Bayesian Modeling of the Impact of HBOT on the Reduction in Cytokine Storms
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Study Design
2.3. Detection of Cytokines and Growth Factors in Serum
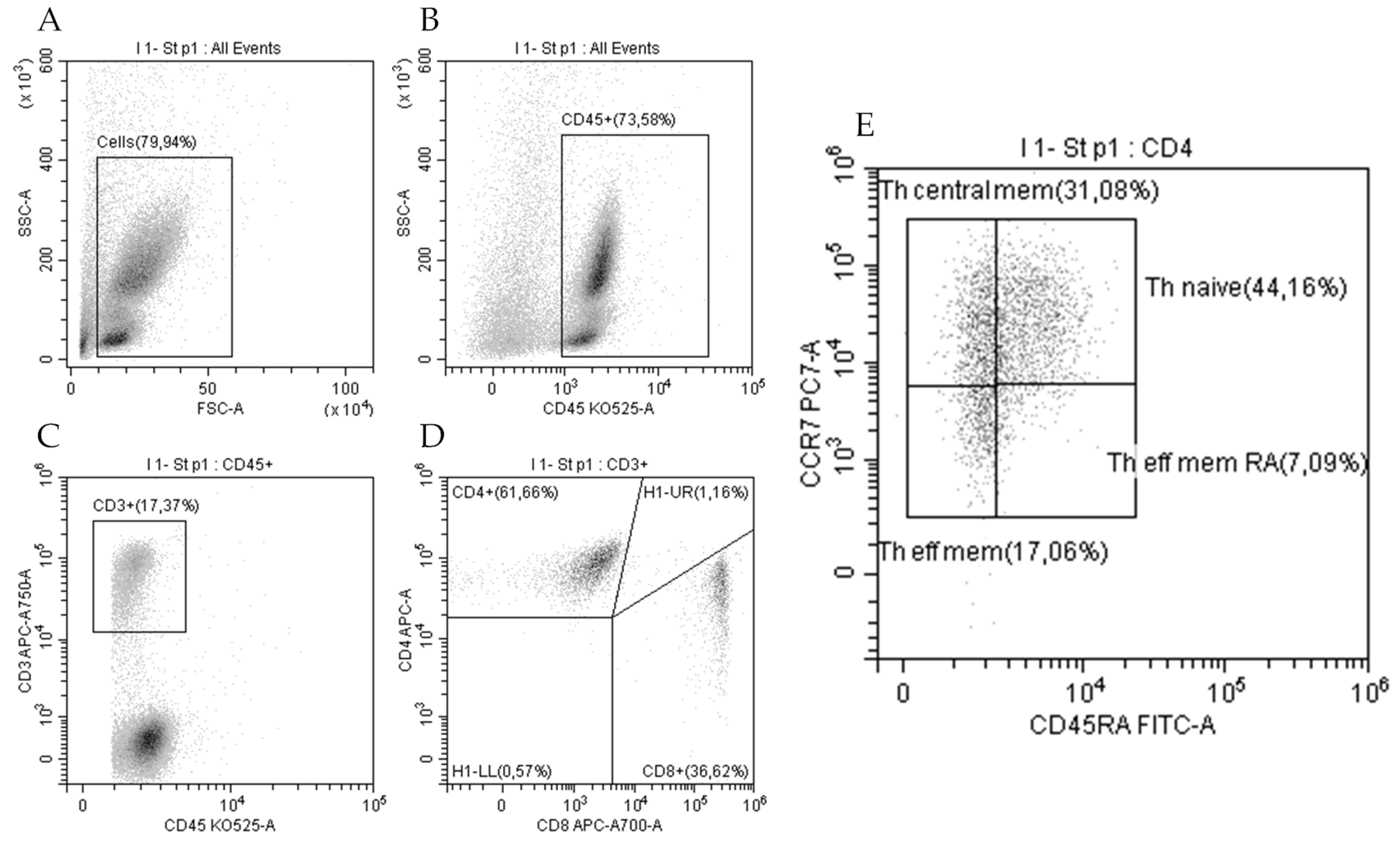
2.4. T-Cell Immunophenotyping
2.5. Model Construction and Validation
3. Results
3.1. Patients
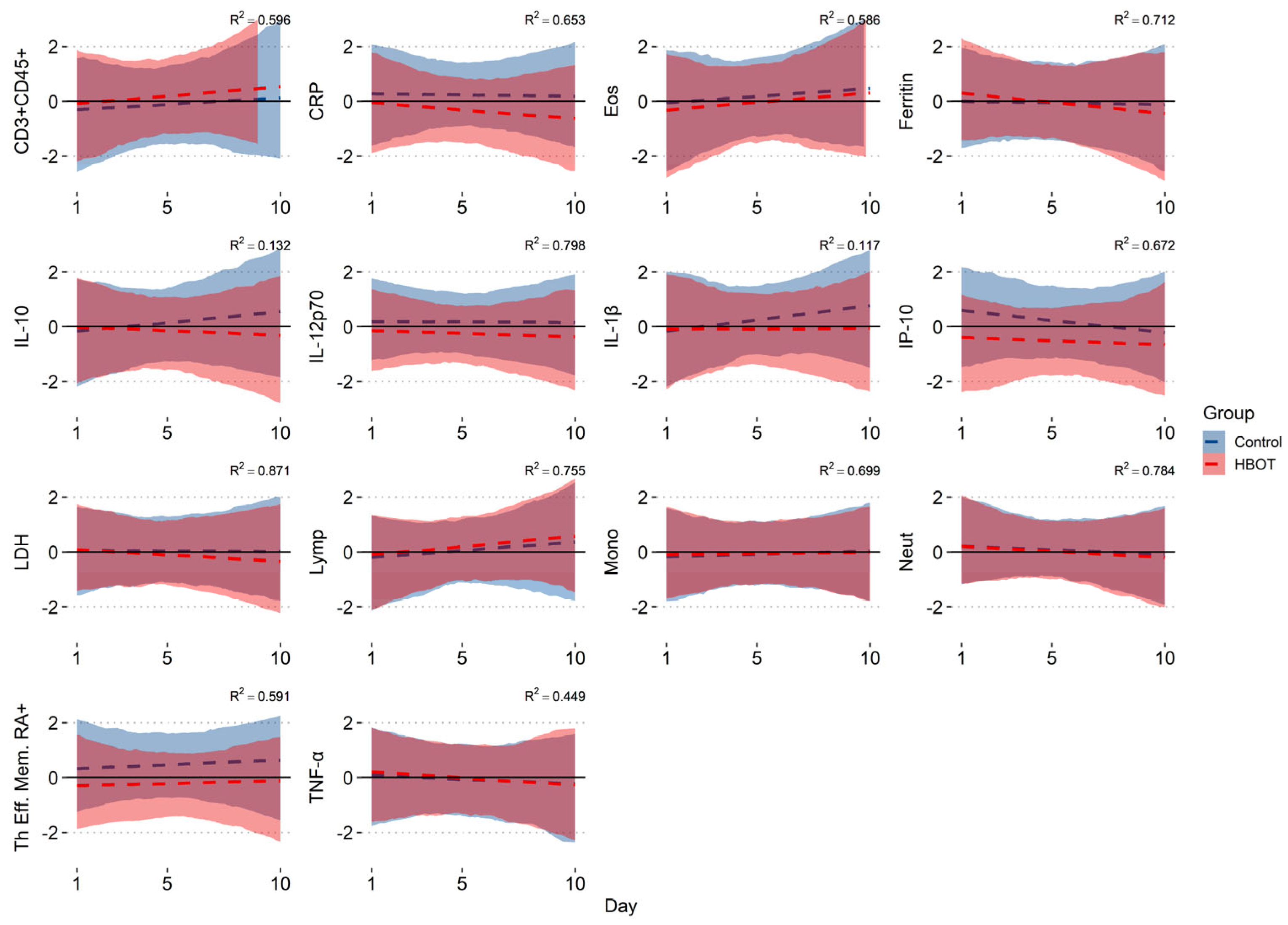
3.2. Bayesian Model Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 22 January 2025).
- WHO. Currently Circulating Variants of Interest (VOIs) (as of 28 June 2024). Available online: https://gisaid.org/hcov19-variants/ (accessed on 22 January 2025).
- Oliaei, S.; Paranjkhoo, P.; SeyedAlinaghi, S.; Mehraeen, E.; Hackett, D. Is There a Role for Hyperbaric Oxygen Therapy in Reducing Long-Term COVID-19 Sequelae? J. Clin. Med. 2023, 12, 2270. [Google Scholar] [CrossRef] [PubMed]
- Kjellberg, A.; Hassler, A.; Boström, E.; El Gharbi, S.; Al-Ezerjawi, S.; Kowalski, J.; Rodriguez-Wallberg, K.A.; Bruchfeld, J.; Ståhlberg, M.; Nygren-Bonnier, M.; et al. Hyperbaric oxygen therapy for long COVID (HOT-LoCO), an interim safety report from a randomised controlled trial. BMC Infect. Dis. 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- Zamani Rarani, F.; Zamani Rarani, M.; Hamblin, M.R.; Rashidi, B.; Hashemian, S.M.R.; Mirzaei, H. Comprehensive overview of COVID-19-related respiratory failure: Focus on cellular interactions. Cell Mol. Biol. Lett. 2022, 27, 63. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.S.; Bertolino, L.; Zampino, R.; Durante-Mangoni, E.; Monaldi Hospital Cardiovascular Infection Study Group. Cardiac sequelae after coronavirus disease 2019 recovery: A systematic review. Clin. Microbiol. Infect. 2021, 27, 1250–1261. [Google Scholar] [CrossRef]
- Cothran, T.P.; Kellman, S.; Singh, S.; Beck, J.S.; Powell, K.J.; Bolton, C.J.; Tam, J.W. A brewing storm: The neuropsychological sequelae of hyperinflammation due to COVID-19. Brain Behav. Immun. 2020, 88, 957–958. [Google Scholar] [CrossRef]
- Gheware, A.; Ray, A.; Rana, D.; Bajpai, P.; Nambirajan, A.; Arulselvi, S.; Mathur, P.; Trikha, A.; Arava, S.; Das, P.; et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci. Rep. 2022, 12, 4058. [Google Scholar] [CrossRef]
- Perkins, G.D.; Ji, C.; Connolly, B.A.; Couper, K.; Lall, R.; Baillie, J.K.; Bradley, J.M.; Dark, P.; Dave, C.; De Soyza, A.; et al. Effect of Noninvasive Respiratory Strategies on Intubation or Mortality Among Patients with Acute Hypoxemic Respiratory Failure and COVID-19: The RECOVERY-RS Randomized Clinical Trial. JAMA 2022, 327, 546–558. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.J.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef]
- Gil-Etayo, F.J.; Suarez-Fernandez, P.; Cabrera-Marante, O.; Arroyo, D.; Garcinuno, S.; Naranjo, L.; Pleguezuelo, D.E.; Allende, L.M.; Mancebo, E.; Lalueza, A.; et al. T-Helper Cell Subset Response Is a Determining Factor in COVID-19 Progression. Front. Cell. Infect. Microbiol. 2021, 11, 624483. [Google Scholar]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology–Current perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Habel, J.R.; Nguyen, T.H.O.; van de Sandt, C.E.; Juno, J.A.; Chaurasia, P.; Wragg, K.; Koutsakos, M.; Hensen, L.; Jia, X.; Chua, B.; et al. Suboptimal SARS-CoV-2-specific CD8(+) T cell response associated with the prominent HLA-A*02:01 phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 24384–24391. [Google Scholar] [CrossRef] [PubMed]
- Laing, A.G.; Lorenc, A.; del Molino del Barrio, I.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Gorenstein, S.A.; Castellano, M.L.; Slone, E.S.; Gillette, B.; Liu, H.; Alsamarraie, C.; Jacobson, A.M.; Wall, S.P.; Adhikari, S.; Swartz, J.L.; et al. Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: Treated cases versus propensity-matched controls. Undersea Hyperb. Med. 2020, 47, 405–413. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Li, Y.; Huang, J.-A.; Jiang, J.; Su, N. Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction. Virol. J. 2021, 18, 117. [Google Scholar] [CrossRef]
- Rarani, F.Z.; Rashidi, B.; Jafari Najaf Abadi, M.H.; Hamblin, M.R.; Reza Hashemian, S.M.; Mirzaei, H. Cytokines and microRNAs in SARS-CoV-2: What do we know? Mol. Ther. Nucleic Acids. 2022, 29, 219–242. [Google Scholar] [CrossRef]
- Mulchandani, R.; Lyngdoh, T.; Kakkar, A.K. Deciphering the COVID-19 cytokine storm: Systematic review and meta-analysis. Eur. J. Clin. Investig. 2021, 51, e13429. [Google Scholar] [CrossRef]
- Kuppalli, K.; Rasmussen, A.L. A glimpse into the eye of the COVID-19 cytokine storm. EBioMedicine 2020, 55, 102789. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, H.; Yan, X.; Ma, S.; Yao, X.; Shi, Y.; Ping, Y.; Cao, M.; Peng, C.; Wang, S.; et al. Neutrophil infiltration and myocarditis in patients with severe COVID-19: A post-mortem study. Front. Cardiovasc. Med. 2022, 9, 1026866. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Stojkov, D.; Fettrelet, T.; Bilyy, R.; Yousefi, S.; Simon, H.U. Transcriptional Insights of Oxidative Stress and Extracellular Traps in Lung Tissues of Fatal COVID-19 Cases. Int. J. Mol. Sci. 2023, 24, 2646. [Google Scholar] [CrossRef] [PubMed]
- Veenith, T.; Martin, H.; Le Breuilly, M.; Whitehouse, T.; Gao-Smith, F.; Duggal, N.; Lord, J.M.; Mian, R.; Sarphie, D.; Moss, P. High generation of reactive oxygen species from neutrophils in patients with severe COVID-19. Sci. Rep. 2022, 12, 10484. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516, Erratum in Nat. Rev. Immunol. 2020, 20, 579. [Google Scholar] [CrossRef]
- Mathieu, D.; Marroni, A.; Kot, J. Tenth European Consensus Conference on Hyperbaric Medicine: Recommendations for accepted and non-accepted clinical indications and practice of hyperbaric oxygen treatment. Diving Hyperbar. Med. J. South Pac. Under. Med. Soc. 2017, 47, 24–32. [Google Scholar]
- Shinomiya, N.; Asai, Y. Hyperbaric Oxygenation Therapy: Molecular Mechanisms and Clinical Applications; Springer Nature: Singapore, 2019; pp. 55–65. [Google Scholar]
- Boet, S.; Martin, L.; Cheng-Boivin, O.; Etherington, C.; Louge, P.; Pignel, R.; Pellégrini, M.; Magnan, M.-A.; Bennett, M. Can preventive hyperbaric oxygen therapy optimise surgical outcome?: A systematic review of randomised controlled trials. Eur. J. Anaesthesiol. 2020, 37, 636–648. [Google Scholar] [CrossRef]
- Fosen, K.M.; Thom, S.R. Hyperbaric oxygen, vasculogenic stem cells, and wound healing. Antioxid. Redox. Signal. 2014, 21, 1634–1647. [Google Scholar] [CrossRef]
- de Wolde, S.D.; Hulskes, R.H.; de Jonge, S.W.; Hollmann, M.W.; van Hulst, R.A.; Weenink, R.P.; Kox, M. The Effect of Hyperbaric Oxygen Therapy on Markers of Oxidative Stress and the Immune Response in Healthy Volunteers. Front. Physiol. 2022, 13, 826163. [Google Scholar] [CrossRef]
- Bosco, G.; Paganini, M.; Giacon, T.A.; Oppio, A.; Vezzoli, A.; Dellanoce, C.; Moro, T.; Paoli, A.; Zanotti, F.; Zavan, B.; et al. Oxidative Stress and Inflammation, MicroRNA, and Hemoglobin Variations after Administration of Oxygen at Different Pressures and Concentrations: A Randomized Trial. Int. J. Environ. Res. Public Health 2021, 18, 9755. [Google Scholar] [CrossRef]
- Lalieu, R.C.; Brouwer, R.J.; Ubbink, D.T.; Hoencamp, R.; Bol Raap, R.; van Hulst, R.A. Hyperbaric oxygen therapy for nonischemic diabetic ulcers: A systematic review. Wound Repair Regen. 2020, 28, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.J.; Lalieu, R.C.; Hoencamp, R.; van Hulst, R.A.; Ubbink, D.T. A systematic review and meta-analysis of hyperbaric oxygen therapy for diabetic foot ulcers with arterial insufficiency. J. Vasc. Surg. 2020, 71, 682–692.e681. [Google Scholar] [CrossRef] [PubMed]
- Löndahl, M.; Boulton, A.J. Hyperbaric oxygen therapy in diabetic foot ulceration: Useless or useful? A battle. Diabetes/Metab. Res. Rev. 2020, 36, e3233. [Google Scholar] [CrossRef] [PubMed]
- Lerche, C.J.; Schwartz, F.; Pries-Heje, M.M.; Fosbøl, E.L.; Iversen, K.; Jensen, P.Ø.; Høiby, N.; Hyldegaard, O.; Bundgaard, H.; Moser, C. Potential advances of adjunctive hyperbaric oxygen therapy in infective endocarditis. Front. Cell. Infect. Microbiol. 2022, 12, 805964. [Google Scholar] [CrossRef]
- Hajhosseini, B.; Kuehlmann, B.A.; Bonham, C.A.; Kamperman, K.J.; Gurtner, G.C. Hyperbaric oxygen therapy: Descriptive review of the technology and current application in chronic wounds. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3136. [Google Scholar] [CrossRef]
- Vinkel, J.; Rib, L.; Buil, A.; Hedetoft, M.; Hyldegaard, O. Key pathways and genes that are altered during treatment with hyperbaric oxygen in patients with sepsis due to necrotizing soft tissue infection (HBOmic study). Eur. J. Med. Res. 2023, 28, 507. [Google Scholar] [CrossRef]
- Wu, X.; Liang, T.Y.; Wang, Z.; Chen, G. The role of hyperbaric oxygen therapy in inflammatory bowel disease: A narrative review. Med. Gas. Res. 2021, 11, 66–71. [Google Scholar] [CrossRef]
- Zhong, X.T.Y.; Chen, R. Effect of Hyperbaric Oxygen Therapy on HBOT in Patients with Severe New Coronavirus Pneumonia: First Report Chinese. Chin. J. Naut. Med. Hyperb. Med. 2020, 27, 132–135. [Google Scholar]
- ECHM. European Committee for Hyperbaric Medicine (ECHM) Position on Hyperbaric Oxygen Therapy (HBOT) in Multiplace Chambers During Coronavirus Disease (COVID-19) Outbreak. Available online: http://www.echm.org/documents/ECHM%20position%20on%20HBOT%20and%20COVID-19%20(16th%20March%202020).pdf (accessed on 8 September 2024).
- Cannellotto, M.; Duarte, M.; Keller, G.; Larrea, R.; Cunto, E.; Chediack, V.; Mansur, M.; Brito, D.M.; García, E.; Di Salvo, H.E.; et al. Hyperbaric oxygen as an adjuvant treatment for patients with COVID-19 severe hypoxaemia: A randomised controlled trial. Emerg. Med. J. 2022, 39, 88–93. [Google Scholar] [CrossRef]
- Siewiera, J.; Brodaczewska, K.; Jermakow, N.; Lubas, A.; Kłos, K.; Majewska, A.; Kot, J. Effectiveness of Hyperbaric Oxygen Therapy in SARS-CoV-2 Pneumonia: The Primary Results of a Randomised Clinical Trial. J. Clin. Med. 2023, 12, 8. [Google Scholar] [CrossRef]
- Keller, G.A.; Colaianni, I.; Coria, J.; Di Girolamo, G.; Miranda, S. Clinical and biochemical short-term effects of hyperbaric oxygen therapy on SARS-Cov-2+ hospitalized patients with hypoxemic respiratory failure. Respir. Med. 2023, 209, 107155. [Google Scholar] [CrossRef] [PubMed]
- Bhaiyat, A.M.; Sasson, E.; Wang, Z.; Khairy, S.; Ginzarly, M.; Qureshi, U.; Fikree, M.; Efrati, S. Hyperbaric oxygen treatment for long coronavirus disease-19: A case report. J. Med. Case. Rep. 2022, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Leitman, M.; Fuchs, S.; Tyomkin, V.; Hadanny, A.; Zilberman-Itskovich, S.; Efrati, S. The effect of hyperbaric oxygen therapy on myocardial function in post-COVID-19 syndrome patients: A randomized controlled trial. Sci. Rep. 2023, 13, 9473. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.; Gonevski, M.; Clark, C.; Baitule, S.; Sharma, K.; Magar, A.; Patel, K.; Sankar, S.; Kyrou, I.; Ali, A.; et al. Hyperbaric oxygen therapy for the treatment of long COVID: Early evaluation of a highly promising intervention. Clin. Med. 2021, 21, e629–e632. [Google Scholar] [CrossRef]
- Ceban, F.; Leber, A.; Jawad, M.Y.; Yu, M.; Lui, L.M.W.; Subramaniapillai, M.; Di Vincenzo, J.D.; Gill, H.; Rodrigues, N.B.; Cao, B.; et al. Registered clinical trials investigating treatment of long COVID: A scoping review and recommendations for research. Infect. Dis. 2022, 54, 467–477. [Google Scholar] [CrossRef]
- Odak, I.; Barros-Martins, J.; Bošnjak, B.; Stahl, K.; David, S.; Wiesner, O.; Busch, M.; Hoeper, M.M.; Pink, I.; Welte, T.; et al. Reappearance of effector T cells is associated with recovery from COVID-19. EBioMedicine 2020, 57, 102885. [Google Scholar] [CrossRef]
- Bergersen, K.V.; Pham, K.; Li, J.; Ulrich, M.T.; Merrill, P.; He, Y.; Alaama, S.; Qiu, X.; Harahap-Carrillo, I.S.; Ichii, K.; et al. Health disparities in COVID-19: Immune and vascular changes are linked to disease severity and persist in a high-risk population in Riverside County, California. BMC Public Health 2023, 23, 1584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jermakow, N.; Brodaczewska, K.; Kot, J.; Lubas, A.; Kłos, K.; Siewiera, J. Bayesian Modeling of the Impact of HBOT on the Reduction in Cytokine Storms. J. Clin. Med. 2025, 14, 1180. https://doi.org/10.3390/jcm14041180
Jermakow N, Brodaczewska K, Kot J, Lubas A, Kłos K, Siewiera J. Bayesian Modeling of the Impact of HBOT on the Reduction in Cytokine Storms. Journal of Clinical Medicine. 2025; 14(4):1180. https://doi.org/10.3390/jcm14041180
Chicago/Turabian StyleJermakow, Natalia, Klaudia Brodaczewska, Jacek Kot, Arkadiusz Lubas, Krzysztof Kłos, and Jacek Siewiera. 2025. "Bayesian Modeling of the Impact of HBOT on the Reduction in Cytokine Storms" Journal of Clinical Medicine 14, no. 4: 1180. https://doi.org/10.3390/jcm14041180
APA StyleJermakow, N., Brodaczewska, K., Kot, J., Lubas, A., Kłos, K., & Siewiera, J. (2025). Bayesian Modeling of the Impact of HBOT on the Reduction in Cytokine Storms. Journal of Clinical Medicine, 14(4), 1180. https://doi.org/10.3390/jcm14041180






