Peak Eccentric Cycling Exercise and Cardiorespiratory Responses to Normobaric Hypoxia Versus Normobaric Normoxia in Healthy Adults: A Randomized, Controlled Crossover Trial
Abstract
1. Introduction
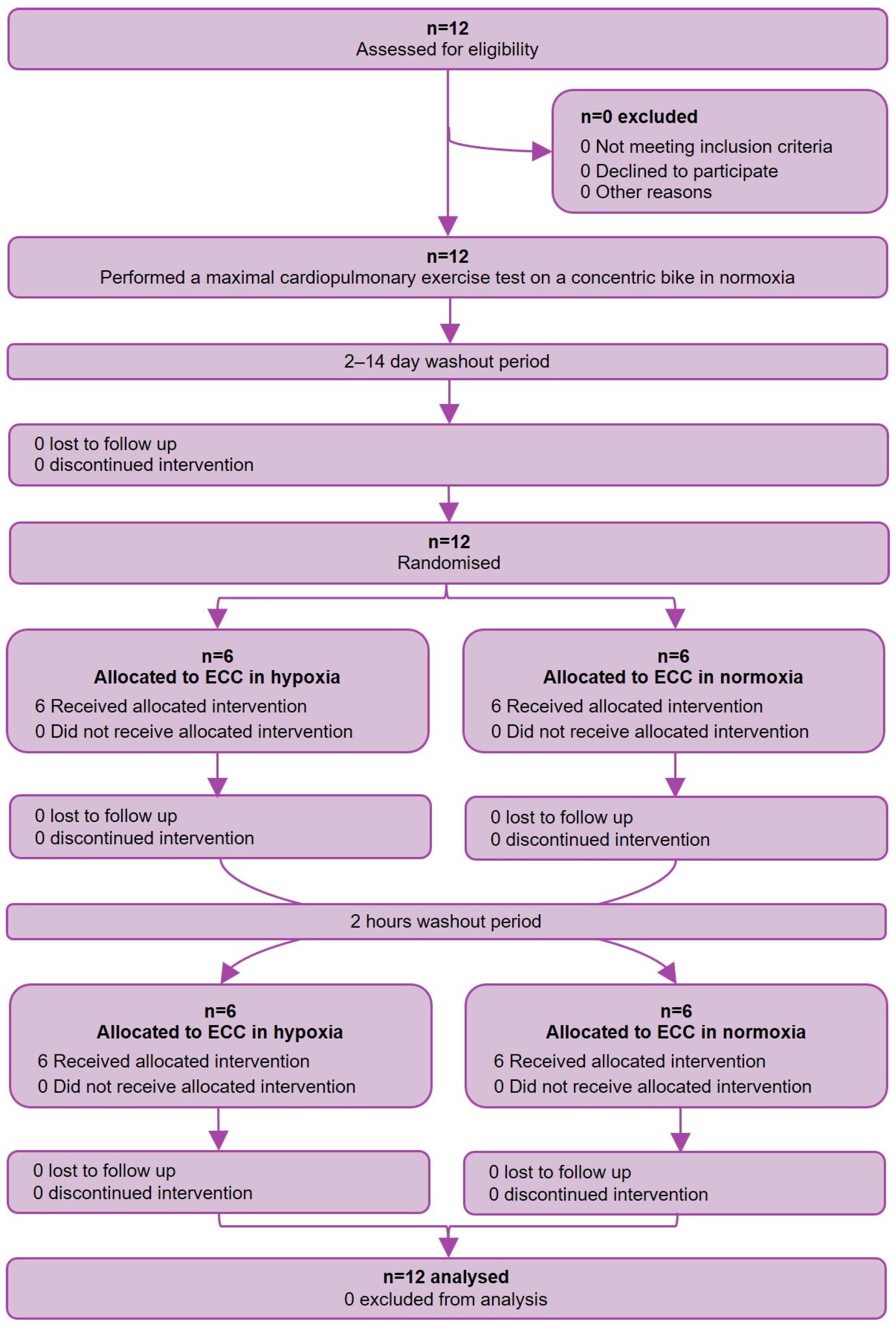
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Procedure, Assessments, and Outcomes
2.4. Statistical Analysis and Sample Size
3. Results
3.1. Baseline Characteristics
3.2. ECC Normoxia Versus Hypoxia
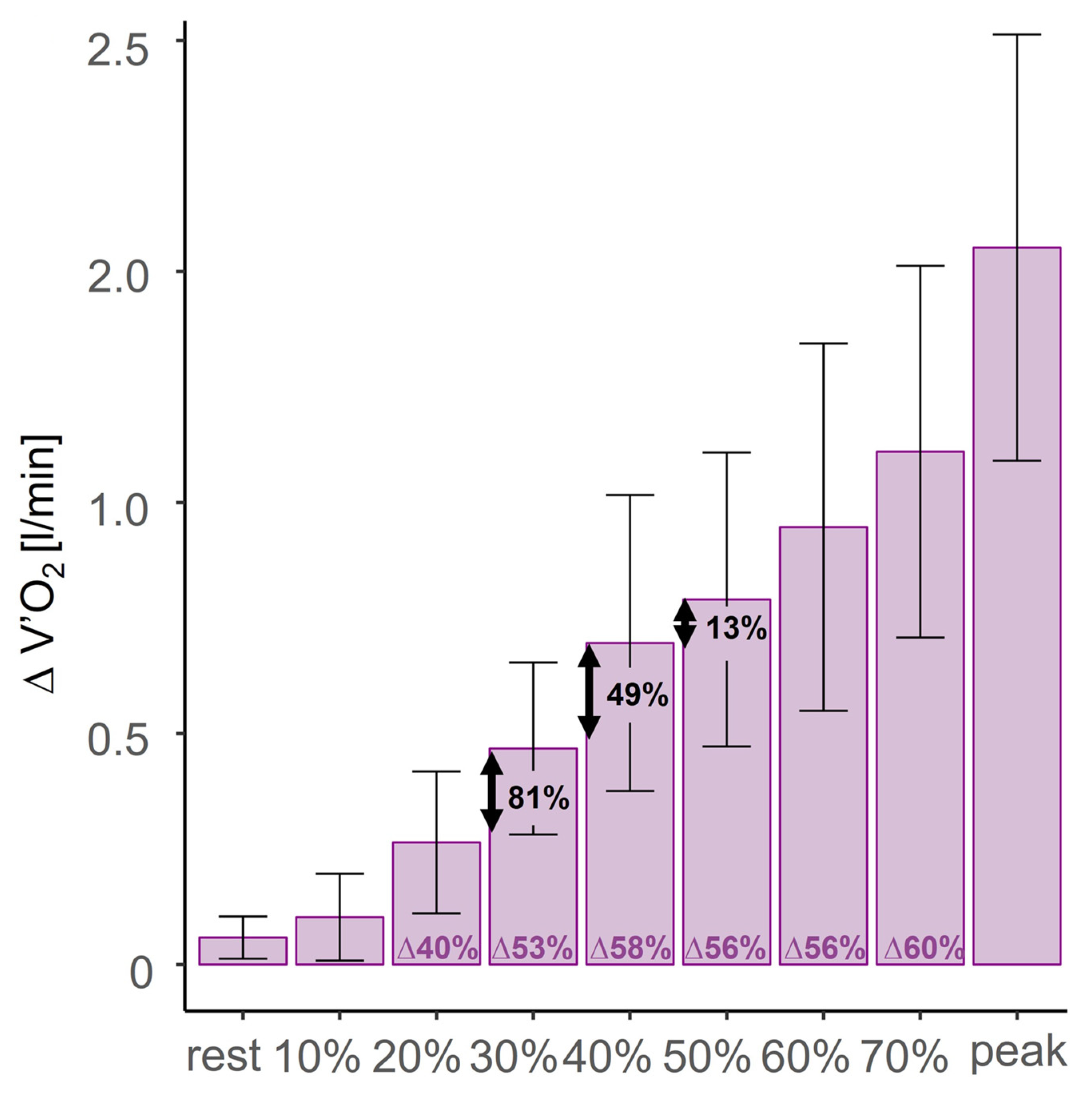
3.3. Normoxic CON Versus Normoxic ECC
4. Discussion
4.1. Minimal Intensity for Maximal Metabolic Cost Reduction in Normoxic ECC
4.2. Implication for ECC in HA Rehabilitation Settings
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CI | Confidence interval |
| CON | Concentric cycling |
| CR10 | Clinical rating scale 10 |
| ECC | Eccentric cycling |
| HA | High altitude |
| HR | Heart rate |
| O2 | Oxygen |
| O2Pulse | Oxygen pulse |
| RPM | Repetitions per minute |
| SD | Standard deviation |
| SpO2 | Oxygen saturation |
| V’CO2 | Carbon dioxide output |
| V’E | Minute ventilation |
| V’E/VO2 | Ventilatory equivalent for O2 |
| V’E/V’CO2 | Ventilatory equivalent for CO2 |
| V’O2 | Oxygen uptake |
| LD | Linear dichroism |
References
- Desplanches, D.; Hoppeler, H.; Linossier, M.T.; Denis, C.; Claassen, H.; Dormois, D.; Lacour, J.R.; Geyssant, A. Effects of training in normoxia and normobaric hypoxia on human muscle ultrastructure. Pflugers Arch. 1993, 425, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.; Puntschart, A.; Sundberg, C.J.; Jansson, E. Related expression of vascular endothelial growth factor and hypoxia-inducible factor-1 mRNAs in human skeletal muscle. Acta Physiol. Scand. 1999, 165, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Vogt, M.; Weibel, E.R.; Flück, M. Response of skeletal muscle mitochondria to hypoxia. Exp. Physiol. 2003, 88, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Lundby, C. Effects of exercise training in hypoxia versus normoxia on vascular health. Sports Med. 2016, 46, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Geiser, J.; Vogt, M.; Billeter, R.; Zuleger, C.; Belforti, F.; Hoppeler, H. Training high—Living low: Changes of aerobic performance and muscle structure with training at simulated altitude. Int. J. Sports Med. 2001, 22, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H.; Vogt, M. Muscle tissue adaptations to hypoxia. J. Exp. Biol. 2001, 204, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.; Puntschart, A.; Geiser, J.; Zuleger, C.; Billeter, R.; Hoppeler, H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J. Appl. Physiol. 2001, 91, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Zoll, J.; Ponsot, E.; Dufour, S.; Doutreleau, S.; Ventura-Clapier, R.; Vogt, M.; Hoppeler, H.; Richard, R.; Flück, M. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J. Appl. Physiol. 2006, 100, 1258–1266. [Google Scholar] [CrossRef]
- Bahenský, P.; Bunc, V.; Tlustý, P.; Grosicki, G.J. Effect of an eleven-day altitude training program on aerobic and anaerobic performance in adolescent runners. Medicina 2020, 56, 184. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B.; Baker, J. Training in hypoxia: Modulation of metabolic and cardiovascular risk factors in men. Med. Sci. Sports Exerc. 2000, 32, 1058–1066. [Google Scholar] [CrossRef]
- Chen, B.; Wu, Z.; Huang, X.; Li, Z.; Wu, Q.; Chen, Z. Effect of altitude training on the aerobic capacity of athletes: A systematic review and meta-analysis. Heliyon 2023, 9, e20188. [Google Scholar] [CrossRef] [PubMed]
- Ponsot, E.; Dufour, S.P.; Zoll, J.; Doutrelau, S.; N’Guessan, B.; Geny, B.; Hoppeler, H.; Lampert, E.; Mettauer, B.; Ventura-Clapier, R.; et al. Exercise training in normobaric hypoxia in endurance runners. II. Improvement of mitochondrial properties in skeletal muscle. J Appl Physiol (1985) 2006, 100, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.P.; Ponsot, E.; Zoll, J.; Doutreleau, S.; Lonsdorfer-Wolf, E.; Geny, B.; Lampert, E.; Flück, M.; Hoppeler, H.; Billat, V.; et al. Exercise training in normobaric hypoxia in endurance runners. I. Improvement in aerobic performance capacity. J Appl Physiol (1985) 2006, 100, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Titz, A.; Schneider, S.R.; Bauer, M.; Mayer, L.; Lüönd, L.; Ulrich, T.; Furian, M.; Forrer, A.; Schwarz, E.I.; et al. The effect of high altitude (2500 m) on incremental cycling exercise in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: A randomised controlled crossover trial. Eur. Respir. J. 2024, 63, 2301001. [Google Scholar] [CrossRef]
- Furian, M.; Flueck, D.; Latshang, T.D.; Scheiwiller, P.M.; Segitz, S.D.; Mueller-Mottet, S.; Murer, C.; Steiner, A.; Ulrich, S.; Rothe, T.; et al. Exercise performance and symptoms in lowlanders with COPD ascending to moderate altitude: Randomized trial. Int. J. Chron. Obstr. Pulm. Dis. 2018, 13, 3529–3538. [Google Scholar] [CrossRef] [PubMed]
- Furian, M.; Hartmann, S.E.; Latshang, T.D.; Flueck, D.; Murer, C.; Scheiwiller, P.M.; Osmonov, B.; Ulrich, S.; Kohler, M.; Poulin, M.J.; et al. Exercise Performance of Lowlanders with COPD at 2,590 m: Data from a Randomized Trial. Respiration 2018, 95, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Wehrlin, J.P.; Hallén, J. Linear decrease in .VO2max and performance with increasing altitude in endurance athletes. Eur. J. Appl. Physiol. 2006, 96, 404–412. [Google Scholar] [CrossRef]
- Dufour, S.P.; Lampert, E.; Doutreleau, S.; Lonsdorfer-Wolf, E.; Billat, V.L.; Piquard, F.; Richard, R. Eccentric cycle exercise: Training application of specific circulatory adjustments. Med. Sci. Sports Exerc. 2004, 36, 1900–1906. [Google Scholar] [CrossRef]
- Hody, S.; Croisier, J.-L.; Bury, T.; Rogister, B.; Leprince, P. Eccentric muscle contractions: Risks and benefits. Front. Physiol. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Steiner, R.; Lastayo, P.; Lippuner, K.; Allemann, Y.; Eberli, F.; Schmid, J.; Saner, H.; Hoppeler, H. Eccentric exercise in coronary patients: Central hemodynamic and metabolic responses. Med. Sci. Sports Exerc. 2003, 35, 1076–1082. [Google Scholar] [CrossRef]
- Perrey, S.; Betik, A.; Candau, R.; Rouillon, J.D.; Hughson, R.L. Comparison of oxygen uptake kinetics during concentric and eccentric cycle exercise. J. Appl. Physiol. 2001, 91, 2135–2142. [Google Scholar] [CrossRef]
- Touron, J.; Costes, F.; Coudeyre, E.; Perrault, H.; Richard, R. Aerobic metabolic adaptations in endurance eccentric exercise and training: From whole body to mitochondria. Front. Physiol. 2021, 11, 596351. [Google Scholar] [CrossRef]
- Lipski, M.; Abbiss, C.R.; Nosaka, K. Cardio-pulmonary responses to incremental eccentric and concentric cycling tests to task failure. Eur. J. Appl. Physiol. 2018, 118, 947–957. [Google Scholar] [CrossRef]
- Barreto, R.V.; de Lima, L.C.R.; Borszcz, F.K.; de Lucas, R.D.; Denadai, B.S. Chronic adaptations to eccentric cycling training: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2023, 20, 2861. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.L.; Bauldoff, G.S.; Carlin, B.W.; Casaburi, R.; Emery, C.F.; Mahler, D.A.; Make, B.; Rochester, C.L.; ZuWallack, R.; Herrerias, C. Pulmonary rehabilitation. Chest 2007, 131, 4S–42S. [Google Scholar] [CrossRef] [PubMed]
- Rochester, C.L.; Alison, J.A.; Carlin, B.; Jenkins, A.R.; Cox, N.S.; Bauldoff, G.; Bhatt, S.P.; Bourbeau, J.; Burtin, C.; Camp, P.G.; et al. Pulmonary rehabilitation for adults with chronic respiratory disease: An official american thoracic society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2023, 208, e7–e26. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzis, I. Strategies of muscle training in very severe COPD patients. Eur. Respir. J. 2011, 38, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.V.; De Lima, L.C.R.; Denadai, B.S. Moving forward with backward pedaling: A review on eccentric cycling. Eur. J. Appl. Physiol. 2021, 121, 381–407. [Google Scholar] [CrossRef] [PubMed]
- LaStayo, P.C.; Pierotti, D.J.; Pifer, J.; Hoppeler, H.; Lindstedt, S.L. Eccentric ergometry: Increases in locomotor muscle size and strength at low training intensities. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2000, 278, R1282–R1288. [Google Scholar] [CrossRef] [PubMed]
- Beijst, C. The Accuracy and Precision of Equipment for Cardiopulmonary Exercise Testing. Master’s Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2011. [Google Scholar]
- American Thoracic Society. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R. Physiological adaptation of the cardiovascular system to high altitude. Prog. Cardiovasc. Dis. 2010, 52, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Treml, B.; Gatterer, H.; Burtscher, J.; Kleinsasser, A.; Burtscher, M. A focused review on the maximal exercise responses in hypo- and normobaric hypoxia: Divergent oxygen uptake and ventilation responses. Int. J. Environ. Res. Public Health 2020, 17, 5239. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Schneider, S.R.; Bloch, K.E. Effect of hypoxia and hyperoxia on exercise performance in healthy individuals and in patients with pulmonary hypertension: A systematic review. J. Appl. Physiol. 2017, 123, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.D.; Gore, C.J.; Kemp, J. Application of ‘live low-train high’ for enhancing normoxic exercise performance in team sport athletes. Sports Med. 2014, 44, 1275–1287. [Google Scholar] [CrossRef]
- Coppel, J.; Hennis, P.; Gilbert-Kawai, E.; Grocott, M.P. The physiological effects of hypobaric hypoxia versus normobaric hypoxia: A systematic review of crossover trials. Extrem. Physiol. Med. 2015, 4, 2. [Google Scholar] [CrossRef]
- Luks, A.M.; Hackett, P.H. Medical conditions and high-altitude travel. N. Engl. J. Med. 2022, 386, 364–373. [Google Scholar] [CrossRef]
- Schneider, S.R.; Lichtblau, M.; Furian, M.; Mayer, L.C.; Berlier, C.; Müller, J.; Saxer, S.; Schwarz, E.I.; Bloch, K.E.; Ulrich, S. Cardiorespiratory adaptation to short-term exposure to altitude vs. Normobaric hypoxia in patients with pulmonary hypertension. J. Clin. Med. 2022, 11, 2769. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.T.; Wardlow, G.C.; Branch, P.A.; Ramos, M.; Black, C.D.; Ade, C.J. Effect of exercise-induced muscle damage on vascular function and skeletal muscle microvascular deoxygenation. Physiol. Rep. 2016, 4, e13032. [Google Scholar] [CrossRef]
- Hamacher, D.; Brennicke, M.; Behrendt, T.; Alt, P.; Törpel, A.; Schega, L. Motor-cognitive dual-tasking under hypoxia. Exp. Brain Res. 2017, 235, 2997–3001. [Google Scholar] [CrossRef]
- Jung, M.; Zou, L.; Yu, J.J.; Ryu, S.; Kong, Z.; Yang, L.; Kang, M.; Lin, J.; Li, H.; Smith, L.; et al. Does exercise have a protective effect on cognitive function under hypoxia? A systematic review with meta-analysis. J. Sport. Health Sci. 2020, 9, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.; Pearson, S.; Ross, A.; McGuigan, M. Eccentric exercise: Physiological characteristics and acute responses. Sports Med. 2017, 47, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Dünnwald, T.; Kienast, R.; Niederseer, D.; Burtscher, M. The use of pulse oximetry in the assessment of acclimatization to high altitude. Sensors 2021, 21, 1263. [Google Scholar] [CrossRef] [PubMed]
- Périard, J.D.; Travers, G.J.S.; Racinais, S.; Sawka, M.N. Cardiovascular adaptations supporting human exercise-heat acclimation. Auton. Neurosci. 2016, 196, 52–62. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Value |
|---|---|
| Total Participants | 12 |
| Female | 6 |
| Male | 6 |
| Age [years] | 30 (12) |
| Height [cm] | 178 (10.1) |
| Weight [kg] | 71.2 (11.1) |
| BMI [kg/m2] | 22.33 (3.78) |
| Peak [V’O2] | 2.54 (0.87) |
| Peak work rate [W] | 269 (91) |
| Eccentric Cycling | Normoxia | ||||||
|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia–Hypoxia | CON | CON–ECC | |||
| Mean (SD) | Mean (SD) | Mean Change [95%CI] | p-Value | Mean (SD) | Mean Change [95%CI] | p-Value | |
| Rest | |||||||
| V’O2 [L/min] | 0.21 (0.04) | 0.20 (0.01) | 0.01 [−0.04, 0.06] | 0.619 | 0.27 (0.08) | 0.06 [0.03, 0.09] | 0.001 * |
| V’O2/kg [L/min/kg] | 2.98 (0.50) | 2.83 (1.44) | 0.15 [−0.58, 0.90] | 0.661 | 3.79 (0.84) | 0.81 [0.45, 1.17] | 0.001 * |
| V’E/V’CO2 | 42.3 (8.10) | 48.1 (10.7) | −5.83 [−12.99, 1.33] | 0.105 | 41.42 (8.74) | −0.89 [−4.78, 3.00] | 0.650 |
| HR [bpm] | 63 (11) | 65 (13) | −2 [−8, 6] | 0.675 | 76 (9) | 13 [7, 18] | <0.001 * |
| V’E [L/min] | 10.3 (2.23) | 11.8 (3.93) | −1.42 [−3.75, −0.91] | 0.220 | 12.25 (3.25) | 1.92 [0.21, 3.63] | 0.043 * |
| O2Puls [mL/beat] | 3.40 (0.97) | 3.00 (1.39) | 0.40 [−0.36, 1.16] | 0.283 | 3.52 (0.92) | 0.13 [−0.04, 0.29] | 0.155 |
| SpO2 [%] | 97 (1) | 94 (3) | 3 [2, 5] | 0.001 * | 97 (1) | 0 [−1, 0] | 0.615 |
| 50% of individual maximal capacity | |||||||
| V’O2 [L/min] | 0.60(0.24) | 0.65 (0.27) | −0.05 [−0.12, −0.03] | 0.199 | 1.38 (0.47) | 0.79 [0.6, 0.98] | <0.001 * |
| V’O2/kg [L/min/kg] | 8.28 (2.36) | 9.05 (2.92) | −0.77 [−1.86, 0.58] | 0.289 | 19.58 (5.25) | 11.34 [8.8, 13.88] | <0.001 * |
| V’E/V’CO2 | 33.5 (6.29) | 35.9 (8.88) | −2.4 [−6.53, 1.58] | 0.217 | 30.63 (5.31) | −3.43 [−5.86, −1] | 0.015 * |
| HR [bpm] | 90 (8) | 97 (18) | −7 [−18, 2] | 0.128 | 122 (14) | 33 [24, 42] | <0.001 * |
| V’E [L/min] | 21.3 (5.69) | 24.0 (8.34) | −2.7 [−7.19, 1.79] | 0.224 | 41.67 (12.07) | 20.08 [13.49, 26.68] | <0.001 * |
| O2Puls [mL/beat] | 6.65 (2.50) | 6.53 (2.18) | 0.12 [−0.44, 0.98] | 0.434 | 11.10 (3.01) | 4.49 [3.63, 5.35] | <0.001 * |
| SpO2 [%] | 97 (1) | 92 (4) | 5 [3, 7] | <0.001 * | 96 (2) | −1 [−2, 0] | 0.095 |
| 70% of individual maximal capacity | |||||||
| V’O2 [L/min] | 0.77 (0.320) | 0.81 (0.352) | −0.04 [−0.11, 0.08] | 0.723 | 1.86 (0.62) | 1.11 [0.87, 1.35] | <0.001 * |
| V’O2/kg [L/min/kg] | 10.7 (3.35) | 11.2 (3.67) | −0.5 [−1.75, 1.48] | 0.862 | 26.46 (7.08) | 16.00 [12.63, 19.37] | <0.001 * |
| V’E/V’CO2 | 33.3 (7.18) | 34.9 (9.54) | −1.6 [−6.01, 2.19] | 0.342 | 30.91 (5.37) | −2.74 [−5.95, 0.47] | 0.110 |
| HR [bpm] | 100 (7) | 107 (20) | −7 [−16, 3] | 0.147 | 143 (15) | 43 [34, 52] | <0.001 * |
| V’E [L/min] | 26.0 (6.89) | 27.7 (10.5) | −1.7 [−5.08, 2.59] | 0.505 | 62.58 (15.30) | 36.92 [29.02, 44.81] | <0.001 * |
| O2Puls [mL/beat] | 7.67 (3.06) | 7.50 (2.73) | 0.17 [−0.47, 1.36] | 0.322 | 12.90 (3.30) | 5.30 [4.25, 6.35] | <0.001 * |
| SpO2 [%] | 97 (1) | 91 (5) | 6 [3, 9] | <0.001 * | 96 (2) | −1 [−2, 0] | 0.045 * |
| Peak exercise | |||||||
| Power [W] | 235 (84) | 213 (86) | 22 [8, 36] | 0.009 * | 269 (91) | 34 [18, 50] | 0.001 * |
| V’O2 [L/min] | 0.99 (0.51) | 0.90 (0.38) | 0.09 [−0.04, 0.22] | 0.172 | 2.54 (0.87) | 1.55 [1.28, 1.82] | <0.001 * |
| V’O2/kg [L/min/kg] | 13.6 (5.70) | 12.4 (4.35) | 1.2 [−0.76, 3.14] | 0.217 | 35.98 (9.95) | 22.35 [18.44, 26.26] | <0.001 * |
| V’E/V’CO2 | 33.3 (11.9) | 32.9 (7.80) | 0.4 [−6.46, 7.26] | 0.904 | 35.32 (4.09) | 2.04 [−4.27, 8.35] | 0.523 |
| HR [bpm] | 111 (15) | 114 (20) | −3 [−11, 5] | 0.459 | 167 (20) | 55 [43, 68] | <0.001 * |
| V’E [L/min] | 30.5 (10.4) | 29.4 (12.0) | 1.1 [−4.63, 6.79] | 0.696 | 116.6 (38.6) | 86.08 [66.43, 105.74] | <0.001 * |
| O2Puls [mL/beat] | 8.60 (3.57) | 7.68 (2.56) | 0.92 [−0.12, 1.96] | 0.081 | 13.20 (4.27) | 5.00 [2.99, 7.66] | 0.001 * |
| SpO2 [%] | 96 (1) | 91 (5) | 5 [3, 8] | 0.001 * | 87 (20) | −9 [−20, 2] | 0.115 |
| Dyspnea [CR10] | 3 (2) | 4 (2) | −1 [−1, 1] | 0.592 | 7 (2) | 4 [3, 5] | <0.001 * |
| Leg fatigue [CR10] | 6 (2) | 7 (2) | −1 [−2, 0] | 0.251 | 8 (2) | 2 [1, 3] | 0.011 * |
| Isotime (individual maximal workload (in watts) that a participant was able to perform under both conditions) | |||||||
| Power [W] | 213 (86) | 213 (86) | - | - | 213 (86) | - | - |
| V’O2 [L/min] | 0.88 (0.44) | 0.86 (0.38) | 0.02 [−0.06, 0.10] | 0.650 | 2.01 (0.964) | 1.13 [0.77, 1.48] | <0.001 * |
| V’O2/kg [L/min/kg] | 12.2 (4.88) | 12.0 (4.40) | 0.2 [−1.04, 1.51] | 0.708 | 28.24 (11.8) | 16.04 [11.03, 21.04] | <0.001 * |
| V’E/V’CO2 | 33.1 (7.50) | 33.2 (7.48) | −0.1 [−4.42, 4.25] | 0.969 | 38.73 (17.5) | 5.63 [−10.08, 7.63] | 0.783 |
| HR [bpm] | 107 (13) | 114 (20) | −7 [−14, 0] | 0.050 | 144 (28) | 37 [23, 53] | <0.001 * |
| V’E [L/min] | 29.7 (7.36) | 29.8 (11.9) | −0.1 [−7.75, 7.59] | 0.983 | 32.2 (4.69) | 2.5 [−4.22, 2.33] | 0.570 |
| O2Puls [mL/beat] | 8.12 (3.32) | 7.38 (2.45) | 0.74 [0.01, 1.47] | 0.048 * | 12.3 (4.15) | 4.18 [2.82, 5.90] | 0.002 * |
| SpO2 [%] | 97 (1) | 91 (5) | 6 [3, 9] | <0.001 * | 94 (3) | −3 [−4, −1] | 0.011 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wick, C.; Constam, E.; Schneider, S.R.; Titz, A.; Furian, M.; Lichtblau, M.; Ulrich, S.; Müller, J. Peak Eccentric Cycling Exercise and Cardiorespiratory Responses to Normobaric Hypoxia Versus Normobaric Normoxia in Healthy Adults: A Randomized, Controlled Crossover Trial. J. Clin. Med. 2025, 14, 1151. https://doi.org/10.3390/jcm14041151
Wick C, Constam E, Schneider SR, Titz A, Furian M, Lichtblau M, Ulrich S, Müller J. Peak Eccentric Cycling Exercise and Cardiorespiratory Responses to Normobaric Hypoxia Versus Normobaric Normoxia in Healthy Adults: A Randomized, Controlled Crossover Trial. Journal of Clinical Medicine. 2025; 14(4):1151. https://doi.org/10.3390/jcm14041151
Chicago/Turabian StyleWick, Carmen, Esther Constam, Simon R. Schneider, Anna Titz, Michael Furian, Mona Lichtblau, Silvia Ulrich, and Julian Müller. 2025. "Peak Eccentric Cycling Exercise and Cardiorespiratory Responses to Normobaric Hypoxia Versus Normobaric Normoxia in Healthy Adults: A Randomized, Controlled Crossover Trial" Journal of Clinical Medicine 14, no. 4: 1151. https://doi.org/10.3390/jcm14041151
APA StyleWick, C., Constam, E., Schneider, S. R., Titz, A., Furian, M., Lichtblau, M., Ulrich, S., & Müller, J. (2025). Peak Eccentric Cycling Exercise and Cardiorespiratory Responses to Normobaric Hypoxia Versus Normobaric Normoxia in Healthy Adults: A Randomized, Controlled Crossover Trial. Journal of Clinical Medicine, 14(4), 1151. https://doi.org/10.3390/jcm14041151






