Assessment of Cerebral Hemodynamic Changes During Roll-Over Test in Healthy Pregnant Women and Those with Mild and Severe Preeclampsia
Abstract
1. Introduction
2. Methods
3. Results
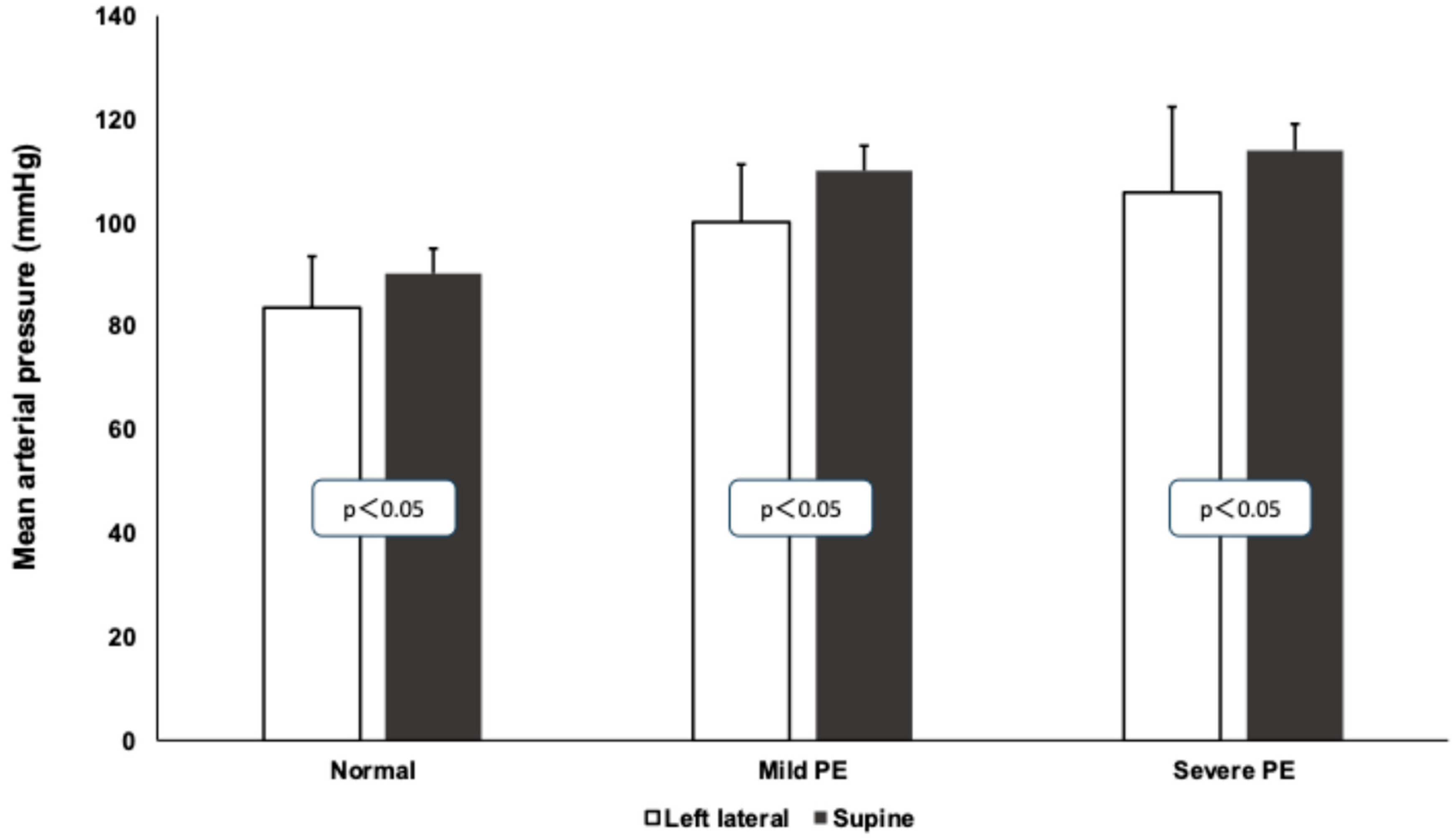
3.1. Mean Arterial Pressure Values During Roll-Over Test
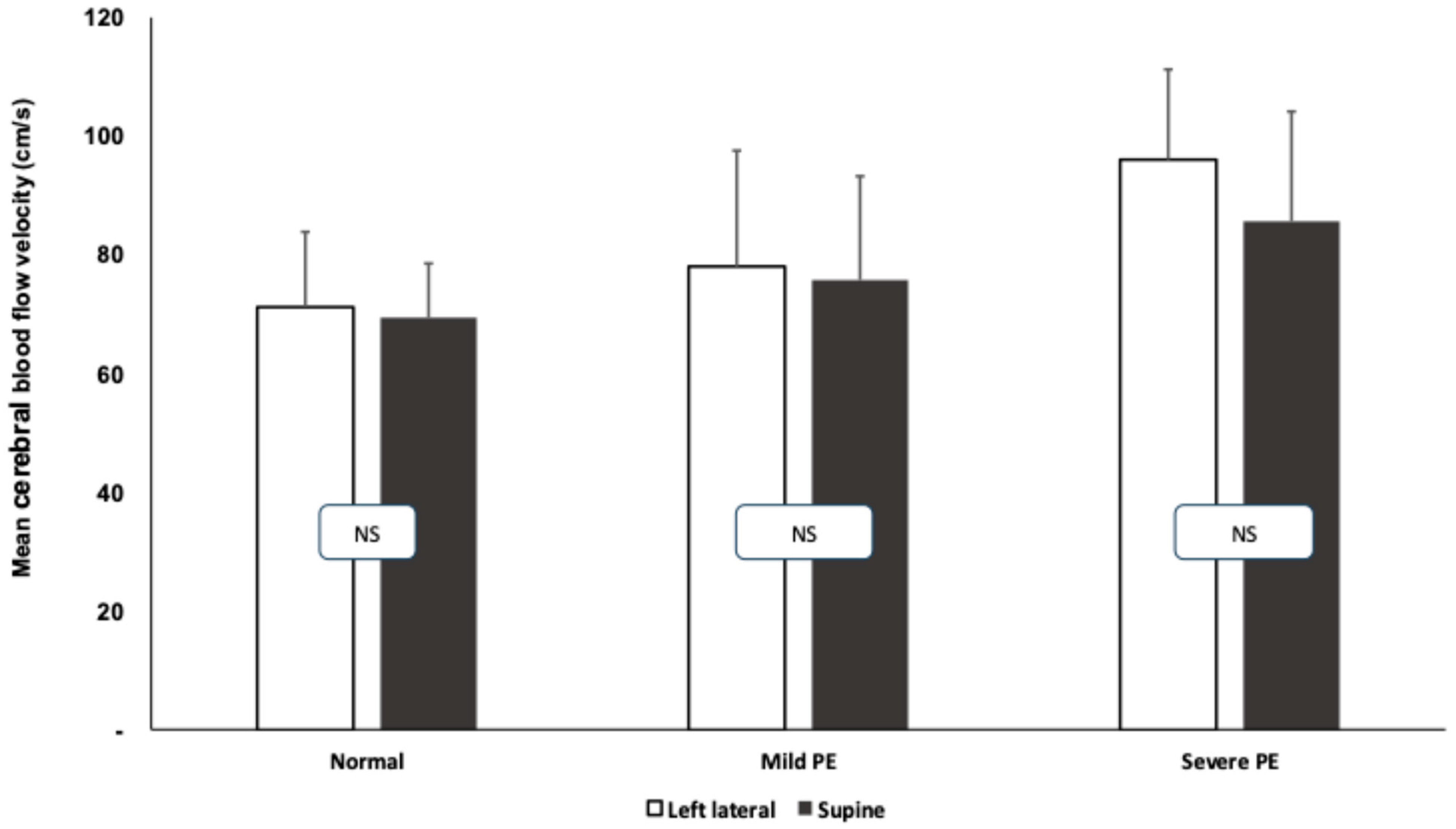
3.2. Transcranial Doppler Measurements During Roll-Over Tests
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar] [PubMed]
- Chaiworapongsa, T.; Espinoza, J.; Gotsch, F.; Kim, Y.M.; Kim, G.J.; Goncalves, L.F.; Edwin, S.; Kusanovic, J.P.; Erez, O.; Than, N.G.; et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J. Matern. Fetal Neonatal Med. 2008, 21, 25–40. [Google Scholar] [CrossRef]
- Crispi, F.; Dominguez, C.; Llurba, E.; Martin-Gallan, P.; Cabero, L.; Gratacos, E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am. J. Obstet. Gynecol. 2006, 195, 201–207. [Google Scholar] [CrossRef]
- Nagamatsu, T.; Fujii, T.; Kusumi, M.; Zou, L.; Yamashita, T.; Osuga, Y.; Momoeda, M.; Kozuma, S.; Taketani, Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: An implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 2004, 145, 4838–4845. [Google Scholar] [CrossRef]
- Nevo, O.; Soleymanlou, N.; Wu, Y.; Xu, J.; Kingdom, J.; Many, A.; Zamudio, S.; Caniggia, I. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1085–R1093. [Google Scholar] [CrossRef]
- Bergman, L.; Escudero, C.A.; Cluver, C.; Hastie, R.; Torres-Vergara, P. Editorial: Preeclampsia and the brain: Pre-clinical and clinical studies of cerebral involvement in preeclampsia. Front. Physiol. 2023, 14, 1151091. [Google Scholar] [CrossRef]
- Jones-Muhammad, M.; Warrington, J.P. Cerebral Blood Flow Regulation in Pregnancy, Hypertension, and Hypertensive Disorders of Pregnancy. Brain Sci. 2019, 9, 224. [Google Scholar] [CrossRef]
- Williams, K.; MacLean, C. Transcranial assessment of maternal cerebral blood flow velocity in normal vs. hypertensive states. Variations with maternal posture. J. Reprod. Med. 1994, 39, 685–688. [Google Scholar]
- Bergman, L.; Cluver, C.; Carlberg, N.; Belfort, M.; Tolcher, M.C.; Panerai, R.B.; van Veen, T. Cerebral perfusion pressure and autoregulation in eclampsia-a case-control study. Am. J. Obstet. Gynecol. 2021, 225, e185.e1–e185.e9. [Google Scholar] [CrossRef]
- Yemini, M.; Lancet, M.; Mass, N.; Feinstein, M.; Katz, Z. Predictive value of roll-over test in women with mild preeclampsia. Am. J. Obstet. Gynecol. 1985, 153, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Goldkrand, J.W.; Jackson, M.J. Blood pressure measurement in pregnant women in the left lateral recumbent position. Am. J. Obstet. Gynecol. 1997, 176, 642–643. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, S.M.; Spencer, J.A.D. Blood pressure measurement in the lateral position. Br. J. Obstet. Gynaecol. 1989, 96, 110–112. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W. Predicting preeclampsia. Obstet. Gynecol. 1990, 75, 445–452. [Google Scholar] [PubMed]
- Annesi, L.; Tossetta, G.; Borghi, C.; Piani, F. The Role of Xanthine Oxidase in Pregnancy Complications: A Systematic Review. Antioxidants 2024, 13, 1234. [Google Scholar] [CrossRef]
- Cipolla, M.J. The adaptation of the cerebral circulation to pregnancy: Mechanisms and consequences. J. Cereb. Blood Flow. Metab. 2013, 33, 465–478. [Google Scholar] [CrossRef]
- Younes, S.T.; Ryan, M.J. Pathophysiology of Cerebral Vascular Dysfunction in Pregnancy-Induced Hypertension. Curr. Hypertens. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Belfort, M.A.; Kennedy, A.; Rassner, U.A. Novel techniques for cerebral evaluation in preeclampsia and eclampsia. Clin. Obstet. Gynecol. 2005, 48, 387–405. [Google Scholar] [CrossRef]
- van Veen, T.R.; Panerai, R.B.; Haeri, S.; Singh, J.; Adusumalli, J.A.; Zeeman, G.G.; Belfort, M.A. Cerebral autoregulation in different hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 2015, 212, 513.e1–513.e7. [Google Scholar] [CrossRef]
- Gant, N.F.; Chand, S.; Worley, R.J.; Whalley, P.J.; Crosby, U.D.; MacDonald, P.C. A clinical test useful for predicting the development of acute hypertension in pregnancy. Am. J. Obstet. Gynecol. 1974, 120, 1–7. [Google Scholar] [CrossRef]
- Kaypour, F.; Masomi Rad, H.; Ranjbar Novin, N. The predictive value of serum uric acid, roll-over test, and body mass index in pre-eclampsia. Int. J. Gynaecol. Obstet. 2006, 92, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Zatik, J.; Major, T.; Aranyosi, J.; Molnár, C.; Limburg, M.; Fülesdi, B. Assessment of cerebral hemodynamics during roll over test in healthy pregnant women and those with pre-eclampsia. BJOG 2001, 108, 353–358. [Google Scholar] [PubMed]
- Williams, K.P.; Wilson, S. Maternal middle cerebral blood flow velocity variation with gestational age. Obstet. Gynecol. 1994, 84, 445–448. [Google Scholar] [PubMed]
- Zatik, J.; Aranyosi, J.; Mihálka, L.; Páll, D.; Major, T.; Fülesdi, B. Comparison of cerebral blood flow velocity as measured in preeclamptic, healthy pregnant, and nonpregnant women by transcranial Doppler sonography. Gynecol. Obstet. Investig. 2001, 51, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Demarin, V.; Rundek, T.; Kodek, B. Maternal cerebral circulation in normal and abnormal pregnancies. Acta Obstet. Gynecol. Scand. 1997, 76, 619–624. [Google Scholar] [CrossRef]
- Hansen, W.F.; Burnham, S.J.; Svendsen, T.O.; Katz, V.L.; Thorp, J.M.; Hansen, A.R. Transcranial Doppler findings of cerebral vasospasm in pre-eclampsia. J. Matern. Fetal Med. 1996, 5, 194–200. [Google Scholar] [CrossRef]
- Zatik, J.; Aranyosi, J.; Molnár, C.; Páll, D.; Borsos, A.; Fülesdi, B. Effect of hyperventilation on cerebral blood flow velocity in preeclamptic pregnancies: Is there evidence for an altered cerebral vasoreactivity? J. Neuroimaging 2001, 11, 179–183. [Google Scholar] [CrossRef]
- Zatik, J.; Aranyosi, J.; Settakis, G.; Páll, D.; Tóth, Z.; Limburg, M.; Fülesdi, B. Breath-holding test in preeclampsia: Lack of evidence for altered cerebral vascular reactivity. Int. J. Obstet. Anesth. 2002, 11, 160–163. [Google Scholar] [CrossRef]
- Belfort, M.A.; Varner, M.W.; Dizon-Townson, D.S.; Grunewald, C.; Nisell, H. Cerebralperfusion pressure, and not cerebral blood flow, may be the critical determinant of intracranial injury in preeclampsia: A new hypothesis. Am. J. Obstet. Gynecol. 2002, 187, 626–634. [Google Scholar] [CrossRef]

| Control (n = 21) | Mild PE (n = 11) | Severe PE (n = 18) | |
|---|---|---|---|
| Age (years) | 26.1 ± 5.6 | 28.2 ± 6.8 | 29.1 ± 7.4 |
| Gestational age (weeks) | 37.2 ± 3.1 | 32.7 ± 4.8 | 35.1 ± 3.4 |
| Previous pregnancies (n) | 1.5 (1–4) | 1 (0–2) | 1 (1–3) |
| Previous births (n) | 1 (0–3) | 0 (0–1) | 1 (0–2) |
| Leading symptoms of PE | NA | H: 9 H + P: 2 | H + P: 14H: 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, D.T.; Fülesdi, B.; Kozma, B.; Páll, D.; Szatmári, S.; Hupuczi, P. Assessment of Cerebral Hemodynamic Changes During Roll-Over Test in Healthy Pregnant Women and Those with Mild and Severe Preeclampsia. J. Clin. Med. 2025, 14, 1182. https://doi.org/10.3390/jcm14041182
Nagy DT, Fülesdi B, Kozma B, Páll D, Szatmári S, Hupuczi P. Assessment of Cerebral Hemodynamic Changes During Roll-Over Test in Healthy Pregnant Women and Those with Mild and Severe Preeclampsia. Journal of Clinical Medicine. 2025; 14(4):1182. https://doi.org/10.3390/jcm14041182
Chicago/Turabian StyleNagy, Dániel T., Béla Fülesdi, Bence Kozma, Dénes Páll, Szilárd Szatmári, and Petronella Hupuczi. 2025. "Assessment of Cerebral Hemodynamic Changes During Roll-Over Test in Healthy Pregnant Women and Those with Mild and Severe Preeclampsia" Journal of Clinical Medicine 14, no. 4: 1182. https://doi.org/10.3390/jcm14041182
APA StyleNagy, D. T., Fülesdi, B., Kozma, B., Páll, D., Szatmári, S., & Hupuczi, P. (2025). Assessment of Cerebral Hemodynamic Changes During Roll-Over Test in Healthy Pregnant Women and Those with Mild and Severe Preeclampsia. Journal of Clinical Medicine, 14(4), 1182. https://doi.org/10.3390/jcm14041182










