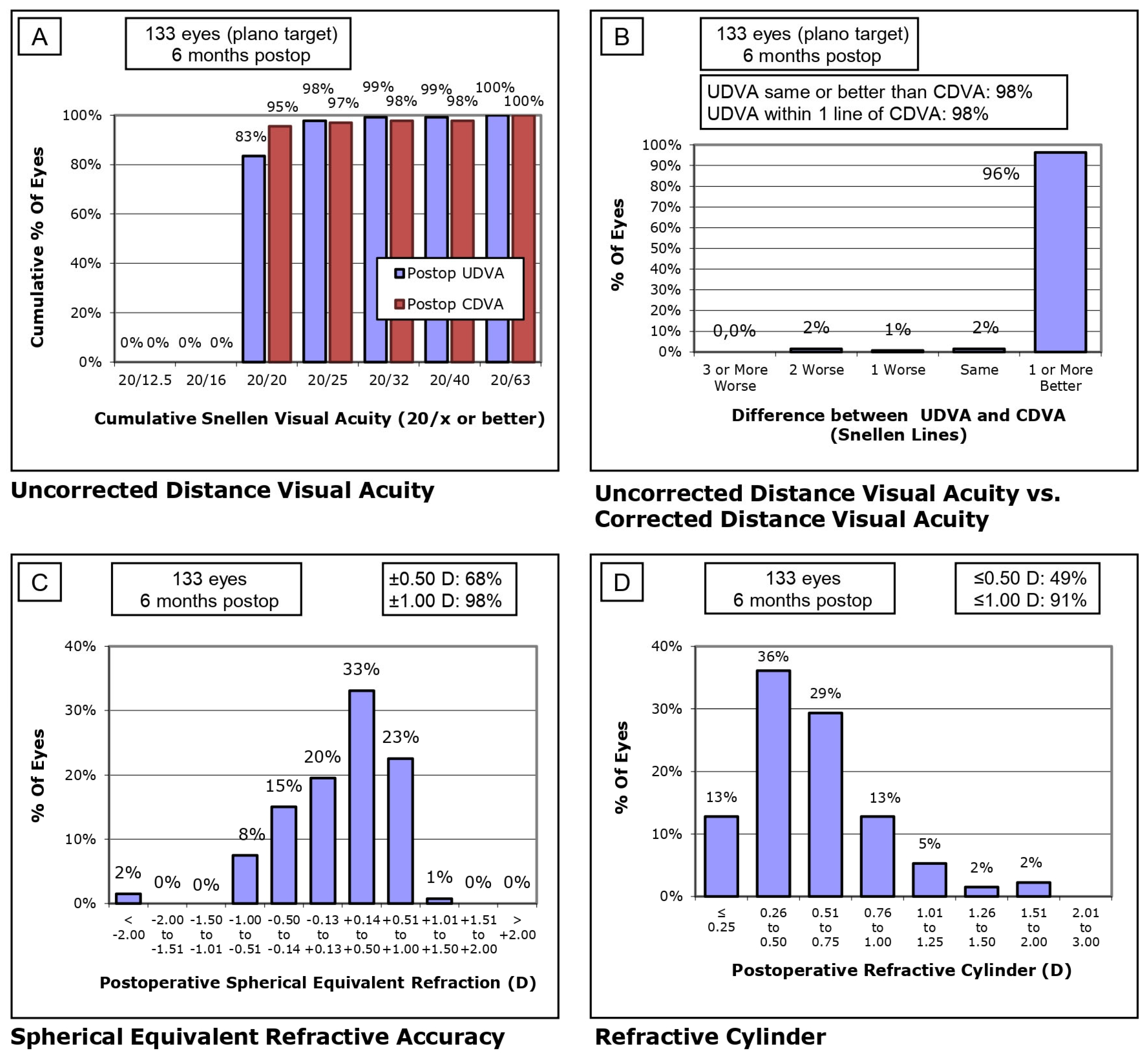
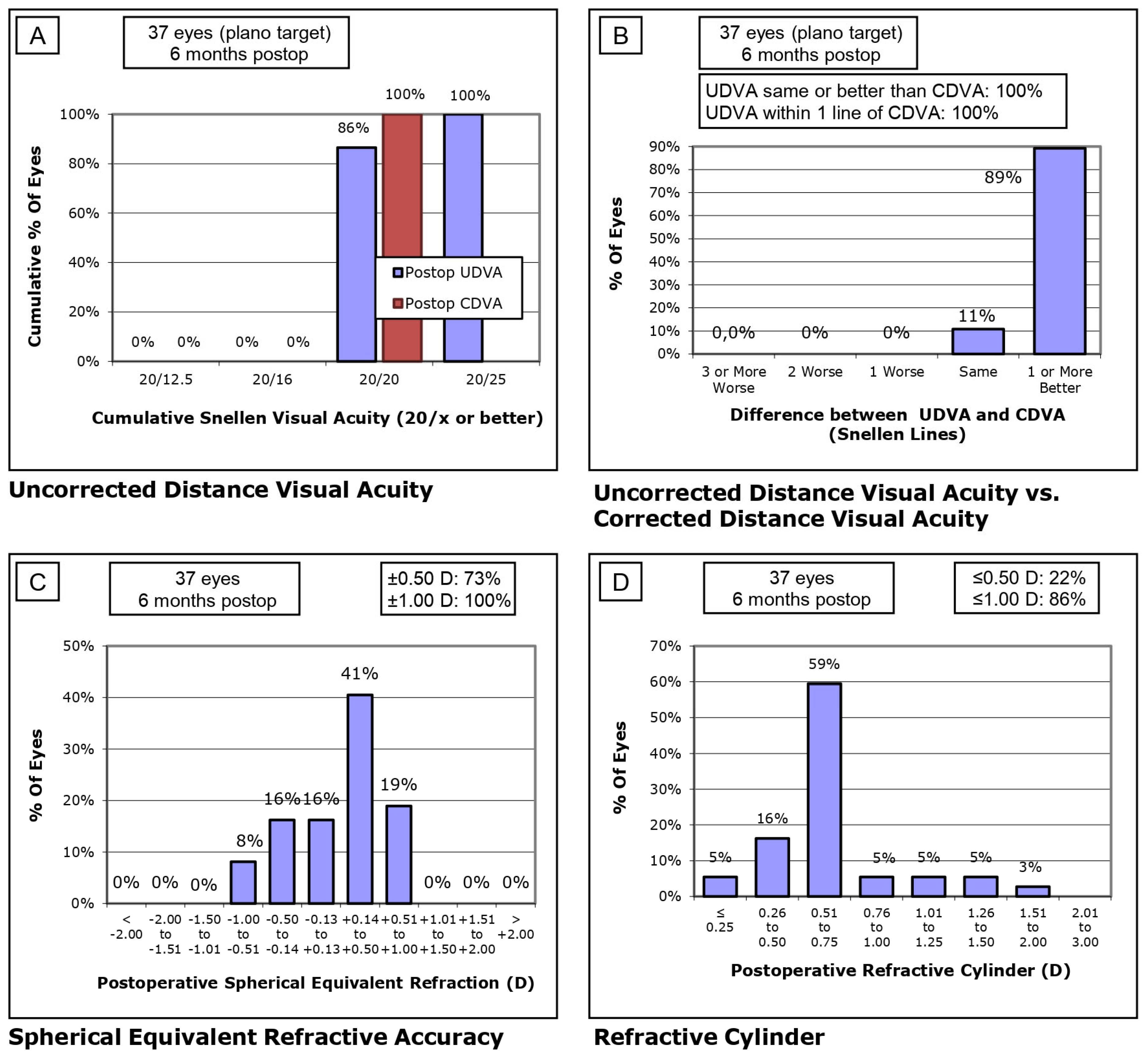
| SE Refractive Prediction ±0.25 D * Eyes (percentage of eyes) | 35 (26.3%) | 10 (27.8%) | - |
| SE Refractive Prediction ±0.50 D * Eyes (percentage of eyes) | 75 (56.4%) | 20 (55.6%) | - |
| SE Refractive Prediction ±0.75 D * Eyes (percentage of eyes) | 110 (82.7%) | 30 (83.3%) | - |
| SE Refractive Prediction ±1.00 D * Eyes (percentage of eyes) | 130 (97.7%) | 35 (97.2%) | - |
| Variable (Units) | SS-OCT | OLCI | p-Value |
|---|---|---|---|
| IOP (mmHg) | 14.03 ± 3.00 (8.0–23.0) | 14.47 ± 2.56 (9.0–22.0) | 0.211 |
| Sphere 1-month (D) | 0.24 ± 0.50 (−3.00 to 1.00) | 0.32 ± 0.38 (−0.50 to 1.00) | 0.172 |
| Cylinder 1-month (D) | −0.15 ± 0.75 (−1.75 to 1.50) | −0.20 ± 0.64 (−1.25 to 1.00) | 0.351 |
| Axis 1-month (degrees) | 89.09 ± 53.91 (1–180) | 90.76 ± 60.90 (0–180) | 0.440 |
| SE 1-month (D) | 0.14 ± 0.59 (−3.88 to 1.38) | 0.18 ± 0.46 (−0.88 to 1.25) | 0.256 |
| UDVA 1-month (LogMAR) | 0.03 ± 0.09 (0.90 to 0.00) | 0.02 ± 0.03 (0.10 to 0.00) | 0.157 |
| CDVA 1-month (LogMAR) | 0.02 ± 0.12 (0.90 to 0.00) | 0.00 ± 0.00 (0.00–0.00) | 0.563 |
| Sphere 6-months (D) | 0.22 ± 0.47 (−2.75 to 1.00) | 0.36 ± 0.40 (−0.50 to 1.00) | 0.053 |
| Cylinder 6-months (D) | −0.07 ± 0.55 (−1.75 to 1.00) | −0.26 ± 0.63 (−1.75 to 1.00) | 0.048 |
| Axis 6-months (degrees) | 78.70 ± 57.91 (0–180) | 92.53 ± 54.60 (0–180) | 0.117 |
| SE 6-months (D) | 0.15 ± 0.51 (−3.00 to 1.13) | 0.19 ± 0.43 (−0.75 to 1.00) | 0.339 |
| UDVA 6-months (LogMAR) | 0.02 ± 0.09 (0.90 to 0.00) | 0.01 ± 0.02 (0.05 to 0.00) | 0.182 |
| CDVA 6-months (LogMAR) | 0.02 ± 0.12 (0.90 to 0.00) | 0.00 ± 0.00 (0.00–0.00) | 0.157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Barri, L.; Mercea, N.; Ionela-Iasmina, Y.; Munteanu, M.; Stanca, H.T. Evaluation of Refractive Predictive Accuracy in Intraocular Lens Power Calculations: A Comparative Study of Swept-Source Optical Coherence Tomography and Optical Low-Coherence Interferometry. J. Clin. Med. 2025, 14, 1201. https://doi.org/10.3390/jcm14041201
Al Barri L, Mercea N, Ionela-Iasmina Y, Munteanu M, Stanca HT. Evaluation of Refractive Predictive Accuracy in Intraocular Lens Power Calculations: A Comparative Study of Swept-Source Optical Coherence Tomography and Optical Low-Coherence Interferometry. Journal of Clinical Medicine. 2025; 14(4):1201. https://doi.org/10.3390/jcm14041201
Chicago/Turabian StyleAl Barri, Leila, Nadina Mercea, Yasar Ionela-Iasmina, Mihnea Munteanu, and Horia T. Stanca. 2025. "Evaluation of Refractive Predictive Accuracy in Intraocular Lens Power Calculations: A Comparative Study of Swept-Source Optical Coherence Tomography and Optical Low-Coherence Interferometry" Journal of Clinical Medicine 14, no. 4: 1201. https://doi.org/10.3390/jcm14041201
APA StyleAl Barri, L., Mercea, N., Ionela-Iasmina, Y., Munteanu, M., & Stanca, H. T. (2025). Evaluation of Refractive Predictive Accuracy in Intraocular Lens Power Calculations: A Comparative Study of Swept-Source Optical Coherence Tomography and Optical Low-Coherence Interferometry. Journal of Clinical Medicine, 14(4), 1201. https://doi.org/10.3390/jcm14041201









