A Systematic Review and Meta-Analysis of Preoperative Biliary Drainage Methods in Periampullary Tumors
Abstract
1. Introduction
- Which PBD method has the lowest postprocedural complications?
- Which type of PBD has a decreased postoperative morbidity?
- Is mortality influenced by the PBD technique used?
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Inclusion Criteria
2.3. Data Exclusion
2.4. Outcomes and Definitions
2.5. Statistical Analysis
- Which PBD method has the lowest postprocedural complications?
- Which type of PBD has a decreased postoperative morbidity?
- Is mortality influenced by the PBD technique used?
3. Results
3.1. Search Tools and Study Characteristics
3.2. Statistical Analysis Among Subgroups
3.2.1. Procedural Approach: ERBD Versus ENBD/PTBD
Postprocedural Complications and Delay Until Surgery
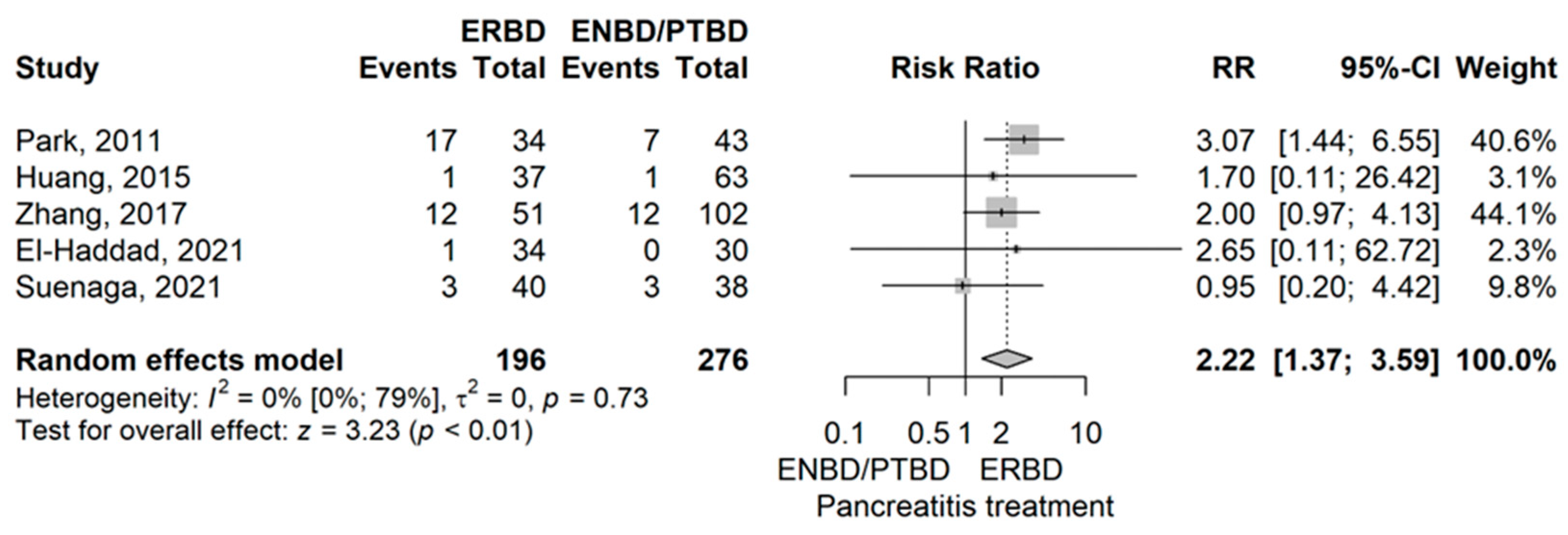
- Pancreatitis
- Cholangitis
- Perforation
- Hemorrhage
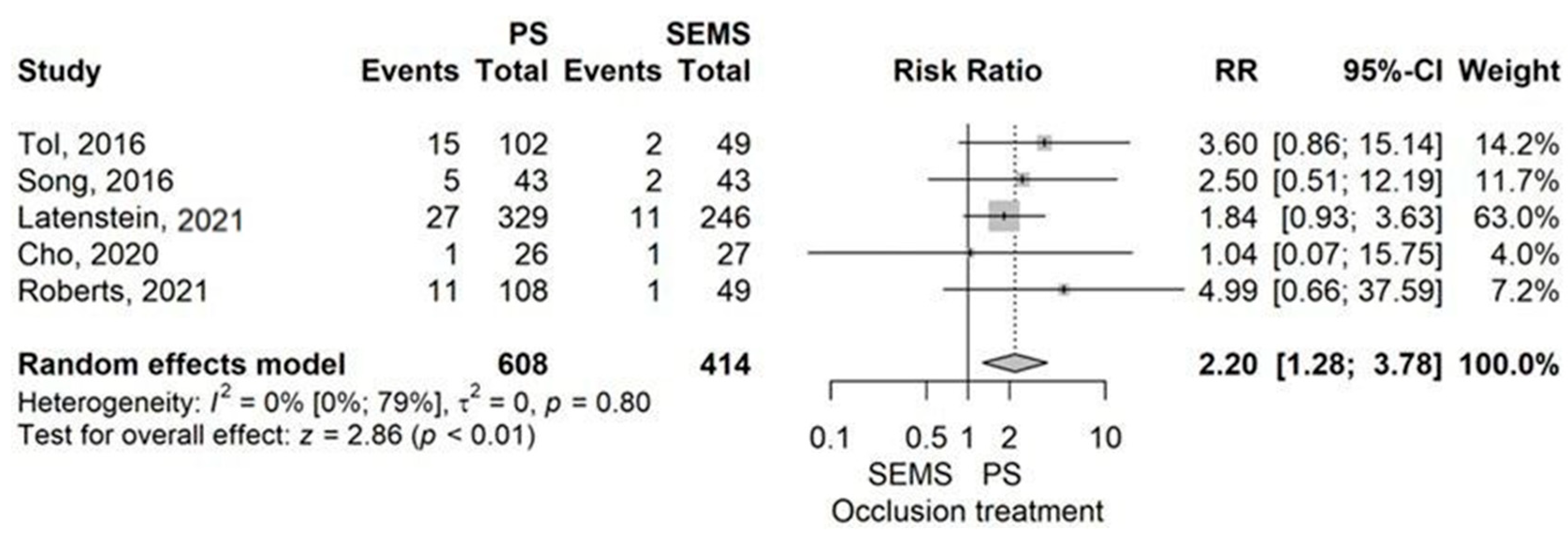
- Catheter occlusion
- Catheter exchange
- Duration of biliary drainage
Postoperative Complications
- Overall complication rate
- Infectious complications
- Sepsis
- Intraabdominal abscess
- Wound infections
- Postpancreatectomy hemorrhage (PPH)
- Chyle leak
- Postoperative Biliary Fistula (POBF)
- Postoperative Pancreatic Fistula (POPF)
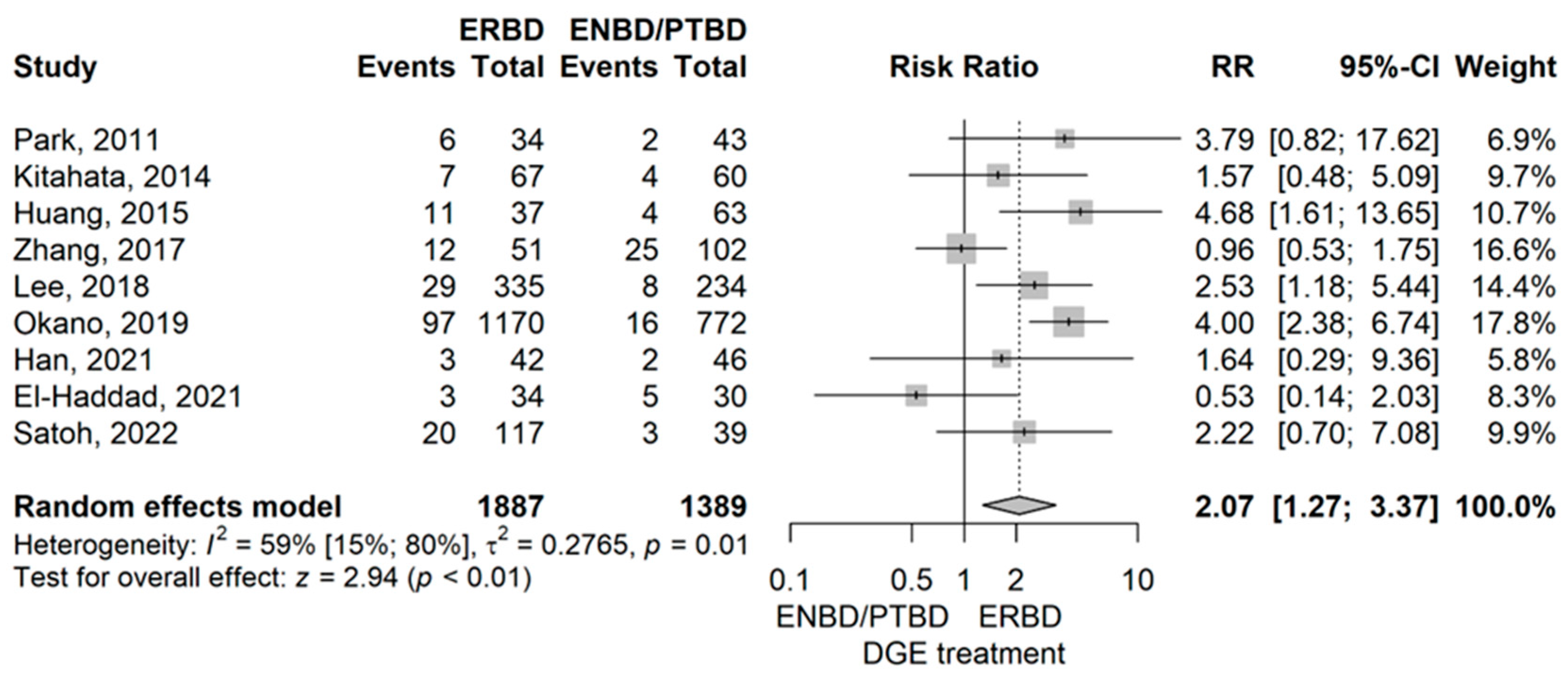
- Delayed gastric emptying (DGE)
Intraoperative Characteristics
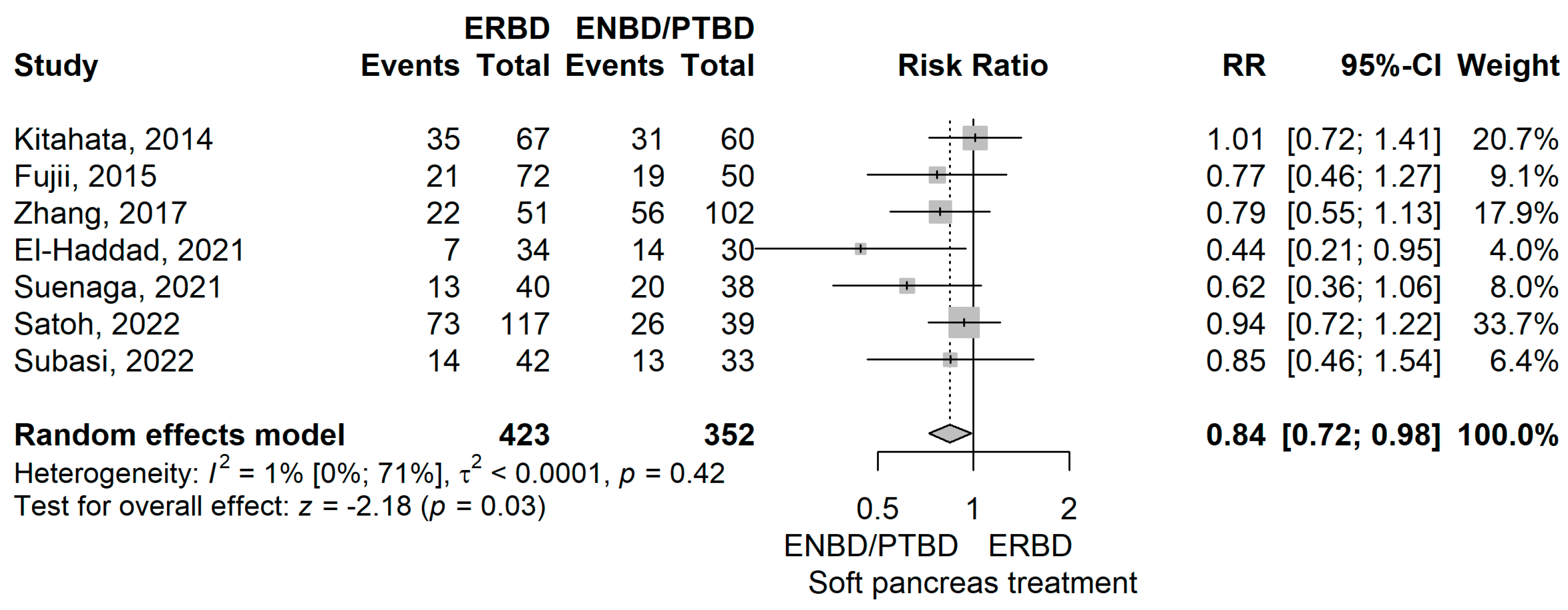
- Soft pancreas
- Main biliary duct (MBD) diameter
- Operative time
- Blood loss
Prognostic Factors
- Hospital stay
- Reoperation rate
- Mortality
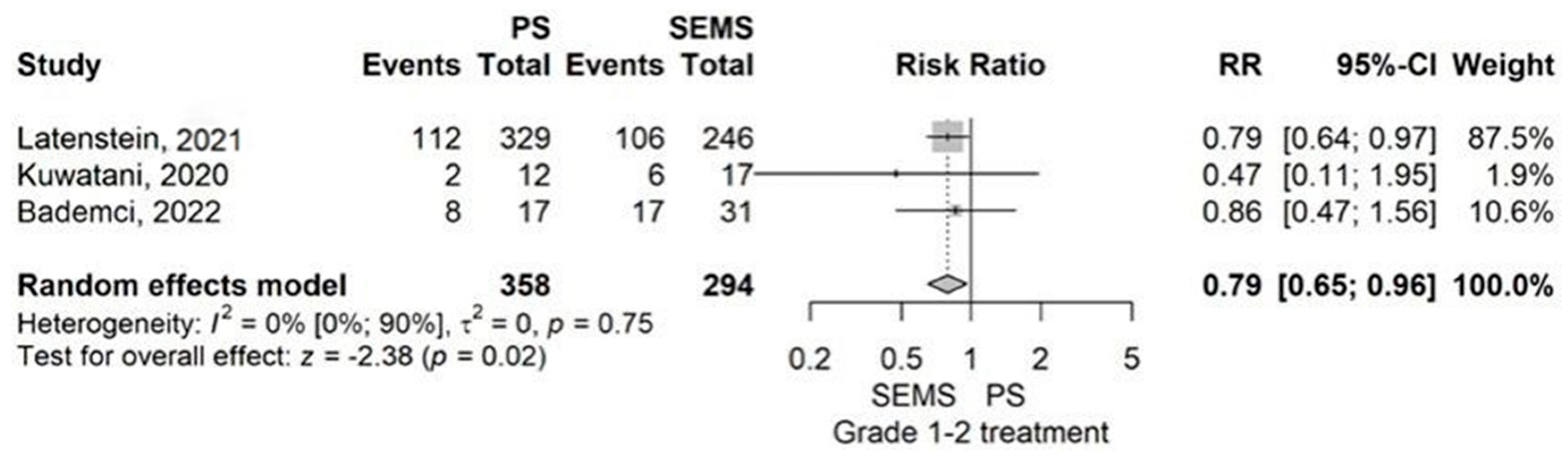
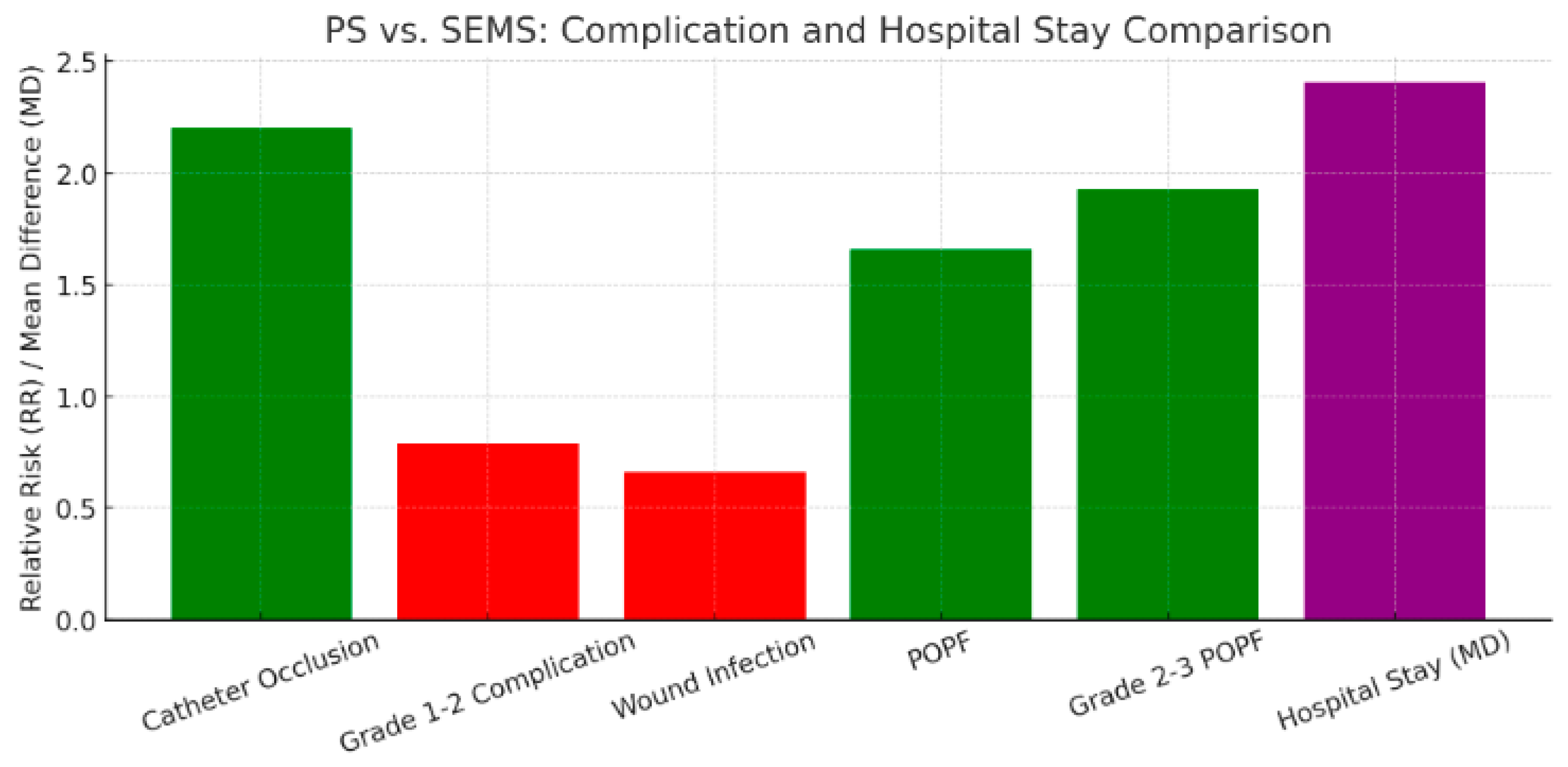
3.2.2. Types of ERBD Stents: PS Versus SEMS
Postprocedural Complications and Delay Until Surgery
- Pancreatitis
- Cholangitis
- Perforation
- Hemorrhage
- Catheter occlusion
- Catheter exchange
- Duration of drainage
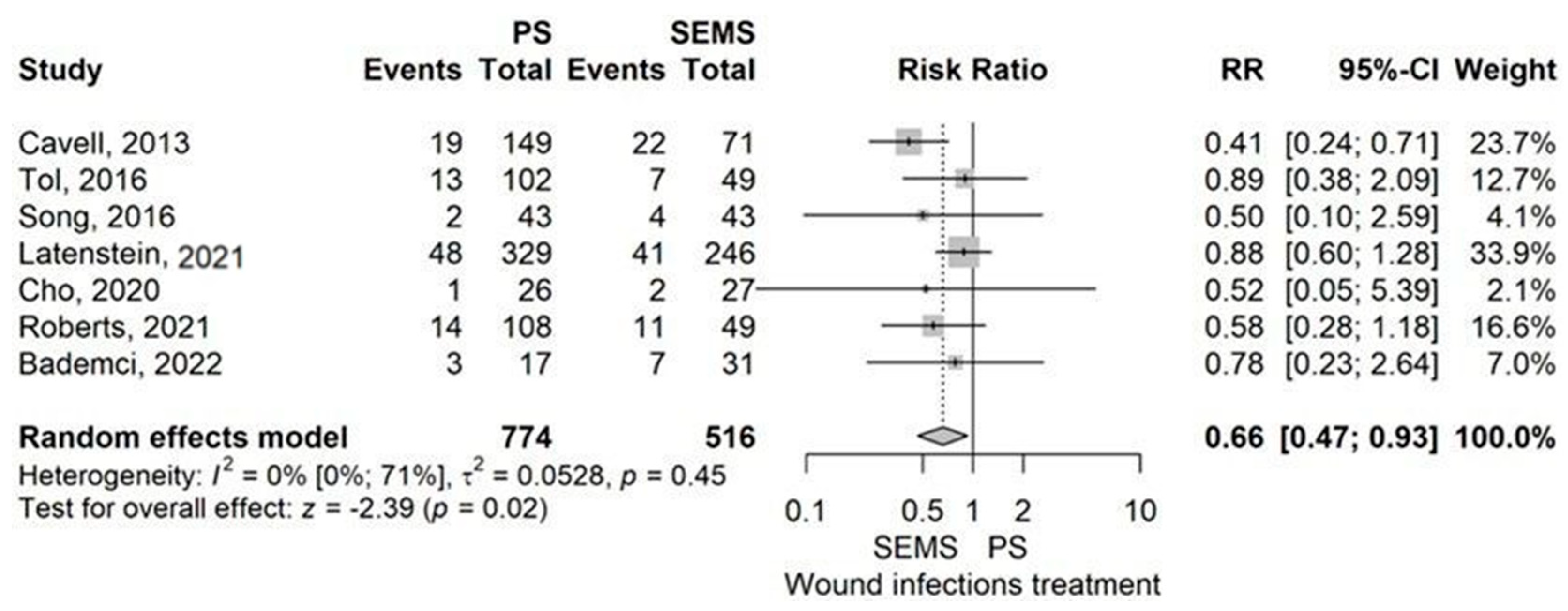
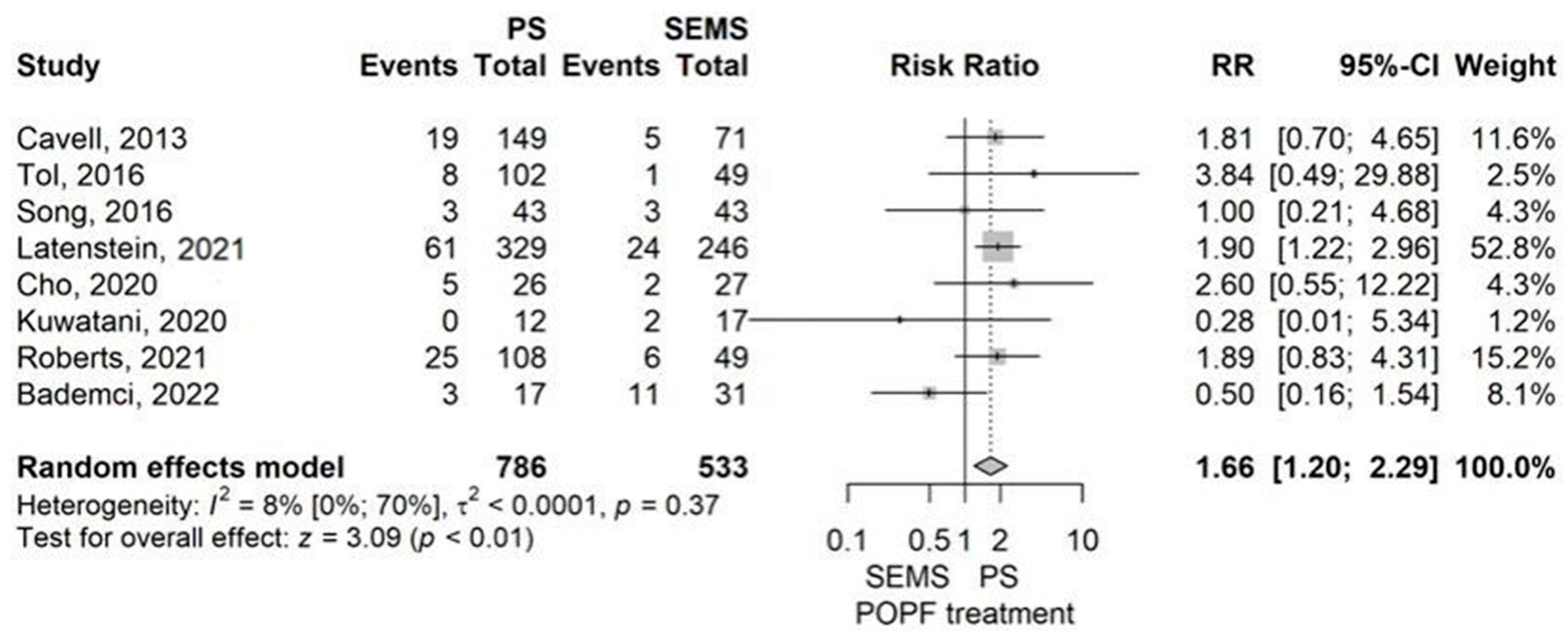
Postoperative Complications
- Overall complication rate
- Infectious complications rate
- Sepsis
- Intraabdominal abscess
- Wound infection
- Postpancreatectomy hemorrhage (PPH)
- Chyle leak
- Postoperative biliary fistula (POBF)
- Postoperative pancreatic fistula (POPF)
- Delayed gastric emptying (DGE)
Intraoperative Characteristics
- Soft pancreas
- Main biliary duct (MBD) diameter
- Operative time
- Blood loss
Prognostic Factors
- Hospital stay
- Reoperation rate
- Mortality
3.3. Summarized Results
4. Discussion
5. Conclusions
6. Limitation of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, D.; Torén, W.; Zhou, Q.; Hu, D.; Andersson, R. Proteomic and Genomic Profiling of Pancreatic Cancer. Cell Biol. Toxicol. 2019, 35, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Kuk, D.; Castillo, C.F.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; et al. Multi-Institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann. Surg. 2017, 265, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Huang, X.; Wang, L.; Xiang, C. The Effect of Preoperative Biliary Stents on Outcomes after Pancreaticoduodenectomy: A Meta-Analysis. Medicine 2020, 99, e22714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Che, X. Comparison of Effect between Nasobiliary Drainage and Biliary Stenting in Malignant Biliary Obstruction: A Systematic Review and Updated Meta-Analysis. World J. Surg. Oncol. 2020, 18, 71. [Google Scholar] [CrossRef]
- Basturk, O.; Cardin, D.B.; Gabriela Chiorean, E.; Jared Christensen, F.A.; Chung, V.; Czito, B.; Del Chiaro, M.; Donahue, T.R.; Fountzilas, C.; Glazer, E.S.; et al. NCCN Guidelines Version 2.2025 Pancreatic Adenocarcinoma; National Comprehensive Cancer Network (NCCN): Plymouth Meeting, PA, USA, 2025. [Google Scholar]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- van der Gaag, N.A.; Rauws, E.A.J.; van Eijck, C.H.J.; Bruno, M.J.; van der Harst, E.; Kubben, F.J.G.M.; Gerritsen, J.J.G.M.; Greve, J.W.; Gerhards, M.F.; de Hingh, I.H.J.T.; et al. Preoperative Biliary Drainage for Cancer of the Head of the Pancreas. N. Engl. J. Med. 2010, 362, 129–137. [Google Scholar] [CrossRef]
- Zhang, B.; Lang, Z.; Zhu, K.; Luo, W.; Zhao, Z.; Zhang, Z.; Wang, Z. Whether Preoperative Biliary Drainage Leads to Better Patient Outcomes of Pancreaticoduodenectomy: A Meta-Analysis and Systematic Review. BMC Gastroenterol. 2025, 25, 161. [Google Scholar] [CrossRef]
- Gong, S.; Song, S.; Cheng, Q.; Huang, Y.; Tian, H.; Jing, W.; Lei, C.; Yang, W.; Yang, K.; Guo, T. Efficacy and Safety of Preoperative Biliary Drainage in Patients Undergoing Pancreaticoduodenectomy: An Updated Systematic Review and Meta-Analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1411–1426. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Li, Y.; Ren, Y.-P.; Fu, N.-T.; Chen, H.-B.; Yang, J.-W.; Xiao, W.-D. Outcomes of Preoperative Endoscopic Nasobiliary Drainage and Endoscopic Retrograde Biliary Drainage for Malignant Distal Biliary Obstruction Prior to Pancreaticoduodenectomy. World J. Gastroenterol. 2017, 23, 5386–5394. [Google Scholar] [CrossRef]
- Hong, S.K.; Jang, J.-Y.; Kang, M.J.; Han, I.W.; Kim, S.-W. Comparison of Clinical Outcome and Cost-Effectiveness after Various Preoperative Biliary Drainage Methods in Periampullary Cancer with Obstructive Jaundice. J. Korean Med. Sci. 2012, 27, 356–362. [Google Scholar] [CrossRef]
- Fujii, T.; Yamada, S.; Suenaga, M.; Kanda, M.; Takami, H.; Sugimoto, H.; Nomoto, S.; Nakao, A.; Kodera, Y. Preoperative Internal Biliary Drainage Increases the Risk of Bile Juice Infection and Pancreatic Fistula after Pancreatoduodenectomy: A Prospective Observational Study. Pancreas 2015, 44, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Tol, J.A.M.G.; van Hooft, J.E.; Timmer, R.; Kubben, F.J.G.M.; van der Harst, E.; de Hingh, I.H.J.T.; Vleggaar, F.P.; Molenaar, I.Q.; Keulemans, Y.C.A.; Boerma, D.; et al. Metal or Plastic Stents for Preoperative Biliary Drainage in Resectable Pancreatic Cancer. Gut 2016, 65, 1981–1987. [Google Scholar] [CrossRef]
- Park, S.-Y.; Park, C.-H.; Cho, S.-B.; Lee, W.-S.; Kim, J.-C.; Cho, C.-K.; Joo, Y.-E.; Kim, H.-S.; Choi, S.-K.; Rew, J.-S. What Is Appropriate Procedure for Preoperative Biliary Drainage in Patients with Obstructive Jaundice Awaiting Pancreaticoduodenectomy? Surg. Laparosc. Endosc. Percutan Tech. 2011, 21, 344–348. [Google Scholar] [CrossRef]
- Lee, P.J.; Podugu, A.; Wu, D.; Lee, A.C.; Stevens, T.; Windsor, J.A. Preoperative Biliary Drainage in Resectable Pancreatic Cancer: A Systematic Review and Network Meta-Analysis. HPB 2018, 20, 477–486. [Google Scholar] [CrossRef]
- Cao, H.; Li, T.; Li, Z.; Zhao, B.; Liu, Z.; Wang, W. Goal-Oriented Preoperative Biliary Drainage Is More Precise and Conducive to Seize the Opportunity for Pancreaticoduodenectomy. World J. Surg. Oncol. 2024, 22, 331. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Chen, Y.; Qin, S.; Chen, J. Value of Preoperative Biliary Drainage in Pancreatic Head Cancer Patients with Severe Obstructive Jaundice: A Multicenter Retrospective Study. Saudi J. Gastroenterol. 2024, 30, 154–161. [Google Scholar] [CrossRef]
- Pawa, S.; Marya, N.B.; Thiruvengadam, N.R.; Ngamruengphong, S.; Baron, T.H.; Bun Teoh, A.Y.; Bent, C.K.; Abidi, W.; Alipour, O.; Amateau, S.K.; et al. American Society for Gastrointestinal Endoscopy Guideline on the Role of Therapeutic EUS in the Management of Biliary Tract Disorders: Summary and Recommendations. Gastrointest. Endosc. 2024, 100, 967–979. [Google Scholar] [CrossRef]
- Nagino, M.; Takada, T.; Miyazaki, M.; Miyakawa, S.; Tsukada, K.; Kondo, S.; Furuse, J.; Saito, H.; Tsuyuguchi, T.; Yoshikawa, T.; et al. Preoperative Biliary Drainage for Biliary Tract and Ampullary Carcinomas. J. Hepatobiliary Pancreat. Surg. 2008, 15, 25–30. [Google Scholar] [CrossRef]
- Lin, H.; Li, S.; Liu, X. The Safety and Efficacy of Nasobiliary Drainage versus Biliary Stenting in Malignant Biliary Obstruction. Medicine 2016, 95, e5253. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H. Current Status of Preoperative Drainage for Distal Biliary Obstruction. World J. Hepatol. 2015, 7, 2171. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Thaker, A.M.; Mosko, J.D.; Berzin, T.M. Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis. Gastroenterol. Rep. 2015, 3, 32–40. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of Acute Pancreatitis—2012: Revision of the Atlanta Classification and Definitions by International Consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Kiriyama, S.; Kozaka, K.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gabata, T.; Hata, J.; Liau, K.; Miura, F.; Horiguchi, A.; et al. Tokyo Guidelines 2018: Diagnostic Criteria and Severity Grading of Acute Cholangitis (with Videos). J. Hepatobiliary Pancreat. Sci. 2018, 25, 17–30. [Google Scholar] [CrossRef]
- Anderson, M.A.; Fisher, L.; Jain, R.; Evans, J.A.; Appalaneni, V.; Ben-Menachem, T.; Cash, B.D.; Decker, G.A.; Early, D.S.; Fanelli, R.D.; et al. Complications of ERCP. Gastrointest. Endosc. 2012, 75, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhara, V.; Khashab, M.A.; Muthusamy, V.R.; Acosta, R.D.; Agrawal, D.; Bruining, D.H.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Gurudu, S.R.; et al. Adverse Events Associated with ERCP. Gastrointest. Endosc. 2017, 85, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 Update of the International Study Group (ISGPS) Definition and Grading of Postoperative Pancreatic Fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Eckmann, C.; Giacobbe, D.R.; Sartelli, M.; Montravers, P. Post-Operative Abdominal Infections: Epidemiology, Operational Definitions, and Outcomes. Intensive Care Med. 2020, 46, 163–172. [Google Scholar] [CrossRef]
- Wente, M.N.; Veit, J.A.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; et al. Postpancreatectomy Hemorrhage (PPH)–An International Study Group of Pancreatic Surgery (ISGPS) Definition. Surgery 2007, 142, 20–25. [Google Scholar] [CrossRef]
- Besselink, M.G.; van Rijssen, L.B.; Bassi, C.; Dervenis, C.; Montorsi, M.; Adham, M.; Asbun, H.J.; Bockhorn, M.; Strobel, O.; Büchler, M.W.; et al. Definition and Classification of Chyle Leak after Pancreatic Operation: A Consensus Statement by the International Study Group on Pancreatic Surgery. Surgery 2017, 161, 365–372. [Google Scholar] [CrossRef]
- Koch, M.; Garden, O.J.; Padbury, R.; Rahbari, N.N.; Adam, R.; Capussotti, L.; Fan, S.T.; Yokoyama, Y.; Crawford, M.; Makuuchi, M.; et al. Bile Leakage after Hepatobiliary and Pancreatic Surgery: A Definition and Grading of Severity by the International Study Group of Liver Surgery. Surgery 2011, 149, 680–688. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed Gastric Emptying (DGE) after Pancreatic Surgery: A Suggested Definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Bademci, R.; Temidayo Talabi, M.O.; Salas, P.; Blanco, M.R.; Riart, G.C.; Bollo, J.; Raventós, V.A. Impact of Biliary Drainage Prior to Pancreatectomy. Acta Chir. Belg. 2022, 122, 390–395. [Google Scholar] [CrossRef]
- Satoh, D.; Matsukawa, H.; Shiozaki, S. The Optimal Type and Management of Biliary Drainage in Patients With Obstructive Jaundice Who Undergo Pancreaticoduodenectomy. In Vivo 2022, 36, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Subasi, O.; Ercan, M.; Aziret, M.; Biricik, A.; Kahraman, Y.S.; Altıntoprak, F.; Celebi, F.; Karaman, K. Effects of Preoperative Biliary Drainage Methods and Time to Postoperative Complications after Biliary Drainage in Periampullary Tumors. Ann. Ital. Chir. 2022, 93, 403–409. [Google Scholar] [PubMed]
- Latenstein, A.E.J.; Mackay, T.M.; van Huijgevoort, N.C.M.; Bonsing, B.A.; Bosscha, K.; Hol, L.; Bruno, M.J.; van Coolsen, M.M.E.; Festen, S.; van Geenen, E.; et al. Nationwide Practice and Outcomes of Endoscopic Biliary Drainage in Resectable Pancreatic Head and Periampullary Cancer. HPB 2021, 23, 270–278. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, J.S.; Hwang, J.W.; Kim, H.S. Preoperative Endoscopic Retrograde Biliary Drainage Increases Postoperative Complications after Pancreaticoduodenectomy Compared to Endoscopic Nasobiliary Drainage. Gland Surg. 2021, 10, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liang, B.; Zhao, X.-Q.; Zhang, F.-B.; Wang, X.-T.; Dong, J.-H. The Effects of Different Preoperative Biliary Drainage Methods on Complications Following Pancreaticoduodenectomy. Medicine 2015, 94, e723. [Google Scholar] [CrossRef] [PubMed]
- Haapamäki, C.; Seppänen, H.; Udd, M.; Juuti, A.; Halttunen, J.; Kiviluoto, T.; Sirén, J.; Mustonen, H.; Kylänpää, L. Preoperative Biliary Decompression Preceding Pancreaticoduodenectomy with Plastic or Self-Expandable Metallic Stent. Scand. J. Surg. 2015, 104, 79–85. [Google Scholar] [CrossRef]
- Okano, K.; Suzuki, Y. Influence of Bile Contamination for Patients Who Undergo Pancreaticoduodenectomy after Biliary Drainage. World J. Gastroenterol. 2019, 25, 6847–6856. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.T.; Jaya, J.; Ha, P.; Thakur, U.; Aldridge, O.; Pilgrim, C.H.C.; Tan, E.; Wong, E.; Fox, A.; Choi, J.; et al. Metal Stents Are Safe and Cost-effective for Preoperative Biliary Drainage in Resectable Pancreaticobiliary Tumours. ANZ J. Surg. 2021, 91, 1841–1846. [Google Scholar] [CrossRef]
- Lee, H.; Han, Y.; Kim, J.R.; Kwon, W.; Kim, S.-W.; Jang, J.-Y. Preoperative Biliary Drainage Adversely Affects Surgical Outcomes in Periampullary Cancer: A Retrospective and Propensity Score-Matched Analysis. J. Hepatobiliary Pancreat. Sci. 2018, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Aoki, T.; Park, K.-H.; Shiraki, T.; Sakuraoka, Y.; Iso, Y.; Kato, M.; Kubota, K. Impact of Preoperative Percutaneous Transhepatic Biliary Drainage on Post-Operative Survival in Patients with Distal Cholangiocarcinoma. ANZ J. Surg. 2019, 89, E363–E367. [Google Scholar] [CrossRef]
- Byun, Y.; Kwon, W.; Han, Y.; Choi, Y.J.; Kang, J.S.; Kim, H.; Jang, J.-Y. Adverse Oncologic Effects of Preoperative Biliary Drainage on Early Stage Ampulla of Vater Cancer. HPB 2021, 23, 253–261. [Google Scholar] [CrossRef]
- Kitahata, Y.; Kawai, M.; Tani, M.; Hirono, S.; Okada, K.; Miyazawa, M.; Shimizu, A.; Yamaue, H. Preoperative Cholangitis during Biliary Drainage Increases the Incidence of Postoperative Severe Complications after Pancreaticoduodenectomy. Am. J. Surg. 2014, 208, 1–10. [Google Scholar] [CrossRef]
- El-Haddad, H.M.; Sabry, A.A.; Shehata, G.M. Endoscopic versus Percutaneous Biliary Drainage for Resectable Pancreatic Head Cancer with Hyperbilirubinemia and Impact on Pancreaticoduodenectomy: A Randomized Controlled Study. Int. J. Surg. 2021, 93, 106043. [Google Scholar] [CrossRef]
- Cho, J.H.; Yoon, Y.-S.; Kim, E.J.; Kim, Y.S.; Cho, J.Y.; Han, H.-S.; Park, Y.H.; Shin, D.W.; Lee, J.-C.; Hwang, J.-H.; et al. A Multicenter Prospective Randomized Controlled Trial for Preoperative Biliary Drainage with Uncovered Metal versus Plastic Stents for Resectable Periampullary Cancer. J. Hepatobiliary Pancreat. Sci. 2020, 27, 690–699. [Google Scholar] [CrossRef]
- Cavell, L.K.; Allen, P.J.; Vinoya, C.; Eaton, A.A.; Gonen, M.; Gerdes, H.; Mendelsohn, R.B.; D’Angelica, M.I.; Kingham, T.P.; Fong, Y.; et al. Biliary Self-Expandable Metal Stents Do Not Adversely Affect Pancreaticoduodenectomy. Am. J. Gastroenterol. 2013, 108, 1168–1173. [Google Scholar] [CrossRef]
- Suenaga, M.; Yokoyama, Y.; Fujii, T.; Yamada, S.; Yamaguchi, J.; Hayashi, M.; Asahara, T.; Nagino, M.; Kodera, Y. Impact of Qualitative and Quantitative Biliary Contamination Status on the Incidence of Postoperative Infection Complications in Patients Undergoing Pancreatoduodenectomy. Ann. Surg. Oncol. 2021, 28, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Murakami, Y.; Satoi, S.; Sho, M.; Motoi, F.; Kawai, M.; Matsumoto, I.; Honda, G.; Kurata, M.; Yanagimoto, H.; et al. Impact of Preoperative Biliary Drainage on Long-Term Survival in Resected Pancreatic Ductal Adenocarcinoma: A Multicenter Observational Study. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), 1238–1246. [Google Scholar] [CrossRef]
- Song, T.J.; Lee, J.H.; Lee, S.S.; Jang, J.W.; Kim, J.W.; Ok, T.J.; Oh, D.W.; Park, D.H.; Seo, D.W.; Lee, S.K.; et al. Metal versus Plastic Stents for Drainage of Malignant Biliary Obstruction before Primary Surgical Resection. Gastrointest. Endosc. 2016, 84, 814–821. [Google Scholar] [CrossRef]
- Kuwatani, M.; Nakamura, T.; Hayashi, T.; Kimura, Y.; Ono, M.; Motoya, M.; Imai, K.; Yamakita, K.; Goto, T.; Takahashi, K.; et al. Clinical Outcomes of Biliary Drainage during a Neoadjuvant Therapy for Pancreatic Cancer: Metal versus Plastic Stents. Gut Liver 2020, 14, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, N.; Hamada, T.; Togawa, O.; Yamamoto, R.; Iwai, T.; Tamada, K.; Kawaguchi, Y.; Shimura, K.; Koike, T.; Yoshida, Y.; et al. Multicenter Study of Endoscopic Preoperative Biliary Drainage for Malignant Distal Biliary Obstruction. World J. Gastroenterol. 2016, 22, 3793. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nagino, M.; Nishio, H.; Ebata, T.; Igami, T.; Nimura, Y. Percutaneous Transhepatic Biliary Drainage Catheter Tract Recurrence in Cholangiocarcinoma. Br. J. Surg. 2010, 97, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Barkay, O.; Mosler, P.; Schmitt, C.M.; Lehman, G.A.; Frakes, J.T.; Johanson, J.F.; Qaseem, T.; Howell, D.A.; Sherman, S. Effect of Endoscopic Stenting of Malignant Bile Duct Obstruction on Quality of Life. J. Clin. Gastroenterol. 2013, 47, 526–531. [Google Scholar] [CrossRef]
- Cheng, C.-L.; Sherman, S.; Watkins, J.L.; Barnett, J.; Freeman, M.; Geenen, J.; Ryan, M.; Parker, H.; Frakes, J.T.; Fogel, E.L.; et al. Risk Factors for Post-ERCP Pancreatitis: A Prospective Multicenter Study. Am. J. Gastroenterol. 2006, 101, 139–147. [Google Scholar] [CrossRef]
- Coté, G.A.; Kumar, N.; Ansstas, M.; Edmundowicz, S.A.; Jonnalagadda, S.; Mullady, D.K.; Azar, R.R. Risk of Post-ERCP Pancreatitis with Placement of Self-Expandable Metallic Stents. Gastrointest. Endosc. 2010, 72, 748–754. [Google Scholar] [CrossRef]
- Cortes, A.; Sauvanet, A.; Bert, F.; Janny, S.; Sockeel, P.; Kianmanesh, R.; Ponsot, P.; Ruszniewski, P.; Belghiti, J. Effect of Bile Contamination on Immediate Outcomes after Pancreaticoduodenectomy for Tumor. J. Am. Coll. Surg. 2006, 202, 93–99. [Google Scholar] [CrossRef]
- Hu, B.-Y.; Wan, T.; Zhang, W.-Z.; Dong, J.-H. Risk Factors for Postoperative Pancreatic Fistula: Analysis of 539 Successive Cases of Pancreaticoduodenectomy. World J. Gastroenterol. 2016, 22, 7797. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Z.; Li, W. Internal Drainage versus External Drainage in Palliation of Malignant Biliary Obstruction: A Meta-Analysis and Systematic Review. Arch. Med. Sci. 2020, 16, 752–763. [Google Scholar] [CrossRef]
- Dorcaratto, D.; Hogan, N.M.; Muñoz, E.; Garcés, M.; Limongelli, P.; Sabater, L.; Ortega, J. Is Percutaneous Transhepatic Biliary Drainage Better than Endoscopic Drainage in the Management of Jaundiced Patients Awaiting Pancreaticoduodenectomy? A Systematic Review and Meta-Analysis. J. Vasc. Interv. Radiol. 2018, 29, 676–687. [Google Scholar] [CrossRef]
- Duan, F.; Cui, L.; Bai, Y.; Li, X.; Yan, J.; Liu, X. Comparison of Efficacy and Complications of Endoscopic and Percutaneous Biliary Drainage in Malignant Obstructive Jaundice: A Systematic Review and Meta-Analysis. Cancer Imaging 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Ramai, D.; Gkolfakis, P.; Khan, S.R.; Papanikolaou, I.S.; Triantafyllou, K.; Tringali, A.; Chandan, S.; Mohan, B.P.; Adler, D.G. Comparative Efficacy of Different Methods for Difficult Biliary Cannulation in ERCP: Systematic Review and Network Meta-Analysis. Gastrointest. Endosc. 2022, 95, 60–71.e12. [Google Scholar] [CrossRef]
- Zarzavadjian Le Bian, A.; Fuks, D.; Dalla Valle, R.; Cesaretti, M.; Violi, V.; Costi, R. Effectiveness and Risk of Biliary Drainage Prior to Pancreatoduodenectomy: Review of Current Status. Surg. Today 2018, 48, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Uemura, K.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Sasaki, H.; Hatano, N.; Kohmo, T.; Sueda, T. Does Preoperative Biliary Drainage Compromise the Long-Term Survival of Patients with Pancreatic Head Carcinoma? J. Surg. Oncol. 2015, 111, 270–276. [Google Scholar] [CrossRef]
- Strom, T.J.; Klapman, J.B.; Springett, G.M.; Meredith, K.L.; Hoffe, S.E.; Choi, J.; Hodul, P.; Malafa, M.P.; Shridhar, R. Comparative Long-Term Outcomes of Upfront Resected Pancreatic Cancer after Preoperative Biliary Drainage. Surg. Endosc. 2015, 29, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Li, L.; Dai, H.; Han, J. Association of Preoperative Obstructive Jaundice with Postoperative Infectious Complications Following Pancreaticoduodenectomy. Hepatogastroenterology 2013, 60, 1274–1279. [Google Scholar] [CrossRef]
- Velanovich, V.; Kheibek, T.; Khan, M. Relationship of Postoperative Complications from Preoperative Biliary Stents after Pancreaticoduodenectomy. A New Cohort Analysis and Meta-Analysis of Modern Studies. JOP 2009, 10, 24–29. [Google Scholar] [PubMed]
- Lai, E.C.H.; Lau, S.H.Y.; Lau, W.Y. The Current Status of Preoperative Biliary Drainage for Patients Who Receive Pancreaticoduodenectomy for Periampullary Carcinoma: A Comprehensive Review. Surgeon 2014, 12, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B.; Spangler, C.C.; Byanova, K.L.; Ripple, G.H.; Rockacy, M.J.; Levenick, J.M.; Smith, K.D.; Colacchio, T.A.; Barth, R.J.; Zaki, B.I.; et al. Cost-Effectiveness and Clinical Efficacy of Biliary Stents in Patients Undergoing Neoadjuvant Therapy for Pancreatic Adenocarcinoma in a Randomized Controlled Trial. Gastrointest. Endosc. 2016, 84, 460–466. [Google Scholar] [CrossRef] [PubMed]


| No. | Author Name | Year of Publication | Type of Study | Population | Groups Compared | Age (Years) | Neoadjuvant Therapy n (%) | MBD Diameter (mm) | Soft Pancreas n (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Bademci et al. [37] | 2022 | Retrospective Cohort Study | 48 | PS N = 17 SEMS N = 31 | 69 vs. 70 (p = 0.773) | - | - | - |
| 2 | Satoh et al. [38] | 2022 | Retrospective Cohort Study | 156 | ERBD N = 117 ENBD N = 39 | 70 vs. 71 (p = 0.217) | - | 3 vs. 4 (p = 0.327) | 73 (62%) vs. 26 (67%) (p = 0.63) |
| 3 | Subasi et al. [39] | 2022 | Retrospective Cohort Study | 75 | ERBD N = 42 PTBD N = 33 | 62.9 vs. 65.2 (p = 0.523) | - | 3.7 vs. 4.3 (p = 0.557) | 14 (33.3%) vs. 13 (39.4%) (p = 0.607) |
| 4 | Latenstein et al. [40] | 2021 | Retrospective Cohort Study | 575 | PS N = 329 SEMS N = 246 | 67 vs. 68 (p = 0.117) | 17 (5.2%) vs. 31 (12.6%) (p = 0.011) | 209 (63.5%) vs. 121 (49.2%) (p = 0.001) | |
| 5 | Han et al. [41] | 2021 | Retrospective Cohort Study | 88 | ERBD N = 42 ENBD N = 46 | 65 vs. 67 (p = 0.529) | - | - | - |
| 6 | Huang et al. [42] | 2015 | Retrospective Cohort Study | 100 | ERBD N = 37 ENBD N = 18 PTBD N = 45 | 58.1 vs. 60.6 vs. 57.5 (p = 0.56) | - | - | - |
| 7 | Haapamäki et al. [43] | 2015 | Retrospective Cohort Study | 191 | PS N = 163 SEMS N = 28 | 64 vs. 64 | 22 (20%) vs. 3 (14%) | - | - |
| 8 | Okano et al. [44] | 2019 | Retrospective Cohort Study | 1942 | ERBD N = 1170 ENBD/PTBD N = 772 | 64 vs. 62 (p = 0.025) | - | - | - |
| 9 | Roberts et al. [45] | 2021 | Retrospective Cohort Study | 157 | PS N = 108 SEMS N = 49 | 66.3 vs. 67.2 (p = 0.63) | 2 (1.9%) vs. 9 (18.4%) (<0.001) | - | - |
| 10 | Lee et al. [46] | 2018 | Retrospective Cohort Study | 569 | ERBD N = 335 PTBD N = 234 | 63.1 vs. 63.5 (p = 0.609) | - | - | - |
| 11 | Mori et al. [47] | 2019 | Retrospective Cohort Study | 84 | ERBD N = 60 PTBD N = 24 | 68 vs. 73 (p = 0.156) | 20 (33%) vs. 9 (37%) (p = 0.717) | - | - |
| 12 | Byun et al. [48] | 2021 | Retrospective Cohort Study | 167 | ERBD N = 106 PTBD N = 61 | 62 vs. 64 (p = 0.805) | - | - | - |
| 13 | Park et al. [14] | 2011 | Retrospective Cohort Study | 77 | ERBD N = 34 PTBD N = 43 | 63.7 vs. 65.9 (p = 0.305) | - | - | - |
| 14 | Kitahata et al. [49] | 2014 | Retrospective Cohort Study | 127 | ERBD N = 67 ENBD/PTBD N = 60 | 68 vs. 70 (p = 0.333) | 3 vs. 0 (p = 0.144) | - | 35 vs. 31 (p = 0.949) |
| 15 | El-Haddad et al. [50] | 2021 | Prospective Cohort Study | 64 | ERBD N = 34 PTBD N = 30 | 53.3 vs. 54 (p = 0.79) | - | - | 7 (23%) vs. 14 (46.7%) (p = 0.16) |
| 16 | Fujii et al. [12] | 2015 | Prospective Cohort Study | 122 | ERBD N = 72 ENBD N = 50 | 67 vs. 66.5 (p = 0.682) | - | 4.9 vs. 4.3 (p = 0.180) | 21 vs. 19 (p = 0.307) |
| 17 | Cho et al. [51] | 2020 | Prospective RCT | 53 | PS N = 26 UCSEMS N = 27 | 69 vs. 67 (p = 0.936) | - | - | - |
| 18 | Tol et al. [13] | 2016 | Prospective Cohort Study | 151 | PS N = 102 FCSEMS N = 49 | 64.7 vs. 67.5 | - | - | - |
| 19 | Cavell et al. [52] | 2013 | Retrospective Study | 220 | PS N = 149 SEMS N = 71 | 68 vs. 67 (p = 0.858) | 10 (7.4%) vs. 23 (34.3%) (p < 0.001) | - | - |
| 20 | Suenaga et al. [53] | 2021 | Prospective Cohort Study | 78 | ERBD N = 40 ENBD/PTBD N = 38 | 67 vs. 70 (p = 0.084) | 13 (33%) vs. 5 (13%) (p = 0.060) | - | 13 (33%) vs. 20 (53%) (p = 0.072) |
| 21 | Uemura et al. [54] | 2015 | Retrospective Cohort Study | 573 | ERBD N = 407 PTBD N = 166 | 67 vs. 67 | 203 (50%) vs. 79 (49%) (p = 0.957) | - | - |
| 22 | Song et al. [55] | 2016 | Prospective RCT | 86 | PS N = 43 FCSEMS N = 43 | 65.72 vs. 65.67 (p = 0.982) | - | - | - |
| 23 | Zhang et al. [10] | 2017 | Retrospective Cohort Study | 153 | ENBD N = 102 ERBD N = 51 | 55.26 vs. 56.24 (p = 0.542) | - | 1.73 vs. 1.78 (p = 0.540) | 56 (54.9%) vs. 22 (43.1%) (p = 0.170) |
| 24 | Kuwatani et al. [56] | 2020 | Retrospective Cohort Study | 29 | SEMS N = 17 PS = 12 | 66 vs. 68 (p = 0.824) | - | - | - |
| Total | 5894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, S.A.; Moiș, E.I.; Graur, F.; Puia, I.C.; Vlad, I.; Nechita, V.I.; Furcea, L.; Zaharie, F.; Popa, C.; Leucuța, D.C.; et al. A Systematic Review and Meta-Analysis of Preoperative Biliary Drainage Methods in Periampullary Tumors. J. Clin. Med. 2025, 14, 7097. https://doi.org/10.3390/jcm14197097
Moldovan SA, Moiș EI, Graur F, Puia IC, Vlad I, Nechita VI, Furcea L, Zaharie F, Popa C, Leucuța DC, et al. A Systematic Review and Meta-Analysis of Preoperative Biliary Drainage Methods in Periampullary Tumors. Journal of Clinical Medicine. 2025; 14(19):7097. https://doi.org/10.3390/jcm14197097
Chicago/Turabian StyleMoldovan, Septimiu Alex, Emil Ioan Moiș, Florin Graur, Ion Cosmin Puia, Iulia Vlad, Vlad Ionuț Nechita, Luminiţa Furcea, Florin Zaharie, Călin Popa, Daniel Corneliu Leucuța, and et al. 2025. "A Systematic Review and Meta-Analysis of Preoperative Biliary Drainage Methods in Periampullary Tumors" Journal of Clinical Medicine 14, no. 19: 7097. https://doi.org/10.3390/jcm14197097
APA StyleMoldovan, S. A., Moiș, E. I., Graur, F., Puia, I. C., Vlad, I., Nechita, V. I., Furcea, L., Zaharie, F., Popa, C., Leucuța, D. C., Mirel, S., Moldovan, M. Ş., Mocan, T., Seicean, A., Ciocan, A., & Hajjar, N. A. (2025). A Systematic Review and Meta-Analysis of Preoperative Biliary Drainage Methods in Periampullary Tumors. Journal of Clinical Medicine, 14(19), 7097. https://doi.org/10.3390/jcm14197097