Evaluation of Cancellous Bone Density from C3 to L5 in 11 Body Donors: CT Versus Micro-CT Measurements
Abstract
1. Background
2. Materials and Methods
2.1. Study Design and Group Allocation
2.2. Recruitment and Ethics
2.3. Inclusion and Exclusion Criteria
2.4. Extraction of Spines and Cancellous Bone
2.5. Diagnostic Imaging
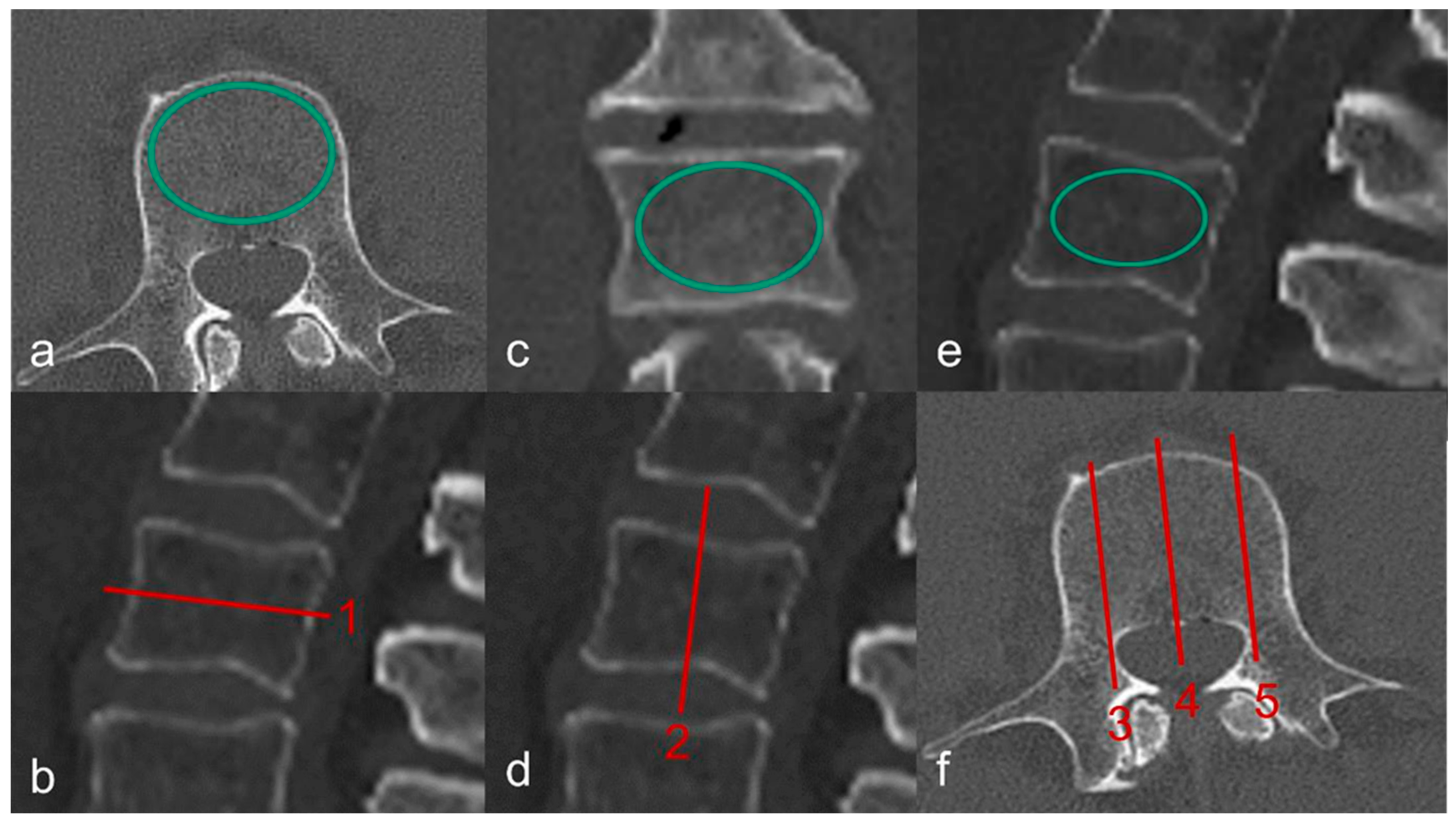
2.5.1. CT and QCT
2.5.2. Micro-CT Images and Evaluation of Microarchitecture
2.6. Statistics
3. Results
3.1. QCT
Density of Cancellous Bone in Hounsfield Units on the CT Image
3.2. Micro-CT Compared with CT
3.2.1. Micro-CT and CT Scans Show Graphically Different Courses of Bone Density Values
3.2.2. Gender and Fracture Status Influence Bone Density Measurements
3.2.3. Micro-CT and CT Investigations Reveal Gender Differences with Varying Clarity
3.2.4. Gender Differences Based on HU Values Were Independent of the Investigated Quadrants
3.2.5. CT and Micro-CT Investigations Differ in Their Depiction of Fracture Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al | aluminum |
| AUC | area under the curve |
| BMD | bone mineral density (mg/cm3) |
| BMI | body mass index |
| BVF | bone volume fraction |
| cm | centimeter |
| CS | cervical spine |
| CT | computed tomography |
| C1-7 | cervical vertebra 1-7 |
| DXA | dual-energy X-ray absorptiometry |
| EMM | estimated marginal means |
| Fig. | figure |
| g/cm3 | gram/cubic centimeter |
| GE | General Electric |
| HU | Hounsfield units |
| IBM | International Business Machines Corporation |
| ICC | intra-class correlation coefficient |
| kV | kilovolt |
| L1-5 | lumbar vertebra 1-5 |
| LS | lumbar spine |
| mg/cm3 | milligram/cubic centimeter |
| mg/mL | milligram/milliliter |
| Micro-CT | micro-computed tomography |
| Mio. | millions |
| Ml | milliliter |
| Mm | millimeter |
| OP | osteoporosis |
| PMMA | polymethylmethacrylat |
| Q I | Quadrant I |
| Q II | Quadrant II |
| Q III | Quadrant III |
| QCT | quantitative computed tomography |
| ROC | receiver operating characteristic |
| ROI | region of interest |
| SD | standard deviation |
| TBS | trabecular bone structure |
| Th1-12 | thoracic vertebra 1-12 |
| TS | thoracic spine |
| VF | vertebral fractures |
| µm | micrometer |
| µA | microampere |
| °C | degrees Celsius |
References
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef] [PubMed]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.; Walter, N.; Pfeifer, C.; Lang, S.; Kerschbaum, M.; Krutsch, W.; Baumann, F.; Alt, V. The Incidence of Fractures Among the Adult Population of Germany—An Analysis from 2009 through 2019. Dtsch. Arztebl. Int. 2021, 118, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. J. Am. Geriatr. Soc. 2019, 67, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Issever, A.S.; Link, T.M. Radiologische Diagnostik der Osteoporose. Z. Rheumatol. 2011, 70, 135–144, quiz 145. [Google Scholar] [CrossRef]
- Scheyerer, M.J.; Ullrich, B.; Osterhoff, G.; Spiegl, U.A.; Schnake, K.J. “Hounsfield units” als Maß für die Knochendichte—Anwendungsmöglichkeiten in der Wirbelsäulenchirurgie. Unfallchirurg 2019, 122, 654–661. [Google Scholar] [CrossRef]
- Burghardt, A.J.; Link, T.M.; Majumdar, S. High-resolution Computed Tomography for Clinical Imaging of Bone Microarchitecture. Clin. Orthop. Relat. Res. 2011, 469, 2179–2193. [Google Scholar] [CrossRef]
- Andresen, R.; Radmer, S.; Banzer, D. Bone mineral density and spongiosa architecture in correlation to vertebral body insufficiency fractures. Acta Radiol. 1998, 39, 538–542. [Google Scholar] [CrossRef]
- Schröder, G.; Flachsmeyer, D.; Kullen, C.M.; Andresen, J.R.; Schulze, M.; Hiepe, L.; Schober, H.-C.; Andresen, R. Insuffizienzfrakturen der Wirbelsäule in Abhängigkeit von der spongiösen Knochendichte: Eine in-vitro-Studie. Orthopadie 2022, 51, 547–555. [Google Scholar] [CrossRef]
- Schröder, G.; Jabke, B.; Schulze, M.; Wree, A.; Martin, H.; Sahmel, O.; Doerell, A.; Kullen, C.M.; Andresen, R.; Schober, H.-C. A comparison, using X-ray micro-computed tomography, of the architecture of cancellous bone from the cervical, thoracic and lumbar spine using 240 vertebral bodies from 10 body donors. Anat. Cell Biol. 2021, 54, 25–34. [Google Scholar] [CrossRef]
- Schröder, G.; Hiepe, L.; Moritz, M.; Vivell, L.-M.; Schulze, M.; Martin, H.; Götz, A.; Andresen, J.R.; Kullen, C.-M.; Andresen, R.; et al. Warum sich in der Halswirbelsäule auch bei Osteoporose nur selten Insuffizienzfrakturen finden. Z. Orthop. Unfall. 2022, 160, 657–669. [Google Scholar] [CrossRef]
- Schröder, G.; Mittlmeier, T.; Gahr, P.; Ulusoy, S.; Hiepe, L.; Schulze, M.; Götz, A.; Andresen, R.; Schober, H.-C. Regional Variations in the Intra- and Intervertebral Trabecular Microarchitecture of the Osteoporotic Axial Skeleton with Reference to the Direction of Puncture. Diagnostics 2024, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- De Laet, C.; Kanis, J.A.; Odén, A.; Johanson, H.; Johnell, O.; Delmas, P.; Eisman, J.A.; Kroger, H.; Fujiwara, S.; Garnero, P.; et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos. Int. 2005, 16, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Engelke, K.; Adams, J.E.; Armbrecht, G.; Augat, P.; Bogado, C.E.; Bouxsein, M.L.; Felsenberg, D.; Ito, M.; Prevrhal, S.; Hans, D.B.; et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 123–162. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Borgstrom, F.; de Laet, C.; Johansson, H.; Johnell, O.; Jonsson, B.; Oden, A.; Zethraeus, N.; Pfleger, B.; Khaltaev, N. Assessment of fracture risk. Osteoporos. Int. 2005, 16, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Bagger, Y.Z.; Tankó, L.B.; Alexandersen, P.; Hansen, H.B.; Qin, G.; Christiansen, C. The long-term predictive value of bone mineral density measurements for fracture risk is independent of the site of measurement and the age at diagnosis: Results from the Prospective Epidemiological Risk Factors study. Osteoporos. Int. 2006, 17, 471–477. [Google Scholar] [CrossRef]
- Pluijm, S.M.F.; Koes, B.; de Laet, C.; van Schoor, N.M.; Kuchuk, N.O.; Rivadeneira, F.; Mackenbach, J.P.; Lips, P.; Pols, H.A.; Steyerberg, E.W. A simple risk score for the assessment of absolute fracture risk in general practice based on two longitudinal studies. J. Bone Miner. Res. 2009, 24, 768–774. [Google Scholar] [CrossRef]
- Bässgen, K.; Westphal, T.; Haar, P.; Kundt, G.; Mittlmeier, T.; Schober, H.-C. Population-based prospective study on the incidence of osteoporosis-associated fractures in a German population of 200,413 inhabitants. J. Public Health 2013, 35, 255–261. [Google Scholar] [CrossRef]
- Christiansen, B.A.; Bouxsein, M.L. Biomechanics of vertebral fractures and the vertebral fracture cascade. Curr. Osteoporos. Rep. 2010, 8, 198–204. [Google Scholar] [CrossRef]
- Montemurro, N.; Cocciaro, A.; Liberti, G.; Cosottini, M.; Perrini, P. The internal trabecular bone structure of the odontoid process of the axis. A retrospective single-center comparative study in patients following cervical trauma. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2022. [Google Scholar] [CrossRef]
- Shin, D.E.; Lee, Y.; An, H.-J.; Hwang, T.-S.; Cho, J.-W.; Oh, J.; Ahn, W.; Lee, J.; Hong, C.G.; Lee, Y.; et al. Trabecular structural difference between the superior and inferior regions of the vertebral body: A cadaveric and clinical study. Front. Endocrinol. 2023, 14, 1238654. [Google Scholar] [CrossRef]
- Perilli, E.; Briggs, A.M.; Kantor, S.; Codrington, J.; Wark, J.D.; Parkinson, I.H.; Fazzalari, N.L. Failure strength of human vertebrae: Prediction using bone mineral density measured by DXA and bone volume by micro-CT. Bone 2012, 50, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.S.; Cleary, R.L.; Butler, J.P.; Antonelli, R.; Mitlak, B.H.; Deraska, D.J.; Zamora-Quezada, J.C.; Neer, R.M. A comparison of lateral versus anterior-posterior spine dual energy x-ray absorptiometry for the diagnosis of osteopenia. J. Clin. Endocrinol. Metab. 1994, 78, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Lunt, M.; Felsenberg, D.; Adams, J.; Benevolenskaya, L.; Cannata, J.; Dequeker, J.; Dodenhof, C.; Falch, J.A.; Johnell, O.; Khaw, K.T.; et al. Population-based geographic variations in DXA bone density in Europe: The EVOS Study. European Vertebral Osteoporosis. Osteoporos. Int. 1997, 7, 175–189. [Google Scholar] [CrossRef]
- Zhao, F.-D.; Pollintine, P.; Hole, B.D.; Adams, M.A.; Dolan, P. Vertebral fractures usually affect the cranial endplate because it is thinner and supported by less-dense trabecular bone. Bone 2009, 44, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, J.J.; Anderson, P.A.; Rosas, H.G.; Buchholz, A.L.; Au, A.G. Hounsfield units for assessing bone mineral density and strength: A tool for osteoporosis management. J. Bone Jt. Surg. Am. 2011, 93, 1057–1063. [Google Scholar] [CrossRef]
- Marinova, M.; Edon, B.; Wolter, K.; Katsimbari, B.; Schild, H.H.; Strunk, H.M. Use of routine thoracic and abdominal computed tomography scans for assessing bone mineral density and detecting osteoporosis. Curr. Med. Res. Opin. 2015, 31, 1871–1881. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, C.; Lee, E.; Lee, J.W. Coronal plane in opportunistic screening of osteoporosis using computed tomography: Comparison with axial and sagittal planes. Skelet. Radiol. 2024, 53, 1103–1109. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, X.; Zhou, R.; Dai, Z.; Guo, C.; Qu, G.; Li, J.; Zhang, Z. The axial and sagittal CT values of the 7th thoracic vertebrae in screening for osteoporosis and osteopenia. Clin. Radiol. 2023, 78, 763–771. [Google Scholar] [CrossRef]
- Kalender, W.A. Computertomographie: Grundlagen, Gerätetechnologie, Bildqualität, Anwendungen; [mit Mehrschicht-Spiral-CT]; Publicis-MCD-Verl.: München, Germany, 2000; ISBN 978-3-89578-082-0. [Google Scholar]
- Zou, D.; Ye, K.; Tian, Y.; Li, W.; Zhou, F.; Zhang, Z.; Lu, Z.; Xu, Z. Characteristics of vertebral CT Hounsfield units in elderly patients with acute vertebral fragility fractures. Eur. Spine J. 2020, 29, 1092–1097. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, Z.; Yu, R.; Liu, X.; Chen, H. Diagnostic Value of Hounsfield Units for Osteoporotic Thoracolumbar Vertebral Non-Compression Fractures in Elderly Patients with Low-Energy Injuries. Int. J. Gen. Med. 2024, 17, 3221–3229. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-C.; Chao, H.; Kao, K.-Y.; Lin, H.-H.; Wang, S.-T.; Chang, M.-C.; Liu, C.-L.; Chou, P.-H. CT Hounsfield unit is a reliable parameter for screws loosening or cages subsidence in minimally invasive transforaminal lumbar interbody fusion. Sci. Rep. 2023, 13, 1620. [Google Scholar] [CrossRef] [PubMed]
- Spiegl, U.J.A.; Schenk, P.; Schnake, K.J.; Ullrich, B.W.; Osterhoff, G.; Scheyerer, M.J.; Schmeiser, G.; Bäumlein, M.; Scherer, M.A.; Müller, M.; et al. Treatment and Outcome of Osteoporotic Thoracolumbar Vertebral Body Fractures With Deformation of Both Endplates With or Without Posterior Wall Involvement (OF 4): Short-Term Results from the Prospective EOFTT Multicenter Study. Glob. Spine J. 2023, 13, 36S–43S. [Google Scholar] [CrossRef]
- Ji, C.; Rong, Y.; Wang, J.; Yu, S.; Yin, G.; Fan, J.; Tang, P.; Jiang, D.; Liu, W.; Gong, F.; et al. Risk Factors for Refracture following Primary Osteoporotic Vertebral Compression Fractures. Pain Physician 2021, 24, E335–E340. [Google Scholar] [CrossRef] [PubMed]
- Korovessis, P. Osteoporotic Vertebral Body Fractures: New Trends in Differential Diagnosis, Bracing and Surgery. J. Clin. Med. 2022, 11, 5172. [Google Scholar] [CrossRef]
- Jiang, L.-M.; Tong, Y.-X.; Jiang, J.-J.; Pi, Y.-W.; Gong, Y.; Tan, Z.; Zhao, D.-X. The vertebral Hounsfield units can quantitatively predict the risk of adjacent vertebral fractures after percutaneous kyphoplasty. Quant. Imaging Med. Surg. 2023, 13, 1036–1047. [Google Scholar] [CrossRef]
- Metzner, F.; Reise, R.; Heyde, C.-E.; von der Höh, N.H.; Schleifenbaum, S. Side specific differences of Hounsfield-Units in the osteoporotic lumbar spine. J. Spine Surg. 2024, 10, 232–243. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Zheng, H.; Li, H.; Wang, H.; Ma, L. Hounsfield unit for assessing bone mineral density distribution within lumbar vertebrae and its clinical values. Front. Endocrinol. 2024, 15, 1398367. [Google Scholar] [CrossRef]
- Xu, F.; Zou, D.; Li, W.; Sun, Z.; Jiang, S.; Zhou, S.; Li, Z. Hounsfield units of the vertebral body and pedicle as predictors of pedicle screw loosening after degenerative lumbar spine surgery. Neurosurg. Focus 2020, 49, E10. [Google Scholar] [CrossRef]
- Ye, K.; Zou, D.; Zhou, F.; Li, W.; Tian, Y. Low vertebral CT Hounsfield units: A risk factor for new osteoporotic vertebral fractures after the treatment of percutaneous kyphoplasty. Arch. Osteoporos. 2022, 17, 137. [Google Scholar] [CrossRef]
- Parsa, A.; Ibrahim, N.; Hassan, B.; van der Stelt, P.; Wismeijer, D. Bone quality evaluation at dental implant site using multislice CT, micro-CT, and cone beam CT. Clin. Oral Implants Res. 2015, 26, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shoumura, S.; Emura, S.; Bunai, Y. Regional variations of vertebral trabecular bone microstructure with age and gender. Osteoporos. Int. 2008, 19, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhang, M.; Yeung, H.Y.; Qin, L. Regional variations in microstructural properties of vertebral trabeculae with aging. J. Bone Miner. Metab. 2005, 23, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Banse, X.; Devogelaer, J.P.; Munting, E.; Delloye, C.; Cornu, O.; Grynpas, M. Inhomogeneity of human vertebral cancellous bone: Systematic density and structure patterns inside the vertebral body. Bone 2001, 28, 563–571. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, X.; Fujita, H.; Onozuka, M.; Kubo, K.-Y. Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013, 2013, 213234. [Google Scholar] [CrossRef]
- Follet, H.; Farlay, D.; Bala, Y.; Viguet-Carrin, S.; Gineyts, E.; Burt-Pichat, B.; Wegrzyn, J.; Delmas, P.; Boivin, G.; Chapurlat, R. Determinants of Microdamage in Elderly Human Vertebral Trabecular Bone. PLoS ONE 2013, 8, e55232. [Google Scholar] [CrossRef]
- Arlot, M.E.; Burt-Pichat, B.; Roux, J.-P.; Vashishth, D.; Bouxsein, M.L.; Delmas, P.D. Microarchitecture influences microdamage accumulation in human vertebral trabecular bone. J. Bone Miner. Res. 2008, 23, 1613–1618. [Google Scholar] [CrossRef]




| Body Donors (n = 11) | |
|---|---|
| Age (years) | 79.1 ± 7.5 |
| Gender (male/female) | 5/6 |
| Body mass index (kg/m2) | 21.9 ± 5.5 |
| Number of sustained fractures (≤1 fracture/>1 fracture) Extracted segments | 3/8 C3-L5 |
| Bone density in the lumbar vertebrae 1 to 3 (mg/cm3) * | 58.7 ± 27.3 |
| Number of vertebral body fractures | 1.8 ± 1.1 |
| Number of vertebral fractures in relation to gender: overall/male/female (n) | |
| Th5 | 1/0/1 |
| Th6 | 1/1/0 |
| Th7 | 3/2/1 |
| Th8 | 3/2/1 |
| Th9 | 2/1/1 |
| Th10 | 1/0/1 |
| Th12 | 2/2/0 |
| L1 | 5/1/4 |
| L2 | 2/0/2 |
| Number of investigated vertebrae (n) | 242 |
| Number of investigated cancellous bone cylinders (n) | 726 |
| Hounsfield units in the sagittal plane in QI to Q III (n) | 726 |
| Comparison of sectional planes in Hounsfield units (axial, coronary, sagittal) (n) | 726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, G.; Akl, E.; Hillebrand, J.; Götz, A.; Mittlmeier, T.; Falk, S.S.I.; Hiepe, L.; Andresen, J.R.; Andresen, R.; Flachsmeyer-Blank, D.; et al. Evaluation of Cancellous Bone Density from C3 to L5 in 11 Body Donors: CT Versus Micro-CT Measurements. J. Clin. Med. 2025, 14, 1059. https://doi.org/10.3390/jcm14041059
Schröder G, Akl E, Hillebrand J, Götz A, Mittlmeier T, Falk SSI, Hiepe L, Andresen JR, Andresen R, Flachsmeyer-Blank D, et al. Evaluation of Cancellous Bone Density from C3 to L5 in 11 Body Donors: CT Versus Micro-CT Measurements. Journal of Clinical Medicine. 2025; 14(4):1059. https://doi.org/10.3390/jcm14041059
Chicago/Turabian StyleSchröder, Guido, Estelle Akl, Justus Hillebrand, Andreas Götz, Thomas Mittlmeier, Steffi S. I. Falk, Laura Hiepe, Julian Ramin Andresen, Reimer Andresen, Dirk Flachsmeyer-Blank, and et al. 2025. "Evaluation of Cancellous Bone Density from C3 to L5 in 11 Body Donors: CT Versus Micro-CT Measurements" Journal of Clinical Medicine 14, no. 4: 1059. https://doi.org/10.3390/jcm14041059
APA StyleSchröder, G., Akl, E., Hillebrand, J., Götz, A., Mittlmeier, T., Falk, S. S. I., Hiepe, L., Andresen, J. R., Andresen, R., Flachsmeyer-Blank, D., Schober, H.-C., & Glass, Ä. (2025). Evaluation of Cancellous Bone Density from C3 to L5 in 11 Body Donors: CT Versus Micro-CT Measurements. Journal of Clinical Medicine, 14(4), 1059. https://doi.org/10.3390/jcm14041059







