Asymmetric Dimethylarginine (ADMA) as a Novel Risk Factor for Progression of Coronary Artery Calcification in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Enrollment and Purpose of This Study
2.2. Measurement of Coronary Artery Calcification
2.3. Patient Assessment and Laboratory Data
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Enrolled Patients
3.2. Association Between CAC Progression and Parameters
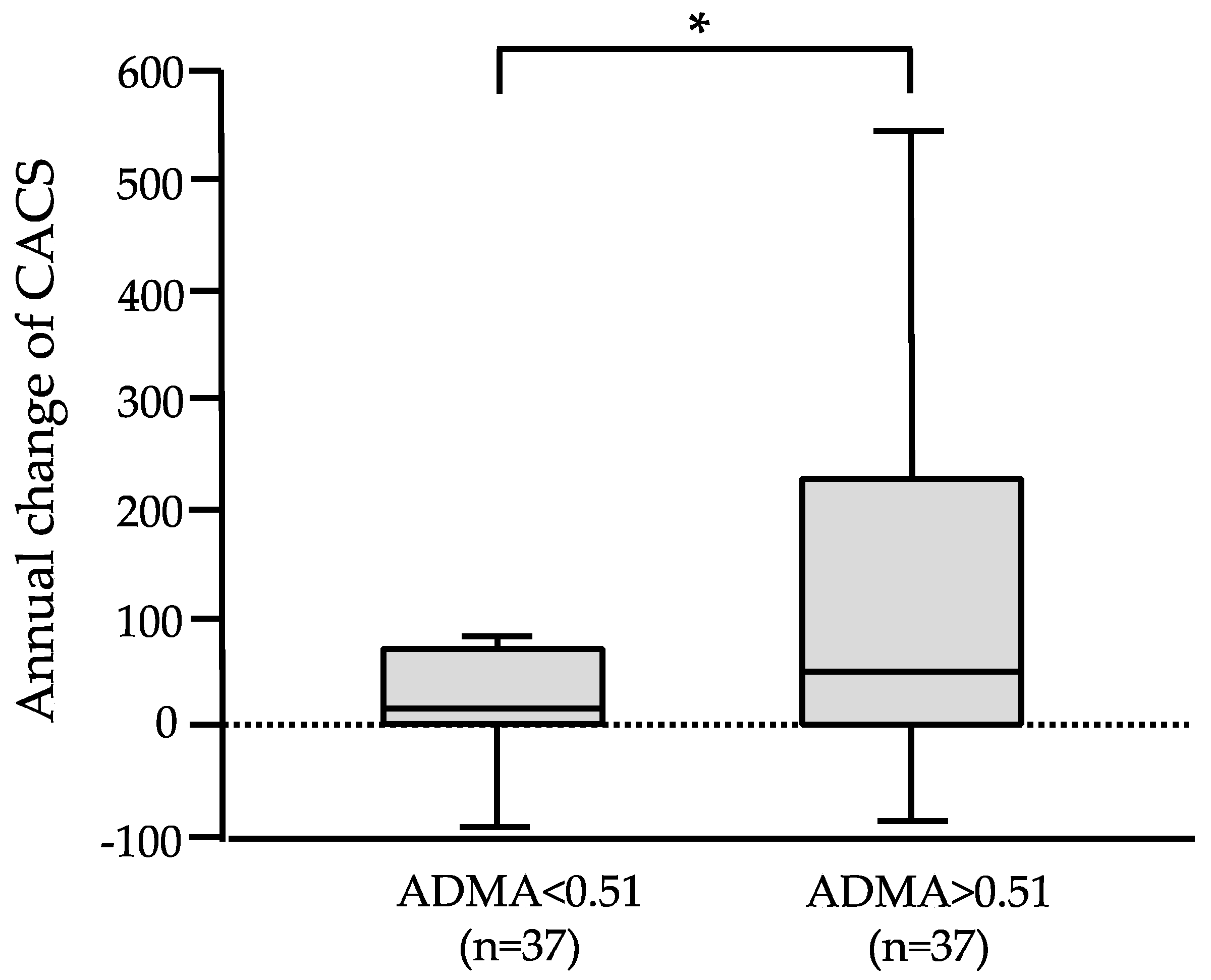
3.3. CAC Progression and Kidney Function Decline According to Stratified ADMA
3.4. Associating Factors for eGFR Decline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodman, G.W.; Goldin, J.; Kuizon, D.B.; Yoon, C.; Gales, B.; Sider, D.; Wang, Y.; Chung, J.; Emerick, A.; Greaser, L.; et al. Coronary artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N. Engl. J. Med. 2000, 342, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Boulay, A.; Chasan-Taber, S.; Amin, N.; Dillon, M.; Burke, S.K.; Chertow, G.M. Cardiac calcification in adult hemodialysis patients: A link between end-stage renal disease and cardiovascular disease? J. Am. Coll. Cardiol. 2002, 39, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Bursztyn, M.; Motro, M.; Grossman, E.; Shemesh, J. Accelerated coronary artery calcification in mildly reduced renal function of high-risk hypertensives: A 3-year prospective observation. J. Hypertens. 2003, 21, 1953–1959. [Google Scholar] [CrossRef]
- Russo, D.; Palmiero, G.; Blasio, A.P.D.; Balletta, M.M.; Andreucci, V.E. Coronary artery calcification in patients with CRF not undergoing dialysis. Am. J. Kidney Dis. 2004, 44, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Larson, M.G.; Keyes, M.G.; Levy, D.; Clouse, M.E.; Culleton, B.; O’Donnell, C.J. Kidney function is inversely associated with coronary artery calcification in men and women free of cardiovascular disease: The Framingham Study. Kidney Int. 2004, 66, 2017–2021. [Google Scholar] [CrossRef]
- Chen, J.; Budoff, M.J.; Reilly, M.P.; Yang, W.; Rosas, S.E.; Rahman, M.; Zhang, X.; Roy, J.A.; Lustigova, E.; Nessel, L.; et al. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol. 2017, 2, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jaeger, B.C.; Scialla, J.J.; Budoff, M.J.; Mehta, R.C.; Jaar, B.G.; Saab, G.; Dobre, M.A.; Reilly, M.P.; Rader, D.J.; et al. CRIC Study Investigators: Progression of coronary artery calcification and risk of clinical events in CRIC Renal Insufficiency Cohort Study. Am. J. Kidney Dis. 2024, 16, S0272–S6386. [Google Scholar]
- Russo, D.; Corrao, S.; Miranda, I.; Ruocco, C.; Manzi, S.; Elefante, R.; Brancaccio, D.; Cozzolino, M.; Biondi, M.L.; Andreucci, V.E. Progression of coronary artery calcification in predialysis patients. Am. J. Nephrol. 2007, 27, 152–158. [Google Scholar] [CrossRef]
- Kobayashi, S.; Oka, M.; Maesato, K.; Ikee, R.; Mano, T.; Moriya, H.; Ohtake, T. Coronary artery calcification, ADMA, and insulin resistance in CKD patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 1289–1295. [Google Scholar] [CrossRef]
- Stuhlinger, M.C.; Abbasi, M.C.; Chu, J.W.; Lamendola, C.; Mclaughlin, T.L.; Cooke, J.P.; Reaven, G.M.; Tsao, P.S. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA 2002, 287, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Chen, J.; Yang, W.; Budoff, M.; Go, A.S.; Grunwald, J.E.; Kallem, R.R.; Post, W.S.; Reilly, M.P.; Ricardo, A.C.; et al. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis 2018, 271, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.R.; Adeney, K.L.; de Boer, L.H.; Ix, J.H.; Shlipak, M.G.; Siscovick, D.S. Incidence and progression of coronary calcification in chronic kidney disease: The Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009, 76, 991–998. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Horiguchi, J.; Yamamoto, H.; Akiyama, Y.; Marukawa, K.; Hirai, N.; Ito, K. Coronary artery calcium scoring using 16-MDCT and a retrospective ECG-gating reconstruction algorithm. Am. J. Roentgenol. 2004, 183, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Bode-Böger, S.M.; Mallamaci, F.; Benedetto, A.; Tripepi, G.; Malatino, L.S.; Cataliotti, A.; Bellanuova, I.; Fermo, I.; Frölich, J.C.; et al. Plasma concentration of asymmetrical dimethylarginine and mortality in patients with end-stage renal disease: A prospective study. Lancet 2001, 358, 2113–2117. [Google Scholar] [CrossRef]
- Kobayashi, S. Cardiovascular events in chronic kidney disease (CKD)—An importance of vascular calcification and microcirculatory impairment. Ren. Replace. Ther. 2016, 2, 55. [Google Scholar] [CrossRef]
- Iribarren, C.; Husson, G.; Sydow, K.; Wang, B.Y.; Sidney, S.; Cooke, J.P. Asymmetric dimethyl-arginine and coronary artery calcification in young adults entering middle age: The CARDIA Stury. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Mantella, D.; Sardella, D.; Beraldi, M.P.; Ferrari, I.; Pierantozzi, A.; Lippi, B.; Giulio, S.D. Asymmetric dimethylarginine, vascular calcifications and parathyroid hormone serum levels in hemodialysis patients. J. Nephrol. 2009, 22, 616–622. [Google Scholar] [PubMed]
- Kumagai, H.; Sakurai, M.; Takita, T.; Maruyama, Y.; Uno, S.; Ikegaya, N.; Hishida, A. Association of homocysteine and asymmetric dimethylarginine with atherosclerosis and cardiovascular events in maintenance hemodialysis patients. Am. J. Kidney Dis. 2006, 48, 797–805. [Google Scholar] [CrossRef]
- Mancin, S.; Mazzoleni, B.; Reggiani, F.; Calatroni, M.; Alterchi, E.; Donizzetti, D.; Finazzi, S.; Soekeland, F.; Sguanci, M.; Badalamanti, S. Integrated protocol for the prevention and treatment of skin ulcers in patients with end-stage renal disease. MethodsX 2023, 11, 102482. [Google Scholar] [CrossRef]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.; Finn, A.V.; Virmani, R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arter. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Bergh, G.V.D.; Branden, A.V.D.; Opdebeeck, B.; Fransen, P.; Neven, E.; De Meyer, G.R.Y.; D’Haese, P.C.; Verhulst, A. Endothelial dysfunction aggravates arterial media calcification in warfarin administered rats. FASEB J. 2022, 36, e22315. [Google Scholar]
- Yuan, C.; Ni, L.; Zhang, C.; Hu, X.; Wu, X. Vascular calcification: New insight into endothelial cells. Microvasc. Res. 2021, 134, 104105. [Google Scholar] [CrossRef]
- Ohtake, T.; Ishioka, K.; Honda, K.; Oka, M.; Maesato, K.; Mano, T.; Ikee, R.; Moriya, H.; Hidaka, S.; Kobayashi, S. Impact of coronary artery calcification in hemodialysis patients: Risk factors and associations with prognosis. Hemodial. Int. 2010, 14, 218–225. [Google Scholar] [CrossRef]
- Obeid, R.; Awwad, H.; Heine, G.H.; Emrich, I.E.; Fliser, D.; Zawada, A.M.; Geisel, J. Plasma concentrations of Trimethylamine-N-oxide, choline, and betaine in patients with moderate to advanced chronic kidney disease and their relation to cardiovascular and renal outcomes. J. Ren. Nutr. 2024, 34, 530–538. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, H.W.; Park, J.T.; Chang, T.I.; Yoo, T.H.; Park, S.K.; Lee, K.B.; Jung, J.Y.; Jeong, J.C.; Oh, K.H.; et al. KNOW-CKD Investigators: Association between progression of coronary artery calcification and development of kidney failure with replacement therapy: Findings from KNOW-CKD study. Atherosclerosis 2024, 395, 117563. [Google Scholar] [CrossRef]
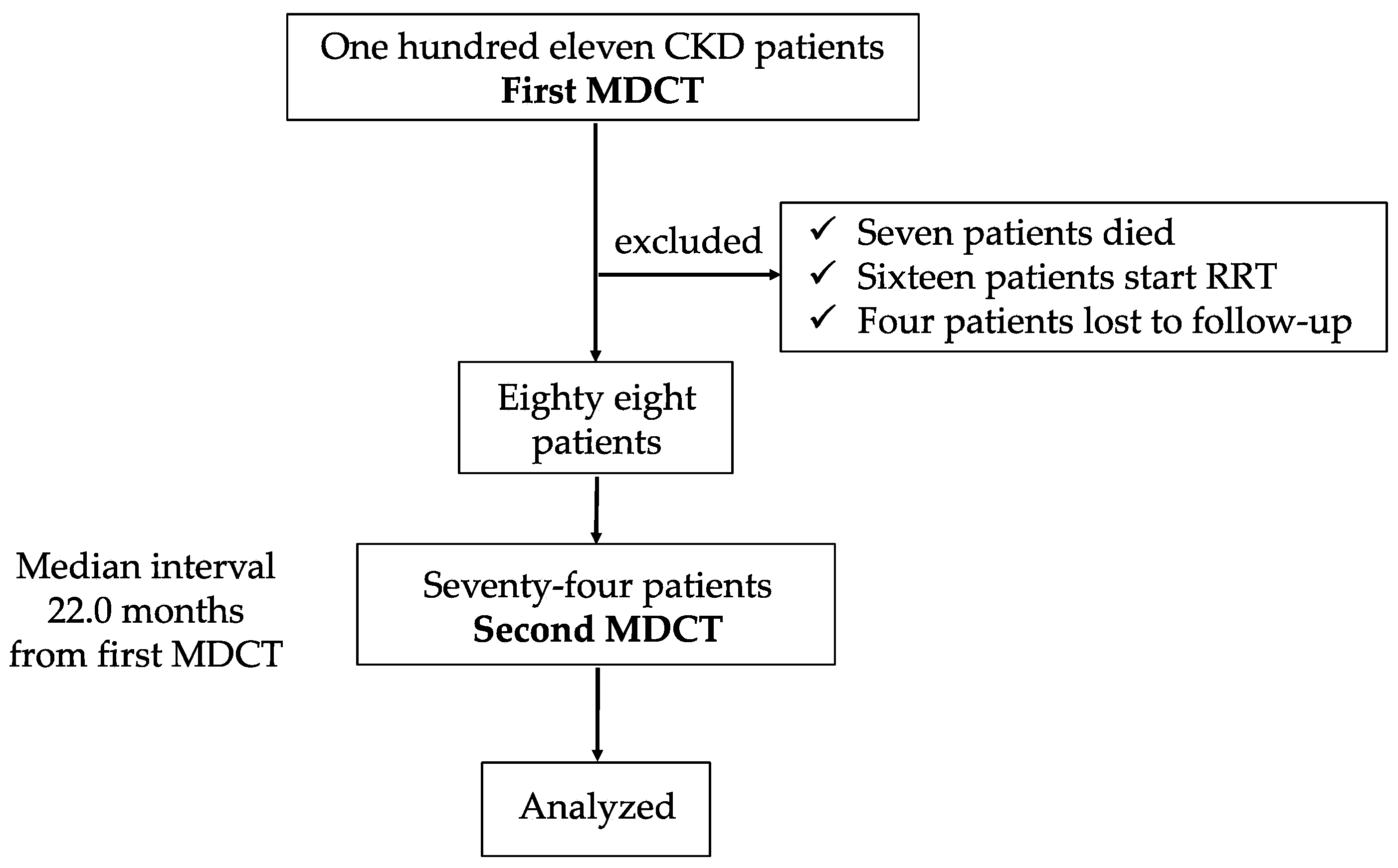
| Variables | (n = 74) | |
|---|---|---|
| Age (years old) | 67.0 ± 11.0 | |
| Sex (male:female) | 46:28 | |
| Comorbidity, n (%) | ||
| Hypertension | 58 (79.4) | |
| Diabetes | 27 (36.5) | |
| Smoking | 7 (9.6) | |
| BMI | 23.0 ± 3.1 | |
| Blood pressure (mmHg) | ||
| Systolic | 131.0 ± 12.0 | |
| Diastolic | 75.0 ± 8.0 | |
| Laboratory parameters | ||
| Creatinine (mg/dL) | 2.02 (1.47–2.67) | |
| eGFR (mL/min/1.73 m2) | 38.0 (26.5–52.2) | |
| Albumin (g/dL) | 4.1 ± 0.4 | |
| T.cho (mg/dL) | 203.0 (172.0–227.0) | |
| TG (mg/L) | 127.0 (97.0–171.0) | |
| HDL-C (mg/dL) | 52.3 (41.9–63.9) | |
| LDL-C (mg/dL) | 115.0 (89.0–137.0) | |
| Ca (mg/dL) | 9.2 ± 0.5 | |
| Pi (mg/dL) | 3.6 ± 0.7 | |
| iPTH (pg/mL) | 81.0 (58.0–146.0) | |
| Fibrinogen (mg/dL) | 337.0 (283.0–384.0) | |
| hsCRP (mg/dL) | 0.08 (0.033–0.174) | |
| urinary protein (g/gCr) | 0.39 (0.20–1.28) | |
| ADMA (µmol/L) | 0.51 (0.44–0.57) | |
| HOMA-IR | 2.22 (1.10–5.90) | |
| Medication | ||
| ARB | 62 (85.0) | |
| Statin | 26 (36.0) | |
| CACS | 42.7 (0–274.2) | |
| First MDCT | Second MDCT | Annual Change | |
|---|---|---|---|
| CACS | 42.7 (0–274.2) | 101.4 (2.6–356.3) | 23.7 (2.0–73.0) |
| Cr (mg/dL) | 2.02 (1.47–2.67) | 2.32 (1.64–3.47) | 0.09 (−0.006–0.59) |
| eGFR (mL/min/1.73 m2) | 38.0 (26.5–52.2) | 27.9 (15.7–42.0) | −6.23 (−14.2–2.3) |
| CACS Zero to Zero Score | CACS Zero to Positive Score | ||||||
|---|---|---|---|---|---|---|---|
| (n = 14) | (n = 7) | ||||||
| at first MDCT | at second MDCT | delta change | at first MDCT | at second MDCT | delta change | p | |
| eGFR(mL/min/1.73 m2) | 47.9 ± 29.8 | 31.6 ± 23.8 | −16.3 ± 36.7 | 29.9 ± 13.3 | 28.4 ± 11.6 | −1.5 ± 9.2 | 0.26 |
| ADMA(µmol/L) | 0.47 ± 0.05 | 0.53 ± 0.07 | 0.059 | ||||
| Univariate Regression Analysis | Multivariate Regression Analysis | |||
|---|---|---|---|---|
| r | p | β | p | |
| Age | 0.129 | 0.33 | ||
| Diabetes | 0.228 | 0.08 | 1.38 | 0.17 |
| Smoking | 0.02 | 0.85 | ||
| Systolic BP | 0.109 | 0.41 | ||
| Diastolic BP | −0.181 | 0.17 | ||
| Cr | 0.129 | 0.33 | ||
| eGFR | −0.224 | 0.08 | 0.048 | 0.78 |
| Albumin | −0.062 | 0.64 | ||
| T.cho | −0.199 | 0.13 | ||
| TG | 0.01 | 0.94 | ||
| HDL-C | −0.175 | 0.18 | ||
| LDL-C | −0.192 | 0.14 | ||
| Ca | −0.211 | 0.09 | −0.033 | 0.84 |
| Pi | 0.264 | 0.04 * | 0.174 | 0.25 |
| Ca×Pi | 0.093 | 0.48 | ||
| iPTH | 0.006 | 0.97 | ||
| hsCRP | −0.021 | 0.87 | ||
| Fibrinogen | 0.024 | 0.86 | ||
| HOMA-IR | −0.083 | 0.53 | ||
| ADMA | 0.389 | 0.005 * | 0.303 | 0.048 * |
| ARB | 0.145 | 0.27 | ||
| Statin | 0.003 | 0.98 | ||
| (n = 74) | Univariate Regression Analysis | |
|---|---|---|
| Baseline Parameters | R | p |
| Age | 0.109 | 0.38 |
| Diabetes | 0.356 | 0.003 * |
| Systolic BP | 0.014 | 0.91 |
| Diastolic BP | −0.26 | 0.035 * |
| Albumin | −0.057 | 0.65 |
| Ca | −0.268 | 0.028 * |
| Pi | 0.245 | 0.045 * |
| hsCRP | −0.008 | 0.95 |
| HOMA-IR | 0.299 | 0.015 * |
| ADMA | 0.332 | 0.012 * |
| Baseline CACS | 0.079 | 0.53 |
| annual change in CACS | 0.64 | 0.061 |
| ARB | 0.02 | 0.87 |
| Urinary protein | 0.104 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, S.; Ohtake, T.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S. Asymmetric Dimethylarginine (ADMA) as a Novel Risk Factor for Progression of Coronary Artery Calcification in Patients with Chronic Kidney Disease. J. Clin. Med. 2025, 14, 1051. https://doi.org/10.3390/jcm14041051
Kobayashi S, Ohtake T, Mochida Y, Ishioka K, Oka M, Maesato K, Moriya H, Hidaka S. Asymmetric Dimethylarginine (ADMA) as a Novel Risk Factor for Progression of Coronary Artery Calcification in Patients with Chronic Kidney Disease. Journal of Clinical Medicine. 2025; 14(4):1051. https://doi.org/10.3390/jcm14041051
Chicago/Turabian StyleKobayashi, Shuzo, Takayasu Ohtake, Yasuhiro Mochida, Kunihiro Ishioka, Machiko Oka, Kyoko Maesato, Hidekazu Moriya, and Sumi Hidaka. 2025. "Asymmetric Dimethylarginine (ADMA) as a Novel Risk Factor for Progression of Coronary Artery Calcification in Patients with Chronic Kidney Disease" Journal of Clinical Medicine 14, no. 4: 1051. https://doi.org/10.3390/jcm14041051
APA StyleKobayashi, S., Ohtake, T., Mochida, Y., Ishioka, K., Oka, M., Maesato, K., Moriya, H., & Hidaka, S. (2025). Asymmetric Dimethylarginine (ADMA) as a Novel Risk Factor for Progression of Coronary Artery Calcification in Patients with Chronic Kidney Disease. Journal of Clinical Medicine, 14(4), 1051. https://doi.org/10.3390/jcm14041051







