Risk of Thrombosis in Women Undergoing In Vitro Fertilization: A Narrative Review
Abstract
1. Introduction
Methodology
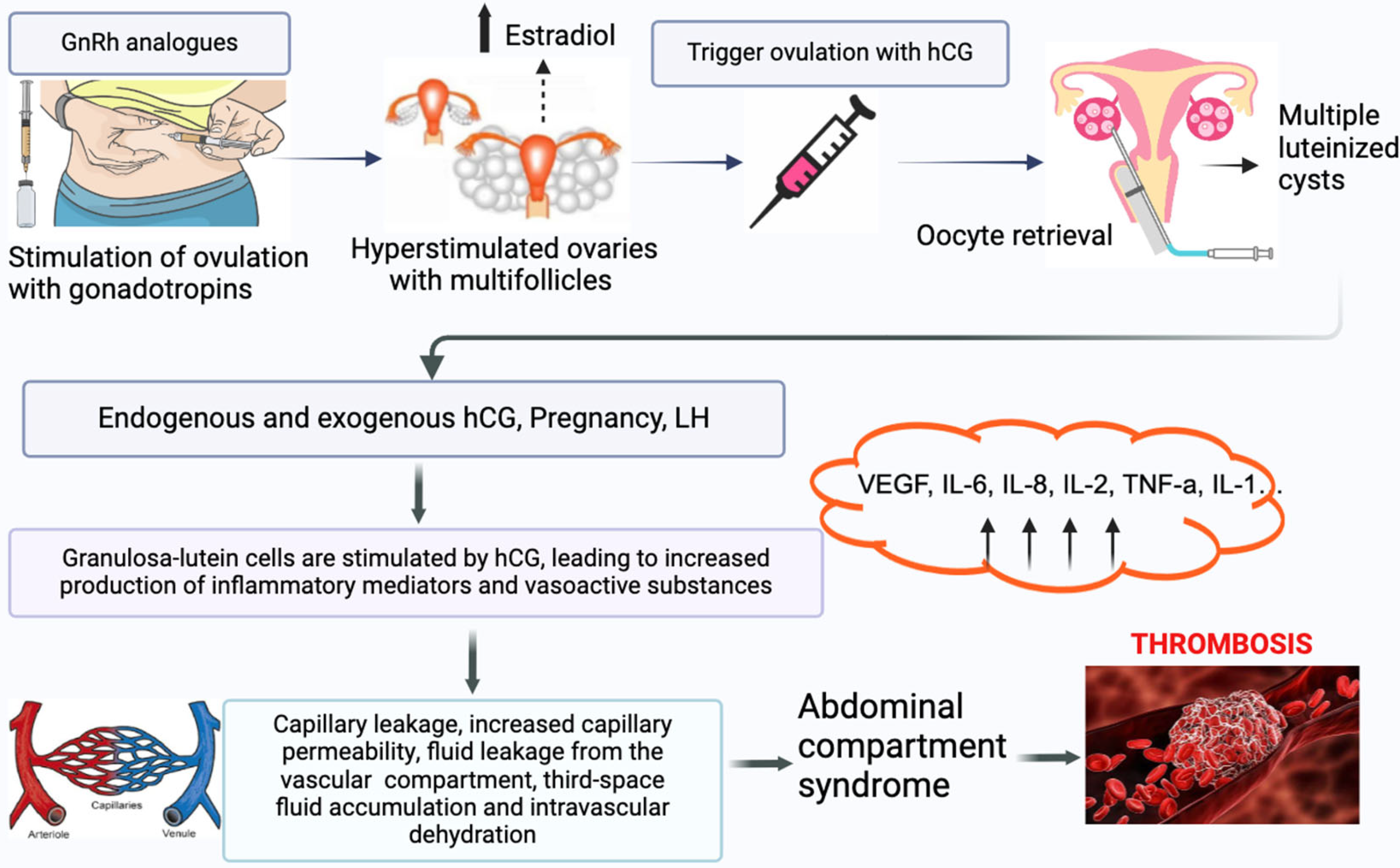
2. Thrombosis in IVF
2.1. OHSS and Thrombotic Risk
2.2. Arterial Thrombosis in IVF Cycles
2.3. Venous Thrombosis in IVF Cycles
2.4. Anatomical Localization
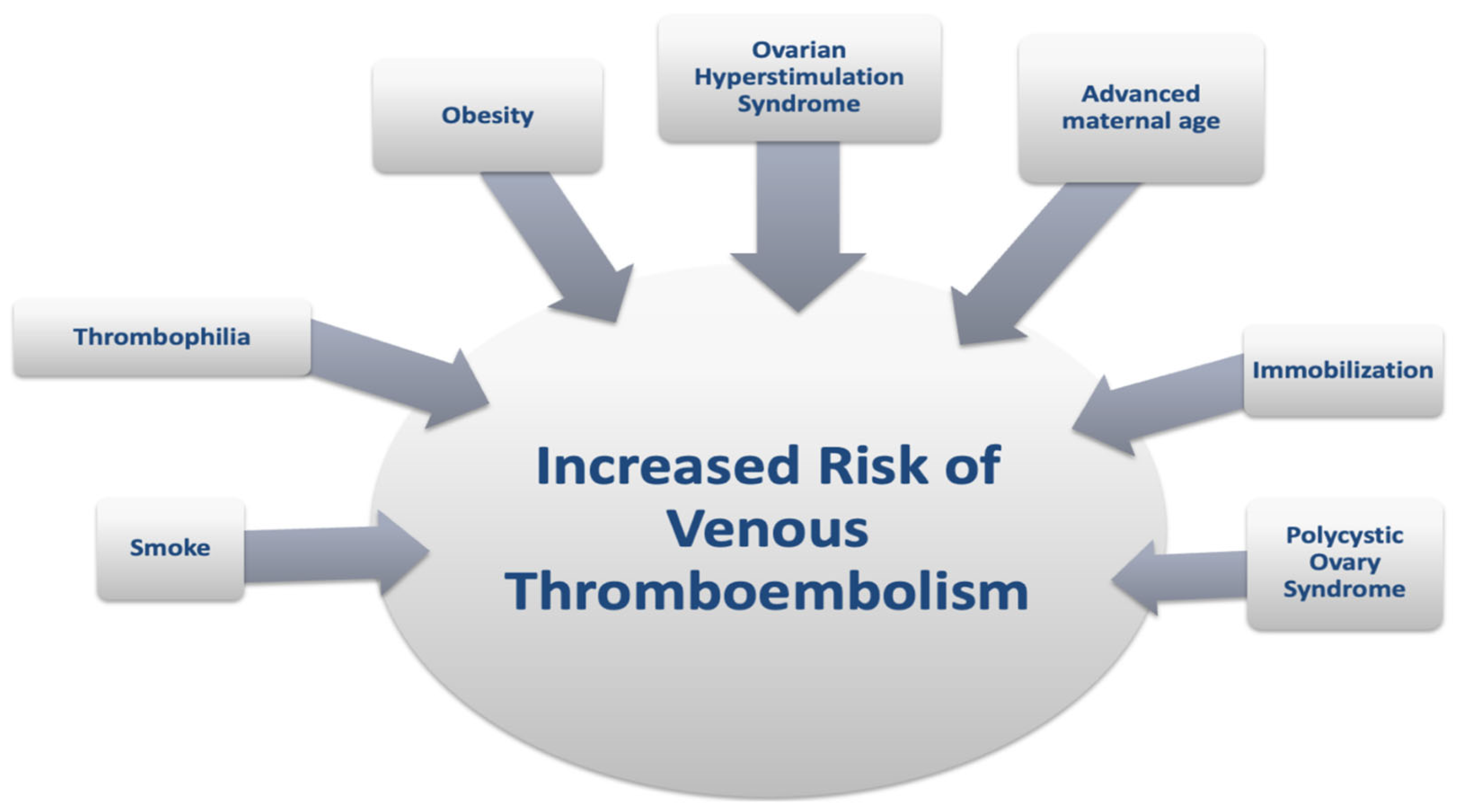
2.5. Thrombophilia
2.6. Other Maternal Risk Factors
2.7. Risk of VTE in Pregnancy After IVF
2.8. Strengths and Limitations
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calhaz-Jorge, C.; De Geyter, C.H.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. Survey on ART and IUI: Legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. Open 2020, 2020, hoz044. [Google Scholar] [CrossRef] [PubMed]
- European IVF Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Smeenk, J.; Wyns, C.; De Geyter, C.; Kupka, M.; Bergh, C.; Cuevas Saiz, I.; De Neubourg, D.; Rezabek, K.; Tandler-Schneider, A.; et al. ART in Europe, 2019: Results generated from European registries by ESHRE. Hum. Reprod. 2023, 38, 2321–2338. [Google Scholar]
- Farquhar, C.; Marjoribanks, J.; Brown, J.; Fauser, B.C.J.M.; Lethaby, A.; Mourad, S.; Rebar, R.; Showell, M.; van der Poel, S. Management of ovarian stimulation for IVF: Narrative review of evidence provided for World Health Organization guidance. Reprod. Biomed. Online 2017, 35, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.M. Venous thrombosis during assisted reproduction: Novel risk reduction strategies. Thromb. Res. 2013, 131 (Suppl. S1), S1–S3. [Google Scholar] [CrossRef]
- Grandone, E.; Villani, M. Assisted reproductive technologies and thrombosis. Thromb. Res. 2015, 135 (Suppl. S1), S44–S45. [Google Scholar] [CrossRef]
- Sennström, M.; Rova, K.; Hellgren, M.; Hjertberg, R.; Nord, E.; Thurn, L.; Lindqvist, P.G. Thromboembolism and in vitro fertilization—A systematic review. Acta Obstet. Gynecol. Scand. 2017, 96, 1045–1052. [Google Scholar] [CrossRef]
- Goualou, M.; Noumegni, S.; de Moreuil, C.; Le Guillou, M.; De Coninck, G.; Hoffmann, C.; Robin, S.; Morcel, K.; Le Moigne, E.; Tremouilhac, C.; et al. Venous Thromboembolism Associated with Assisted Reproductive Technology: A Systematic Review and Meta-analysis. Thromb. Haemost. 2023, 123, 283–294. [Google Scholar] [CrossRef]
- Kupka, M.S.; Ferraretti, A.P.; de Mouzon, J.; Erb, K.; D’Hooghe, T.; Castilla, J.A.; Calhaz-Jorge, C.; De Geyter, C.; Goossens, V.; European IVF-Monitoring Consortium, for the European Society of Human Reproduction and Embryology. Assisted reproductive technology in Europe, 2010: Results generated from European registers by ESHRE. Hum. Reprod. 2014, 29, 2099–2113. [Google Scholar] [CrossRef]
- Practice Committee of American Society for Reproductive Medicine. Ovarian hyperstimulation syndrome. Fertil. Steril. 2008, 90, S188–S193. [Google Scholar] [CrossRef]
- Grandone, E.; Colaizzo, D.; Vergura, P.; Cappucci, F.; Vecchione, G.; Lo Bue, A.; Cittadini, E.; Margaglione, M. Age and homocysteine plasma levels are risk factors for thrombotic complications after ovarian stimulation. Hum. Reprod. 2004, 19, 1796–1799. [Google Scholar] [CrossRef]
- Henriksson, P.; Westerlund, E.; Wallén, H.; Brandt, L.; Hovatta, O.; Ekbom, A. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: Cross sectional study. BMJ 2013, 346, e8632. [Google Scholar] [CrossRef] [PubMed]
- Rova, K.; Passmark, H.; Lindqvist, P.G. Venous thromboembolism in relation to in vitro fertilization: An approach to determining the incidence and increase in risk in successful cycles. Fertil. Steril. 2012, 97, 95–100. [Google Scholar] [CrossRef]
- Nelson, S.M. Prophylaxis of VTE in women—During assisted reproductive techniques. Thromb. Res. 2009, 123 (Suppl 3), S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.T.; Kesmodel, U.S.; Juul, S.; Hvas, A.M. No evidence that assisted reproduction increases the risk of thrombosis: A Danish national cohort study. Hum. Reprod. 2012, 27, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Serour, G.I.; Aboulghar, M.; Mansour, R.; Sattar, M.A.; Amin, Y.; Aboulghar, H. Complications of medically assisted conception in 3500 cycles. Fertil. Steril. 1998, 70, 638–642. [Google Scholar] [CrossRef]
- Palomba, S.; Costanzi, F.; Nelson, S.M.; Besharat, A.; Caserta, D.; Humaidan, P. Beyond the Umbrella: A Systematic Review of the Interventions for the Prevention of and Reduction in the Incidence and Severity of Ovarian Hyperstimulation Syndrome in Patients Who Undergo In Vitro Fertilization Treatments. Int. J. Mol. Sci. 2023, 24, 14185. [Google Scholar] [CrossRef]
- Girolami, A.; Scandellari, R.; Tezza, F.; Paternoster, D.; Girolami, B. Arterial thrombosis in young women after ovarian stimulation: Case report and review of the literature. J. Thromb. Thrombolysis 2007, 24, 169–174. [Google Scholar] [CrossRef]
- Chan, W.S. The ‘ART’ of thrombosis: A review of arterial and venous thrombosis in assisted reproductive technology. Curr. Opin. Obstet. Gynecol. 2009, 21, 207–218. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, J.; Qin, W.; Li, Y.; Yang, L.; Hu, W. The Clinical Characteristics of Acute Cerebrovascular Accidents Resulting from Ovarian Hyperstimulation Syndrome. Eur. Neurol. 2017, 77, 221–230. [Google Scholar] [CrossRef]
- Filipovic-Pierucci, A.; Gabet, A.; Deneux-Tharaux, C.; Plu-Bureau, G.; Olié, V. Arterial and venous complications after fertility treatment: A French nationwide cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 237, 57–63. [Google Scholar] [CrossRef]
- Chan, W.S.; Dixon, M.E. The “ART” of thromboembolism: A review of assisted reproductive technology and thromboembolic complications. Thromb. Res. 2008, 121, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Villani, M.; Favuzzi, G.; Totaro, P.; Chinni, E.; Vecchione, G.; Vergura, P.; Fischetti, L.; Margaglione, M.; Grandone, E. Venous thromboembolism in assisted reproductive technologies: Comparison between unsuccessful versus successful cycles in an Italian cohort. J. Thromb. Thrombolysis 2018, 45, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Dulitzky, M.; Cohen, S.B.; Inbal, A.; Seidman, D.S.; Soriano, D.; Lidor, A.; Mashiach, S.; Rabinovici, J. Increased prevalence of thrombophilia among women with severe ovarian hyperstimulation syndrome. Fertil. Steril. 2002, 77, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Mozes, M.; Bogokowsky, H.; Antebi, E.; Lunenfeld, B.; Rabau, E.; Serr, D.M.; David, A.; Salomy, M. Thromboembolic phenomena after ovarian stimulation with human gonadotrophins. Lancet 1965, 2, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Kermode, A.G.; Churchyard, A.; Carroll, W.M. Stroke complicating severe ovarian hyperstimulation syndrome. Aust. N. Z. J. Med. 1993, 23, 219–220. [Google Scholar] [CrossRef]
- Inbar, O.J.; Levran, D.; Mashiach, S.; Dor, J. Ischemic stroke due to induction of ovulation with clomiphene citrate and menotropins without evidence of ovarian hyperstimulation syndrome. Fertil. Steril. 1994, 62, 1075–1076. [Google Scholar] [CrossRef]
- Cluroe, A.D.; Synek, B.J. A fatal case of ovarian hyperstimulation syndrome with cerebral infarction. Pathology 1995, 27, 344–346. [Google Scholar] [CrossRef]
- Chan, W.S.; Ginsberg, J.S. A review of upper extremity deep vein thrombosis in pregnancy: Unmasking the ‘ART’ behind the clot. J. Thromb. Haemost. 2006, 4, 1673–1677. [Google Scholar] [CrossRef]
- Fleming, T.; Sacks, G.; Nasser, J. Internal jugular vein thrombosis following ovarian hyperstimulation syndrome. Aust. N. Z. J. Obstet. Gynaecol. 2012, 52, 87–90. [Google Scholar] [CrossRef]
- Salomon, O.; Schiby, G.; Heiman, Z.; Avivi, K.; Sigal, C.; Levran, D.; Dor, J.; Itzchak, Y. Combined jugular and subclavian vein thrombosis following assisted reproductive technology--new observation. Fertil. Steril. 2009, 92, 620–625. [Google Scholar] [CrossRef]
- Aboulghar, M.A.; Mansour, R.T.; Serour, G.I.; Amin, Y.M. Moderate ovarian hyperstimulation syndrome complicated by deep cerebrovascular thrombosis. Hum Reprod. 1998, 13, 2088–2091. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Fukuda, J.; Karube, H.; Matsui, T.; Shimizu, Y.; Tanaka, T. Characteristics of blood hemostatic markers in a patient with ovarian hyperstimulation syndrome who actually developed thromboembolism. Fertil. Steril. 1995, 64, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Delvigne, A.; Demoulin, A.; Smitz, J.; Donnez, J.; Koninckx, P.; Dhont, M.; Englert, Y.; Delbeke, L.; Darcis, L.; Gordts, S.; et al. The ovarian hyperstimulation syndrome in in-vitro fertilization: A Belgian multicentric study. I. Clinical and biological features. Hum. Reprod. 1993, 8, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Grandone, E.; Di Micco, P.P.; Villani, M.; Colaizzo, D.; Fernández-Capitán, C.; Del Toro, J.; Rosa, V.; Bura-Riviere, A.; Quere, I.; Blanco-Molina, Á.; et al. Venous Thromboembolism in Women Undergoing Assisted Reproductive Technologies: Data from the RIETE Registry. Thromb. Haemost. 2018, 118, 1962–1968. [Google Scholar] [CrossRef]
- Olausson, N.; Discacciati, A.; Nyman, A.I.; Lundberg, F.; Hovatta, O.; Westerlund, E.; Wallén, H.N.; Mobarrez, F.; Bottai, M.; Ekbom, A.; et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilization with fresh respectively frozen-thawed embryo transfer: Nationwide cohort study. J. Thromb. Haemost. 2020, 18, 1965–1973. [Google Scholar] [CrossRef]
- Abramov, Y.; Elchalal, U.; Schenker, J.G. Obstetric outcome of in vitro fertilized pregnancies complicated by severe ovarian hyperstimulation syndrome: A multicenter study. Fertil. Steril. 1998, 70, 1070–1076. [Google Scholar] [CrossRef]
- Bauersachs, R.M.; Manolopoulos, K.; Hoppe, I.; Arin, M.J.; Schleussner, E. More on: The ‘ART’ behind the clot: Solving the mystery. J. Thromb. Haemost. 2007, 5, 438–439. [Google Scholar] [CrossRef]
- Richardson, M.A.; Berg, D.T.; Calnek, D.S.; Ciaccia, A.V.; Joyce, D.E.; Grinnell, B.W. 17β-estradiol, but not raloxifene, decreases thrombomodulin in the antithrombotic protein C pathway. Endocrinology 2000, 141, 3908–3911. [Google Scholar] [CrossRef]
- Rogolino, A.; Coccia, M.E.; Fedi, S.; Gori, A.M.; Cellai, A.P.; Scarselli, G.F.; Prisco, D.; Abbate, R. Hypercoagulability, high tissue factor and low tissue factor pathway inhibitor levels in severe ovarian hyperstimulation syndrome: Possible association with clinical outcome. Blood Coagul. Fibrinolysis 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Hansen, A.T.; Kesmodel, U.S.; Juul, S.; Hvas, A.M. Increased venous thrombosis incidence in pregnancies after in vitro fertilization. Hum. Reprod. 2014, 29, 611–617. [Google Scholar] [CrossRef]
- Villani, M.; Dentali, F.; Colaizzo, D.; Tiscia, G.L.; Vergura, P.; Petruccelli, T.; Petruzzelli, F.; Ageno, W.; Margaglione, M.; Grandone, E. Pregnancy-related venous thrombosis: Comparison between spontaneous and ART conception in an Italian cohort. BMJ Open 2015, 5, e008213. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Bogatti, P.; Fischer-Tamaro, L.; Giolo, E.; Luppi, S.; Montico, M.; Ronfani, L.; Morgutti, M. Factor V Leiden and prothrombin gene G20210A mutation and in vitro fertilization: Prospective cohort study. Hum. Reprod. 2011, 26, 3068–3077. [Google Scholar] [CrossRef]
- Grandone, E.; Ageno, W. The Legacy of Edwards and Steptoe and the Windy Roads of Assisted Reproduction: Where Do We Stand with Venous Thromboembolism? Thromb. Haemost. 2023, 123, 267–269. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Prevention of moderate and severe ovarian hyperstimulation syndrome: A guideline. Fertil. Steril. 2024, 121, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Rosendaal, F.R. Risk factors for venous thrombotic disease. Thromb Haemost. 1999, 82, 610–619. [Google Scholar] [CrossRef]
- Yinon, Y.; Pauzner, R.; Dulitzky, M.; Elizur, S.E.; Dor, J.; Shulman, A. Safety of IVF under anticoagulant therapy in patients at risk for thrombo-embolic events. Reprod. Biomed. Online 2006, 12, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.M.; Greer, I.A.; Middeldorp, S.; Veenstra, D.L.; Prabulos, A.M.; Vandvik, P.O. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e691S–e736S. [Google Scholar] [CrossRef] [PubMed]
- Grandone, E.; Piazza, G. Thrombophilia, Inflammation, and Recurrent Pregnancy Loss: A Case-Based Review. Semin. Reprod. Med. 2021, 39, 62–68. [Google Scholar] [CrossRef]
- Available online: https://www.rcog.org.uk/media/qejfhcaj/gtg-37a.pdf (accessed on 23 December 2024).
- Balachandren, N.; Seshadri, S.; Yasmin, E.; Saab, W.; Gates, C.; Sayar, Z.; Cohen, H.; Webber, L. Venous thromboembolism associated with medically assisted reproduction (MAR): British fertility society policy and practice guidance for assessment and prevention. Hum. Fertil. 2024, 27, 2352387. [Google Scholar] [CrossRef]
- Sticchi, E.; Romagnuolo, I.; Cellai, A.P.; Lami, D.; Fedi, S.; Prisco, D.; Noci, I.; Abbate, R.; Fatini, C. Fibrinolysis alterations in infertile women during controlled ovarian stimulation: Influence of BMI and genetic components. Thromb. Res. 2012, 130, 919–924. [Google Scholar] [CrossRef]
- Gurunath, S.; Vinekar, S.; Biliangady, R. Assisted Reproductive Techniques in a Patient with History of Venous Thromboembolism: A Case Report and Review of Literature. J. Hum. Reprod. Sci. 2018, 11, 193–197. [Google Scholar] [CrossRef]
- Westerlund, E.; Antovic, A.; Hovatta, O.; Eberg, K.P.; Blombäck, M.; Wallén, H.; Henriksson, P. Changes in von Willebrand factor and ADAMTS13 during IVF. Blood Coagul. Fibrinolysis 2011, 22, 127–131. [Google Scholar] [CrossRef]
| Ref. | Author | Study Design | IVF Population (n) | Cycles (n) | ATEs (n) | Incidence of ATEs per Cycle (n) | ATE Localization | Timing of ATEs (Mean Days after hCG) | |
|---|---|---|---|---|---|---|---|---|---|
| 2002 | [23] | Dulitzky, M. | Cohort | 61 | na | 1 | na | Ischemic stroke | na |
| 2004 | [10] | Grandone, E. | Case–control | 305 | 747 | 3 | 0.40% | Ischemic stroke, upper extremity | na |
| 2007 | [17] | Girolami, A. | Review case series | 34 | na | 34 | na | Ischemic stroke, carotid, AMI, peripheral, other | 13.7 |
| 2009 | [18] | Chan, W.S. | Review case series | 96 | na | 35 | na | Ischemic stroke, neck, AMI, peripheral | 10.7 |
| 2012 | [14] | Hansen, A.T. | Cohort | 30,884 | 75,141 | 2 | 0.003% | na | na |
| 2017 | [19] | Yang, S. | Case series | 38 | na | 29 | na | Ischemic stroke | 8 |
| 2019 | [20] | Filipovic- Pierrucci, A. | Cohort | 277,913 | 788,007 | 78 | 0.01% | na | na |
| Ref. | Author | Study Design | IVF Population (n) | Cycles (n) | VTEs (n) | Incidence of VTEs per Cycle (n) | VTE Localization | Timing of VTEs (Mean Days After hCG) | |
|---|---|---|---|---|---|---|---|---|---|
| 1993 | [33] | Delvigne, A. | Case–control | 384 | na | 1 | 0.01% | CSVT | na |
| 1995 | [32] | Kodama, H. | Case series | 23 | 1316 | 1 | 0.08% | CSVT | 11 |
| 1998 | [36] | Abramov, Y. | Case–control | 163 | 163 | 4 | 2.50% | PE | na |
| 1998 | [15] | Serour, G.I. | Case–control | 2924 | 3500 | 4 | 0.12% | upper extremity | na |
| 1998 | [31] | Aboulghar, M.A. | Case reports | 2 | 2 | 2 | 100% | CSVT | 5 |
| 2002 | [23] | Dulitzky, M. | Cohort | 61 | na | 2 | na | PE | na |
| 2004 | [10] | Grandone, E. | Case–control | 305 | 747 | 2 | 0.30% | CSVT; SVT | na |
| 2006 | [28] | Chan, W.S. | Review case series | 37 | 2500 | 37 | 1.48% | upper extremity; neck | 28 |
| 2009 | [30] | Salomon, O. | Case series | 5 | na | 5 | na | upper extremity; neck | 16 |
| 2009 | [18] | Chan, W.S. | Review case series | 96 | na | 61 | na | upper extremity; neck | 26.6 |
| 2012 | [12] | Rova, K. | Cohort | 19,194 | na | 32 | na | na | 45 |
| 2012 | [14] | Hansen, A.T. | Cohort | 30,884 | 75,141 | 7 | 0.009% | na | na |
| 2012 | [29] | Fleming, T. | Case series | 2 | 2 | 2 | 100% | neck | 39.5 |
| 2013 | [11] | Henriksson, P. | Cohort | 23,498 | na | 99 | na | PE; other not specified | na |
| 2014 | [40] | Hansen, A.T. | Cohort | 18,787 | na | 36 | na | PE; other not specified | na |
| 2015 | [41] | Villani, M. | Cohort | 234 | 684 | 6 | 0.88% | PE; lower limb | na |
| 2017 | [19] | Yang, S. | Case series | 38 | na | 2 | na | CSVT | 8.33 |
| 2018 | [22] | Villani, M. | Cohort | 661 | 1836 | 5 | 0.27% | PE; lower limb | na |
| 2018 | [34] | Grandone, E. | Cohort | 41 | ns | 41 | na | PE; lower limb, upper extremity | na |
| 2019 | [20] | Filipovic-Pierrucci, A. | Cohort | 277,913 | 788,007 | 282 | 0.04% | PE; lower limb | na |
| 2020 | [35] | Olausson, N. | Cohort | 30,328 | na | 161 | na | Lower limb PE, SVT, CSVT, others | na |
| Risk Factor | Magnitude of the Risk |
|---|---|
| OHSS | RR: 14.83; 95% CI: 0.86–255.62 |
| IVF | OR 1.77, 95% CI: 1.41–2.23 HR 4.99, 95% CI: 1.24–20.05 |
| Hyperhomocysteinemia | OR: 15.2; 95% CI: 2.0–115.0 |
| PCOS | RR: 4.8; 95% CI: 1.7–13.4 |
| Successful ART leading to pregnancy | OR: 13.94; 95% CI: 1.41–137.45 |
| Guideline | Intervention | Grade of Evidence |
|---|---|---|
| ACCP 2012 [47] | LMWHs | 2C |
| RCOG 2015 [49] | LMWHs | 2+/C |
| British Fertil. Society 2024 [50] | Subcutaneous LMWHs are the anticoagulant of choice | A |
| British Fertil. Society 2024 [50] | If risk factor(s) for VTE, transfer single embryo | D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grandone, E.; Bitsadze, V.; Khizroeva, J.; Chinni, E.; Mastroianno, M.; Nappi, L.; Tretyakova, M.; Makatsariya, N.; Grigoreva, K.; Gashimova, N.; et al. Risk of Thrombosis in Women Undergoing In Vitro Fertilization: A Narrative Review. J. Clin. Med. 2025, 14, 1053. https://doi.org/10.3390/jcm14041053
Grandone E, Bitsadze V, Khizroeva J, Chinni E, Mastroianno M, Nappi L, Tretyakova M, Makatsariya N, Grigoreva K, Gashimova N, et al. Risk of Thrombosis in Women Undergoing In Vitro Fertilization: A Narrative Review. Journal of Clinical Medicine. 2025; 14(4):1053. https://doi.org/10.3390/jcm14041053
Chicago/Turabian StyleGrandone, Elvira, Victoria Bitsadze, Jamilya Khizroeva, Elena Chinni, Mario Mastroianno, Luigi Nappi, Maria Tretyakova, Natalia Makatsariya, Kristina Grigoreva, Nilufar Gashimova, and et al. 2025. "Risk of Thrombosis in Women Undergoing In Vitro Fertilization: A Narrative Review" Journal of Clinical Medicine 14, no. 4: 1053. https://doi.org/10.3390/jcm14041053
APA StyleGrandone, E., Bitsadze, V., Khizroeva, J., Chinni, E., Mastroianno, M., Nappi, L., Tretyakova, M., Makatsariya, N., Grigoreva, K., Gashimova, N., Lazarchuk, A., Kapanadze, D., Polyakova, T., Shatilina, A., Lyadnova, E., Blbulyan, A., Kuneshko, N., Zainulina, M., Gerotziafas, G., & Makatsariya, A. (2025). Risk of Thrombosis in Women Undergoing In Vitro Fertilization: A Narrative Review. Journal of Clinical Medicine, 14(4), 1053. https://doi.org/10.3390/jcm14041053









