Prospective Survey of Postoperative Pain in Japan: A Multicenter, Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Background for Seven Organ Surgeries
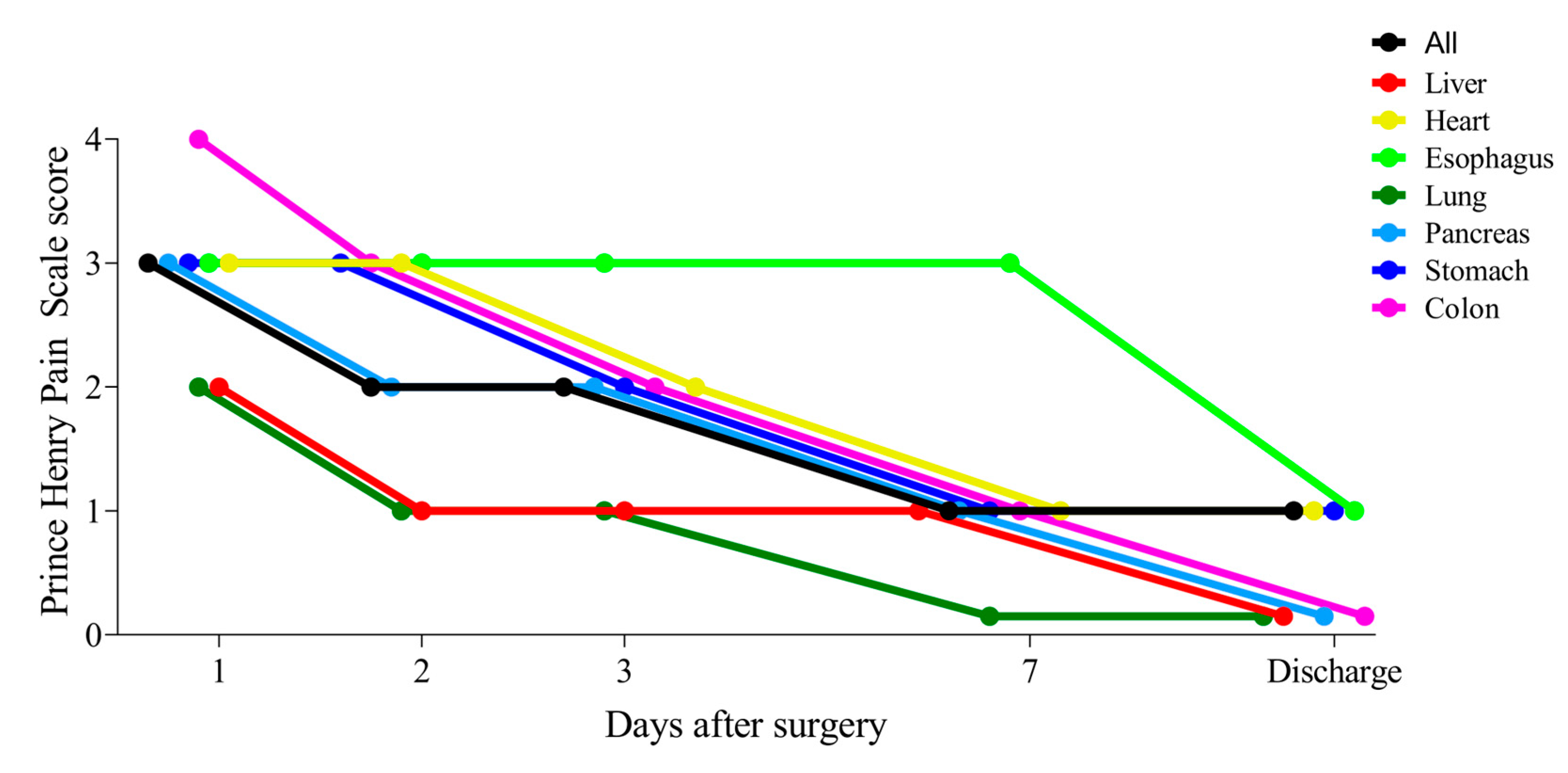
3.2. Changes in Postoperative Pain Following Seven Organ Surgeries
3.3. Correlations Between Clinical Factors and the Three Endpoints
3.4. Comparison of Clinical Factors on the Three Endpoints
3.5. Univariate and Multivariate Logistic Regression Analyses of Risk Factors for the Three Endpoints
3.6. Effects of Risk Factors Selected from Multivariate Analyses on Each Endpoint
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ERAS | Enhanced Recovery After Surgery |
| ESSENSE | Essential Strategy for Early Normalization after Surgery with Patient’s Excellent satisfaction |
| JSSMN | Japanese Society for Surgical Metabolism and Nutrition |
| QoR-15 | Quality of Recovery-15 |
| CRP | C-reactive Protein |
| ASA-PS | American Society of Anesthesiologists Physical Status |
| IV-PCA | Intravenous Patient-Controlled Analgesia |
References
- Fearon, K.C.H.; Ljungqvist, O.; Von Meyenfeldt, M.; Revhaug, A.; Dejong, C.H.C.; Lassen, K.; Nygren, J.; Hausel, J.; Soop, M.; Andersen, J.; et al. Enhanced Recovery after Surgery: A Consensus Review of Clinical Care for Patients Undergoing Colonic Resection. Clin. Nutr. 2005, 24, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN Guideline: Clinical Nutrition in Surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef] [PubMed]
- Visioni, A.; Shah, R.; Gabriel, E.; Attwood, K.; Kukar, M.; Nurkin, S. Enhanced Recovery After Surgery for Noncolorectal Surgery?: A Systematic Review and Meta-Analysis of Major Abdominal Surgery. Ann. Surg. 2018, 267, 57. [Google Scholar] [CrossRef]
- Makaryus, R.; Miller, T.E.; Gan, T.J. Current Concepts of Fluid Management in Enhanced Recovery Pathways. Br. J. Anaesth. 2018, 120, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Kaibori, M.; Miyata, G.; Yoshii, K.; Fukushima, R. Perioperative Management for Gastrointestinal Surgery after Instituting Interventions Initiated by the Japanese Society of Surgical Metabolism and Nutrition. Asian J. Surg. 2020, 43, 124–129. [Google Scholar] [CrossRef]
- Yamagata, Y.; Yoshikawa, T.; Yura, M.; Otsuki, S.; Morita, S.; Katai, H.; Nishida, T. Current Status of the “Enhanced Recovery after Surgery” Program in Gastric Cancer Surgery. Ann. Gastroenterol. Surg. 2019, 3, 231–238. [Google Scholar] [CrossRef]
- Taniguchi, H.; Sasaki, T.; Fujita, H.; Kobayashi, H.; Kawasaki, R.; Goloubev, M.; Ishikawa, T.; Takano, O.; Ogata, T.; Cho, H.; et al. Modified ERAS Protocol Using Preoperative Oral Rehydration Therapy: Outcomes and Issues. J. Anesth. 2014, 28, 143–147. [Google Scholar] [CrossRef]
- Kehlet, H. Postoperative pain, analgesia, and recovery—Bedfellows that cannot be ignored. Pain 2018, 159, S11. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Yamanaka, M.; Shono, A.; Fukuda, N.; Saito, Y. Preoperative Epidural Fentanyl Reduces Postoperative Pain after Upper Abdominal Surgery. J. Anesth. 2007, 21, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Yokouchi, T.; Tanaka, M. Effects of Intraoperative High-Dose vs Low-Dose Remifentanil for Postoperative Epidural Analgesia after Gynecological Abdominal Surgery: A Randomized Clinical Trial. J. Clin. Anesth. 2016, 32, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Shi, J.; Gao, T.; Shen, J.; Zhao, C.; Xi, F.; Li, W.; Li, Q.; Yu, W. The Addition of Dexmedetomidine to Analgesia for Patients After Abdominal Operations: A Prospective Randomized Clinical Trial. World J. Surg. 2017, 41, 39–46. [Google Scholar] [CrossRef]
- Stark, P.A.; Myles, P.S.; Burke, J.A. Development and Psychometric Evaluation of a Postoperative Quality of Recovery Score: The QoR-15. Anesthesiology 2013, 118, 1332–1340. [Google Scholar] [CrossRef]
- Campfort, M.; Cayla, C.; Lasocki, S.; Rineau, E.; Léger, M. Early Quality of Recovery According to QoR-15 Score Is Associated with One-Month Postoperative Complications after Elective Surgery. J. Clin. Anesth. 2022, 78, 110638. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Buvanendran, A.; Kroin, J.S. Multimodal Analgesia for Controlling Acute Postoperative Pain. Curr. Opin. Anesthesiol. 2009, 22, 588. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, E.H.; Degenhardt, E.; et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists Task Force on Acute Pain Management. Practice Guidelines for Acute Pain Management in the Perioperative Setting: An Updated Report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology 2012, 116, 248–273. [Google Scholar] [CrossRef]
- Sinatra, R. Causes and Consequences of Inadequate Management of Acute Pain. Pain Med. 2010, 11, 1859–1871. [Google Scholar] [CrossRef]
- Guo, T.Z.; Jiang, J.Y.; Buttermann, A.E.; Maze, M. Dexmedetomidine Injection into the Locus Ceruleus Produces Antinociception. Anesthesiology 1996, 84, 873–881. [Google Scholar] [CrossRef]
- Meiser, A.; Sirtl, C.; Bellgardt, M.; Lohmann, S.; Garthoff, A.; Kaiser, J.; Hügler, P.; Laubenthal, H.J. Desflurane Compared with Propofol for Postoperative Sedation in the Intensive Care Unit. Br. J. Anaesth. 2003, 90, 273–280. [Google Scholar] [CrossRef]
- Gustafsson, U.O.; Scott, M.J.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.E.; Macfie, J.; Liberman, A.S.; Soop, M.; et al. Guidelines for Perioperative Care in Elective Colonic Surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations. World J. Surg. 2013, 37, 259–284. [Google Scholar] [CrossRef]
- Hughes, M.; Yim, I.; Deans, D.A.C.; Couper, G.W.; Lamb, P.J.; Skipworth, R.J.E. Systematic Review and Meta-Analysis of Epidural Analgesia Versus Different Analgesic Regimes Following Oesophagogastric Resection. World J. Surg. 2018, 42, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Huang, Q.; Ye, S.; Rong, T. Short and Long-Term Outcomes of Epidural or Intravenous Analgesia after Esophagectomy: A Propensity-Matched Cohort Study. PLoS ONE 2016, 11, e0154380. [Google Scholar] [CrossRef] [PubMed]
- Hausken, J.; Fretland, Å.A.; Edwin, B.; Andersen, M.H.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kristiansen, R.; Røysland, K.; Kvarstein, G.; Tønnessen, T.I. Intravenous Patient-Controlled Analgesia Versus Thoracic Epidural Analgesia After Open Liver Surgery: A Prospective, Randomized, Controlled, Noninferiority Trial. Ann. Surg. 2019, 270, 193. [Google Scholar] [CrossRef]
- Pirrera, B.; Alagna, V.; Lucchi, A.; Berti, P.; Gabbianelli, C.; Martorelli, G.; Mozzoni, L.; Ruggeri, F.; Ingardia, A.; Nardi, G.; et al. Transversus Abdominis Plane (TAP) Block versus Thoracic Epidural Analgesia (TEA) in Laparoscopic Colon Surgery in the ERAS Program. Surg. Endosc. 2018, 32, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Sondekoppam, R.V.; Uppal, V.; Brookes, J.; Ganapathy, S. Bilateral Thoracic Paravertebral Blocks Compared to Thoracic Epidural Analgesia After Midline Laparotomy: A Pragmatic Noninferiority Clinical Trial. Anesth. Analg. 2019, 129, 855. [Google Scholar] [CrossRef] [PubMed]
- Hübner, M.; Blanc, C.; Roulin, D.; Winiker, M.; Gander, S.; Demartines, N. Randomized Clinical Trial on Epidural Versus Patient-Controlled Analgesia for Laparoscopic Colorectal Surgery Within an Enhanced Recovery Pathway. Ann. Surg. 2015, 261, 648. [Google Scholar] [CrossRef]
- Mann, C.; Pouzeratte, Y.; Boccara, G.; Peccoux, C.; Vergne, C.; Brunat, G.; Domergue, J.; Millat, B.; Colson, P. Comparison of Intravenous or Epidural Patient-Controlled Analgesia in the Elderly after Major Abdominal Surgery. Anesthesiology 2000, 92, 433–441. [Google Scholar] [CrossRef]
- Ferguson, S.E.; Malhotra, T.; Seshan, V.E.; Levine, D.A.; Sonoda, Y.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. A Prospective Randomized Trial Comparing Patient-Controlled Epidural Analgesia to Patient-Controlled Intravenous Analgesia on Postoperative Pain Control and Recovery after Major Open Gynecologic Cancer Surgery. Gynecol. Oncol. 2009, 114, 111–116. [Google Scholar] [CrossRef]
- Nishimori, M.; Low, J.H.; Zheng, H.; Ballantyne, J.C. Epidural Pain Relief versus Systemic Opioid-based Pain Relief for Abdominal Aortic Surgery—Nishimori, M—2012|Cochrane Library. Cochrane Database Syst. Rev. 2016, CD005059. [Google Scholar] [CrossRef]
- Jørgensen, H.; Wetterslev, J.; Møiniche, S.; Dahl, J.B. Epidural Local Anaesthetics versus Opioid-Based Analgesic Regimens on Postoperative Gastrointestinal Paralysis, PONV and Pain after Abdominal Surgery. Cochrane Database Syst. Rev. 2000, CD001893. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Li, N.; Sun, Y.; Wang, C.; An, K. Combination of Thoracic Epidural Analgesia with Patient-Controlled Intravenous Analgesia versus Traditional Thoracic Epidural Analgesia for Postoperative Analgesia and Early Recovery of Laparotomy: A Prospective Single-Centre, Randomized Controlled Trial. BMC Anesthesiol. 2022, 22, 341. [Google Scholar] [CrossRef]
| Variable | Liver | Cardiovascular | Esophagus | Lung | Pancreas | Stomach | Colorectal | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 105 | 110 | 32 | 30 | 62 | 66 | 188 | |||||||
| Number of participating hospitals | 3 | 2 | 2 | 2 | 4 | 5 | 7 | |||||||
| Age | 72 (65–77) | 72 (65–76) | 71 (63–74) | 71 (69–76) | 73 (66–77) | 74 (68–79) | 71 (60–79) | |||||||
| Gender (male/female) | 72 (69%)/33 (31%) | 84 (76%)/26 (24%) | 25 (78%)/7 (22%) | 20 (67%)/10 (33%) | 35 (56%)/27 (44%) | 44 (67%)/22 (33%) | 103 (55%)/85 (45%) | |||||||
| Diagnosis | Hepatocellular carcinoma | 47 | Aortic disease | 18 | Esophageal cancer | 32 | Lung cancer | 25 | Pancreatic cancer | 42 | Gastric cancer | 65 | Right side colon cancer | 68 |
| Metastatic tumor | 44 | Valvular disease | 35 | Metastatic tumor | 3 | Cholangiocarcinoma | 6 | Others | 1 | Left side colon cancer | 59 | |||
| Diagnosis | Cholangiocarcinoma | 9 | Ischemic heart disease | 25 | Others | 2 | Duodenal cancer | 7 | Rectal cancer | 54 | ||||
| Others | 5 | AAA | 32 | NET or benign disease | 7 | Inflammatory bowel disease | 3 | |||||||
| Others | 4 | |||||||||||||
| Stage (UICC) | ||||||||||||||
| I | 12 (11%) | – | 5 (16%) | 19 (63%) | 21 (34%) | 42 (64%) | 47 (25%) | |||||||
| II | 32 (30%) | – | 11 (34%) | 7 (23%) | 31 (50%) | 11 (17%) | 61 (32%) | |||||||
| III | 9 (9%) | – | 13 (41%) | 0 (0%) | 3 (5%) | 7 (11%) | 61 (32%) | |||||||
| IV | 47 (45%) | – | 3 (9%) | 2 (7%) | 0 (0%) | 5 (7%) | 12 (7%) | |||||||
| No malignancy | 5 (4%) | 110 (100%) | 0 (0%) | 2 (7%) | 7 (11%) | 1 (1%) | 7 (4%) | |||||||
| Surgical Procedures | Partial hepatectomy | 14 | Thoracic aortic surgery | 17 | Thoracoscopic esophagectomy | 30 | Thoracoscopic pulmonary resection | 12 | Pancreatoduodenectomy | 40 | Distal gastrectomy | 8 | Laparoscopic colectomy | 120 |
| Segmentectomy | 19 | Valve replacement | 36 | Robotic-assisted | 2 | Robotic-assisted | 18 | Distal pancreatectomy | 4 | Total gastrectomy | 3 | Robotic-assisted colectomy | 9 | |
| Sectionectomy | 9 | CABG | 25 | Laparoscopic DP | 3 | Laparoscopic DG | 33 | Laparoscopic proctectomy | 38 | |||||
| Bisectionectomy | 12 | Y-grafting | 32 | Robotic-assisted PD | 10 | Laparoscopic TG | 3 | Robotic-assisted proctectomy | 13 | |||||
| Laparoscopic partial hepatectomy | 27 | Robotic-assisted DP | 4 | Robotic-assisted gastrectomy | 18 | Abdominoperineal resection of rectum | 3 | |||||||
| Laparoscopic segmentectomy | 17 | Total pancreatectomy | 1 | Laparoscopic PG | 1 | Open colectomy | 4 | |||||||
| Laparoscopic sectionectomy | 3 | Laparoscopic small intestine resection | 1 | |||||||||||
| Laparoscopic bisectionectomy | 4 | |||||||||||||
| Variable | Prince Henry Pain Scale Score | QoR-15 Score | Postoperative Hospital Stay (Days) | |||
|---|---|---|---|---|---|---|
| Correlation Coefficient (ρ) | p | Correlation Coefficient (ρ) | p | Correlation Coefficient (ρ) | p | |
| Age (years) | −0.09 | 0.030 | −0.04 | 0.335 | 0.12 | 0.005 |
| Operative blood loss (mL) | −0.04 | 0.375 | −0.18 | <0.001 | 0.30 | <0.001 |
| Operative time (min) | 0.03 | 0.513 | −0.19 | <0.001 | 0.41 | <0.001 |
| Length of wound (cm) | −0.05 | 0.228 | −0.25 | <0.001 | 0.36 | <0.001 |
| Urine volume (mL) | −0.06 | 0.129 | −0.08 | 0.075 | 0.24 | <0.001 |
| Prince Henry Pain Scale | – | – | −0.31 | <0.001 | 0.20 | <0.001 |
| First gas discharge (days after surgery) | 0.05 | 0.223 | −0.03 | 0.553 | 0.11 | 0.011 |
| First bowel movement (days after surgery) | −0.01 | 0.752 | −0.01 | 0.791 | 0.11 | 0.011 |
| Start of eating (days after surgery) | 0.12 | 0.004 | −0.03 | 0.481 | 0.31 | <0.001 |
| Food intake ratio (%) | −0.16 | <0.001 | 0.25 | <0.001 | −0.26 | <0.001 |
| Five levels of appetite | −0.11 | 0.022 | 0.26 | <0.001 | −0.37 | <0.001 |
| 10 levels of bed exit | 0.01 | 0.802 | 0.11 | 0.011 | −0.33 | <0.001 |
| QOR-15 score on the 7th day after surgery | −0.31 | <0.001 | – | – | −0.39 | <0.001 |
| Postoperative hospital stays (days) | 0.20 | <0.001 | −0.39 | <0.001 | – | – |
| Perioperative total lymphocyte count (/μL) | −0.19 | 0.001 | 0.19 | 0.001 | −0.15 | 0.006 |
| Perioperative neutrophil count (/μL) | 0.25 | <0.001 | −0.30 | <0.001 | 0.27 | <0.001 |
| Perioperative serum albumin level (g/dL) | 0.05 | 0.373 | 0.16 | 0.004 | −0.23 | <0.001 |
| Perioperative serum CRP level (mg/dL) | 0.06 | 0.262 | −0.16 | 0.003 | 0.27 | <0.001 |
| Variable | Prince Henry Pain Scale Score | QoR-15 Score | Postoperative Hospital Stay (Days) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | (IQR) | p | Median | (IQR) | p | Median | (IQR) | p | |
| Gender | 0.027 | 0.221 | 0.043 | ||||||
| Male | 2.3 | (1.3, 3.0) | 124 | (106, 136) | 11 | (8, 16) | |||
| Female | 2.3 | (1.7, 3.3) | 120 | (102, 134) | 10 | (8, 14) | |||
| Preoperative treatment (chemotherapy, radiation, etc.) | 0.600 | 0.005 | <0.001 | ||||||
| No | 2.3 | (1.3, 3.0) | 123 | (107, 136) | 10 | (8, 15) | |||
| Yes | 2.3 | (1.3, 3.0) | 115 | (97, 129) | 14 | (11, 20) | |||
| Laparoscopic or thoracic surgery | 0.336 | <0.001 | <0.001 | ||||||
| No | 2.3 | (1.7, 3.0) | 115 | (100, 127) | 12 | (9, 18) | |||
| Yes | 2.3 | (1.3, 3.0) | 128 | (110, 139) | 10 | (7, 13) | |||
| ASA-PS Classification | 0.016 | <0.001 | <0.001 | ||||||
| I, II | 2.3 | (1.3, 3.0) | 126 | (107, 137) | 10 | (8, 14) | |||
| III | 2.7 | (2.0, 3.3) | 112 | (99, 125) | 14 | (10, 18) | |||
| Postoperative complication | 0.118 | <0.001 | <0.001 | ||||||
| No | 2.3 | (1.3, 3.0) | 125 | (109, 137) | 9 | (7, 13) | |||
| Yes | 2.3 | (1.7, 3.0) | 109 | (91, 127) | 18 | (13, 26) | |||
| PONV | 0.141 | 0.228 | 0.871 | ||||||
| No | 2.3 | (1.3, 3.0) | 123 | (106, 136) | 11 | (8, 15) | |||
| Yes | 2.3 | (1.7, 3.3) | 120 | (100, 131) | 11 | (8, 16) | |||
| Intestinal paralysis | 0.017 | 0.001 | <0.001 | ||||||
| No | 2.3 | (1.3, 3.0) | 123 | (106, 135) | 10 | (8, 15) | |||
| Yes | 2.7 | (2.3, 3.3) | 101 | (79, 118) | 18 | (14, 23) | |||
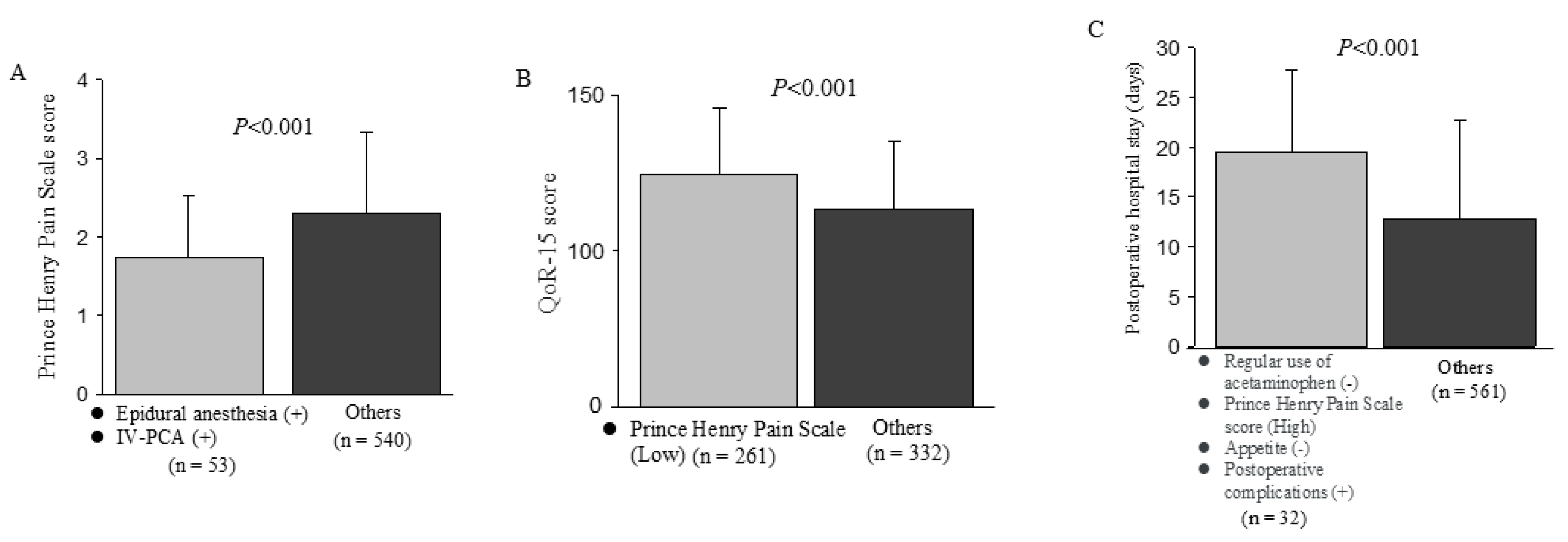
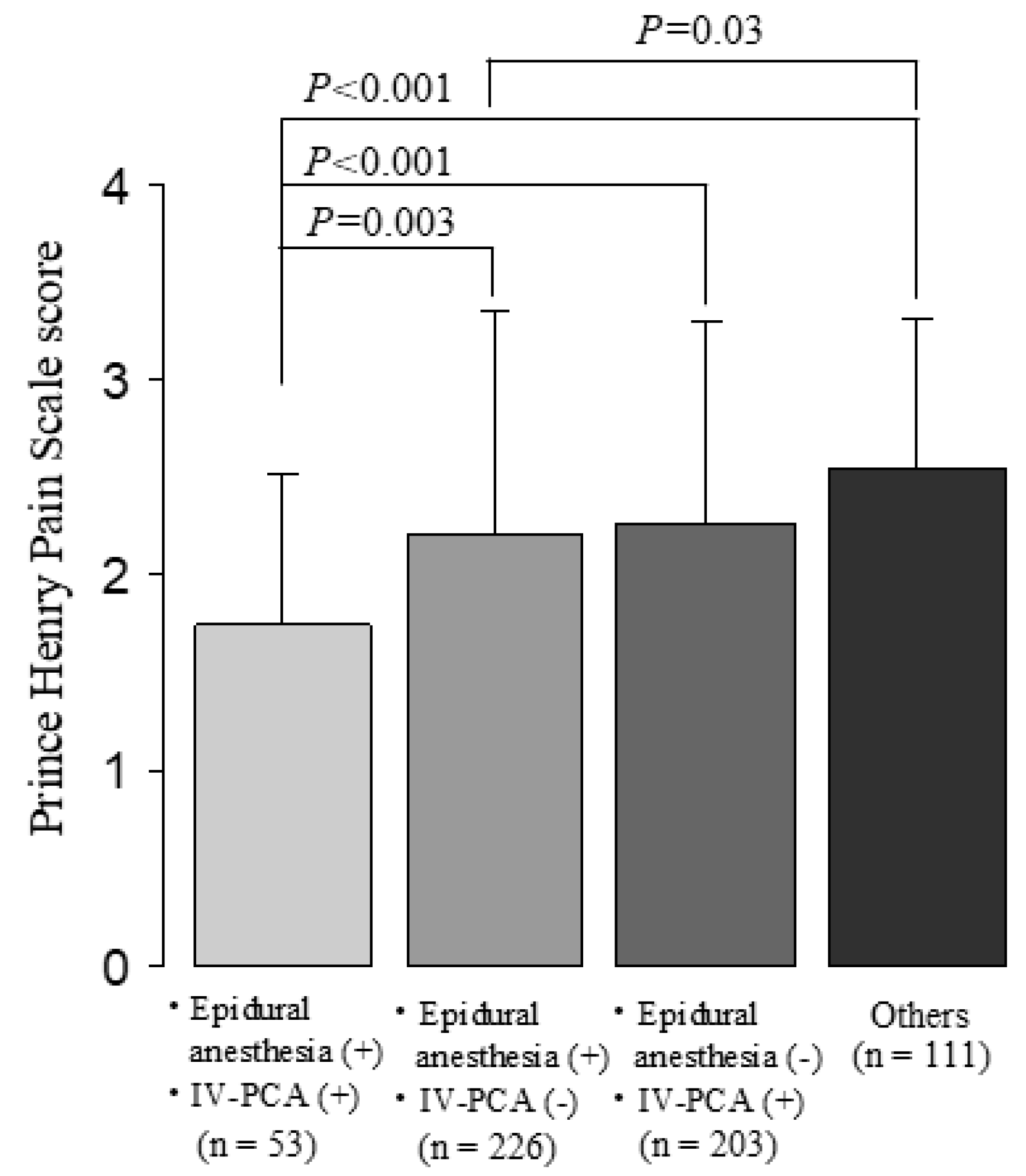
| Postoperative epidural analgesia | 0.003 | 0.004 | 0.005 | ||||||
| No | 2.3 | (1.7, 3.0) | 119 | (105, 131) | 10 | (8, 15) | |||
| Yes | 2.0 | (1.3, 3.0) | 126 | (106, 139) | 11 | (8, 16) | |||
| IV-PCA | 0.031 | 0.051 | 0.620 | ||||||
| No | 2.3 | (1.7, 3.0) | 120 | (105, 133) | 11 | (8, 15) | |||
| Yes | 2.0 | (1.3, 3.0) | 126 | (106, 137) | 10 | (8, 16) | |||
| Regular use of acetaminophen | 0.046 | 0.223 | <0.001 | ||||||
| No | 2.7 | (1.7, 3.3) | 126 | (108, 136) | 13 | (9, 18) | |||
| Yes | 2.3 | (1.3, 3.0) | 120 | (105, 135) | 10 | (8, 14) | |||
| (A) Prince Henry Pain Scale score | ||||
| Variable | Univariate Analysis | Multivariate Analysis | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age ≥ 72 years (vs. <72 years) | 0.97 (0.70–1.34) | 0.838 | 1.00 (0.92–1.08) | 0.957 |
| Gender Female (vs. Male) | 1.34 (0.95–1.90) | 0.095 | 1.06 (0.98–1.16) | 0.161 |
| Preoperative treatment Yes (vs. No) | 1.08 (0.66–1.77) | 0.761 | 1.11 (0.96–1.29) | 0.169 |
| Laparoscopic or thoracic surgery Yes (vs. No) | 0.79 (0.57–1.11) | 0.182 | 1.10 (0.84–1.44) | 0.496 |
| Operative blood loss ≥ 80.0 mL (vs. <80.0 mL) | 0.99 (0.71–1.37) | 0.947 | 1.03 (0.91–1.17) | 0.660 |
| Operative time ≥ 282 min (vs. <282 min) | 1.27 (0.91–1.77) | 0.155 | 0.99 (0.90–1.09) | 0.860 |
| Length of wound ≥ 12.85 cm (vs. <12.85 cm) | 1.10 (0.78–1.56) | 0.585 | 1.12 (0.85–1.47) | 0.439 |
| ASA-PS III (vs. I, II) | 1.88 (1.25–2.85) | 0.003 | 1.05 (0.92–1.20) | 0.498 |
| Postoperative epidural analgesia Yes (vs. No) | 0.52 (0.37–0.73) | <0.001 | 0.71 (0.62–0.81) | <0.001 |
| IV-PCA Yes (vs. No) | 0.70 (0.50–0.98) | 0.039 | 0.74 (0.66–0.83) | <0.001 |
| Regular use of acetaminophen Yes (vs. No) | 0.84 (0.55–1.26) | 0.399 | 1.01 (0.91–1.12) | 0.892 |
| (B) QoR-15 score | ||||
| Variable | Univariate Analysis | Multivariate Analysis | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Laparoscopic or thoracic surgery Yes (vs. No) | 2.98 (2.09–4.26) | <0.001 | 4.01 (0.74–21.70) | 0.107 |
| Operative blood loss ≥ 80.0 mL (vs. <80.0 mL) | 0.49 (0.35–0.69) | <0.001 | 1.06 (0.37–3.01) | 0.912 |
| Operative time ≥ 282 min (vs. <282 min) | 0.65 (0.46–0.91) | 0.012 | 1.69 (0.80–3.59) | 0.172 |
| Length of wound ≥ 12.85 cm (vs. <12.85 cm) | 0.30 (0.21–0.44) | <0.001 | 0.98 (0.14–6.93) | 0.980 |
| ASA-PS III (vs. I, II) | 0.38 (0.25–0.58) | <0.001 | 1.17 (0.40–3.43) | 0.781 |
| Prince Henry Pain Scale ≥ 2.33 (vs. <2.33) | 0.33 (0.23–0.47) | <0.001 | 0.39 (0.18–0.82) | 0.013 |
| Mean food intake ratio ≥ 32.5% (vs. <32.5%) | 1.59 (1.11–2.29) | 0.011 | 2.09 (0.92–4.74) | 0.079 |
| Five levels of appetite ≥ 2.75 (vs. <2.75) | 1.87 (1.46–2.42) | <0.001 | 1.33 (0.86–2.07) | 0.202 |
| Postoperative complication Yes (vs. No) | 0.40 (0.26–0.60) | <0.001 | 0.52 (0.25–1.09) | 0.084 |
| Postoperative hospital stays ≥ 11 days (vs. <11 days) | 0.32 (0.22–0.45) | <0.001 | 0.60 (0.27–1.33) | 0.209 |
| Perioperative neutrophil count ≥ 7847.5 (vs. <7847.5 (/μL)) | 0.28 (0.18–0.44) | <0.001 | 0.57 (0.29–1.13) | 0.108 |
| (C) Postoperative hospital stay (days) | ||||
| Variable | Univariate Analysis | Multivariate Analysis | ||
| OR (95% CI) | p | OR (95% CI) | p | |
| Preoperative treatment Yes (vs. No) | 3.39 (2.00–6.01) | <0.001 | 0.85 (0.28–2.60) | 0.774 |
| Operative blood loss ≥ 80.0 mL (vs. <80.0 mL) | 2.77 (1.98–3.89) | <0.001 | 1.45 (0.61–3.44) | 0.403 |
| Urine volume ≥ 300 mL (vs. <300 mL) | 2.06 (1.48–2.88) | <0.001 | 1.51 (0.77–2.95) | 0.234 |
| ASA-PS III (vs. I, II) | 3.28 (2.15–5.10) | <0.001 | 2.32 (0.54–9.92) | 0.257 |
| Regular use of acetaminophen Yes (vs. No) | 0.53 (0.34–0.80) | 0.003 | 0.07 (0.02–0.31) | <0.001 |
| Prince Henry Pain Scale ≥ 2.33 (vs. <2.33) | 1.95 (1.40–2.74) | <0.001 | 2.30 (1.04–5.06) | 0.039 |
| Five levels of appetite ≥ 2.75 (vs. <2.75) | 0.41 (0.32–0.53) | <0.001 | 0.45 (0.29–0.71) | <0.001 |
| 10 levels of bed exit ≥ 8.25 (vs. <8.25) | 0.33 (0.23–0.48) | <0.001 | 0.50 (0.25–1.02) | 0.058 |
| QoR-15 score on the 7th day after surgery ≥ 122 (vs. <122) | 0.32 (0.22–0.45) | <0.001 | 1.11 (0.53–2.32) | 0.782 |
| Postoperative complication Yes (vs. No) | 14.22 (8.19–26.59) | <0.001 | 9.06 (3.54–23.20) | <0.001 |
| Perioperative neutrophil count ≥ 7847.5 (vs. <7847.5 (/μL)) | 2.10 (1.32–3.36) | 0.002 | 1.00 (0.47–2.15) | 0.993 |
| Perioperative serum albumin level ≥ 3.025 (vs. <3.025) | 0.46 (0.28–0.72) | <0.001 | 0.75 (0.37–1.51) | 0.418 |
| Perioperative serum CRP level ≥ 7.70 (vs. <7.70 (mg/dL)) | 2.00 (1.26–3.18) | 0.003 | 0.89 (0.44–1.77) | 0.734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaibori, M.; Yoshii, K.; Lai, T.T.; Matsushima, H.; Tatsuishi, W.; Inada, R.; Matsugu, Y.; Komeda, K.; Asakuma, M.; Tanaka, K.; et al. Prospective Survey of Postoperative Pain in Japan: A Multicenter, Observational Study. J. Clin. Med. 2025, 14, 1130. https://doi.org/10.3390/jcm14041130
Kaibori M, Yoshii K, Lai TT, Matsushima H, Tatsuishi W, Inada R, Matsugu Y, Komeda K, Asakuma M, Tanaka K, et al. Prospective Survey of Postoperative Pain in Japan: A Multicenter, Observational Study. Journal of Clinical Medicine. 2025; 14(4):1130. https://doi.org/10.3390/jcm14041130
Chicago/Turabian StyleKaibori, Masaki, Kengo Yoshii, Tung Thanh Lai, Hideyuki Matsushima, Wataru Tatsuishi, Ryo Inada, Yasuhiro Matsugu, Koji Komeda, Mitsuhiro Asakuma, Keitaro Tanaka, and et al. 2025. "Prospective Survey of Postoperative Pain in Japan: A Multicenter, Observational Study" Journal of Clinical Medicine 14, no. 4: 1130. https://doi.org/10.3390/jcm14041130
APA StyleKaibori, M., Yoshii, K., Lai, T. T., Matsushima, H., Tatsuishi, W., Inada, R., Matsugu, Y., Komeda, K., Asakuma, M., Tanaka, K., Sato, H., Yamada, T., Miyasaka, T., Hasegawa, Y., Matsui, R., Takehara, K., Ko, S., Yamato, I., Washizawa, N., ... Miyata, G. (2025). Prospective Survey of Postoperative Pain in Japan: A Multicenter, Observational Study. Journal of Clinical Medicine, 14(4), 1130. https://doi.org/10.3390/jcm14041130








