Short-Term Efficacy of Two-Step Treatment of Retinopathy of Prematurity in a Japanese Cohort: Anti-VEGF Therapy Followed by Routine Laser Photocoagulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Laser Photocoagulation
2.2. Intravitreal Injection of Anti-VEGF Agents
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROP | Retinopathy of prematurity |
| A-ROP | Aggressive retinopathy of prematurity |
| VEGF | Vascular endothelial growth factor |
| LPC | Laser photocoagulation |
| IVR | Intravitreal ranibizumab |
| IVB | Intravitreal bevacizumab |
| PMA | Postmenstrual age |
| GA | Gestational age |
| BW | Birth weight |
References
- Terry, T.L. Fibroblastic Overgrowth of Persistent Tunica Vasculosa Lentis in Infants Born Prematurely: II. Report of Cases-Clinical Aspects. Trans. Am. Ophthalmol. Soc. 1942, 40, 262–284. [Google Scholar]
- Heath, P. Pathology of retinopathy of prematurity, RLF. Am. J. Ophthalmol. 1951, 34, 1249–1268. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Kobayashi, Y.; Fukuda, H.; Suekane, K. Photocoagulation for the treatment of the retinopathy of prematurity. Rinsho Ganka (Jpn. J. Clin. Ophthalmol.) 1968, 22, 419–427. [Google Scholar]
- Hardy, R.J.; Good, W.V.; Dobson, V.; Palmer, E.A.; Phelps, D.L.; Quintos, M.; Tung, B. Early Treatment for Retinopathy of Prematurity Cooperative Group. Multicenter trial of early treatment for retinopathy of prematurity: Study design. Control Clin. Trials 2004, 3, 311–325. [Google Scholar]
- Liang, J. Systematic review and meta-analysis of the negative outcomes of retinopathy of prematurity treated with laser photocoagulation. Eur. J. Ophthalmol. 2019, 29, 223–228. [Google Scholar] [CrossRef]
- Fielder, A.R.; Quinn, G.E.; Shah, P.K.; Darlow, B.A.; Marlow, N. Retinopathy of prematurity comes full circle. Arch. Dis. Child Fetal Neonatal Ed. 2024, 110, 8–9. [Google Scholar] [CrossRef]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989, 161, 851–858. [Google Scholar] [CrossRef]
- Mintz-Hittner, H.A.; Kennedy, K.A.; Chuang, A.Z.; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef]
- Stahl, A.; Lepore, D.; Fielder, A.; Fleck, B.; Reynolds, J.D.; Chiang, M.F.; Li, J.; Liew, M.; Maier, R.; Zhu, Q.; et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): An open-label randomised controlled trial. Lancet 2019, 394, 1551–1559. [Google Scholar] [CrossRef]
- Stahl, A.; Sukgen, E.A.; Wu, W.C.; Lepore, D.; Nakanishi, H.; Mazela, J.; Moshfeghi, D.M.; Vitti, R.; Athanikar, A.; Chu, K.; et al. Effect of Intravitreal Aflibercept vs Laser Photocoagulation on Treatment Success of Retinopathy of Prematurity: The FIREFLEYE Randomized Clinical Trial. JAMA 2022, 328, 348–359. [Google Scholar] [CrossRef]
- Alzuabi, A.K.; Alshammari, O.M.; Almousa, A.N.; Abouammoh, M.A. Anti-vascular endothelial growth factor therapy in retinopathy of prematurity: An updated literature review. Saudi J. Ophthalmol. 2022, 36, 260–269. [Google Scholar] [CrossRef]
- Banerjee, M.; Moharana, S.; Padhy, S.K. Systemic Effects of Intravitreal Anti-VEGF Therapy: A Review of Safety across Organ Systems. Ophthalmol. Ther. 2025, 14, 1661–1684. [Google Scholar] [CrossRef]
- Taher, N.O.; Ghaddaf, A.A.; Al-Ghamdi, S.A.; Homsi, J.J.; Al-Harbi, B.J.; Alomari, L.K.; Almarzouki, H.S. Intravitreal Anti-vascular Endothelial Growth Factor Injection for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 884608. [Google Scholar] [CrossRef] [PubMed]
- Toy, B.C.; Schachar, I.H.; Tan, G.S.; Moshfeghi, D.M. Chronic Vascular Arrest as a Predictor of Bevacizumab Treatment Failure in Retinopathy of Prematurity. Ophthalmology 2016, 123, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.F.; Tay, S.A.; Agarwal-Sinha, S.; Tan, G.S.W.; Wu, W.C.; Tsai, A.S.H. Persistent avascular retina in retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 2177–2190. [Google Scholar] [CrossRef] [PubMed]
- Good, W.V. Early Treatment for Retinopathy of Prematurity Cooperative Group. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans. Am. Ophthalmol. Soc. 2004, 102, 233–248, Discussion 248–250. [Google Scholar]
- Nowroozzadeh, M.H.; Sadeghi, E.; Shahriari-Garaee, H.; Badie, M.R.; Banihashemi, J.; Garg, S.J. An Update on Anti-Vascular Endothelial Growth Factor Treatment for Retinopathy of Prematurity. J. Curr. Ophthalmol. 2023, 35, 125–134. [Google Scholar] [CrossRef]
- Dablouk, M.; Chhabra, A.; Masoud, A.T. Recurrence of Retinopathy of Prematurity Following Anti-vascular Endothelial Growth Factor (Anti-VEGF) Therapy: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e73286. [Google Scholar] [CrossRef]
- Mintz-Hittner, H.A.; Geloneck, M.M.; Chuang, A.Z. Clinical Management of Recurrent Retinopathy of Prematurity after Intravitreal Bevacizumab Monotherapy. Ophthalmology 2016, 123, 1845–1855. [Google Scholar] [CrossRef]
- Ling, K.P.; Liao, P.J.; Wang, N.K.; Chao, A.N.; Chen, K.J.; Chen, T.L.; Hwang, Y.S.; Lai, C.C.; Wu, W.C. Rates and risk factors for recurrence of retinopathy of prematurity after laser or intravitreal anti-vascular endothelial growth factor monotherapy. Retina 2020, 40, 1793–1803. [Google Scholar] [CrossRef]
- Jang, J.H. Characteristics of retinal vascularization in reactivated retinopathy of prematurity requiring treatment and clinical outcome after reinjection of ranibizumab. Sci. Rep. 2024, 14, 15647. [Google Scholar] [CrossRef]
- Chan, J.J.T.; Lam, C.P.S.; Kwok, M.K.M.; Wong, R.L.M.; Lee, G.K.Y.; Lau, W.W.Y.; Yam, J.C.S. Risk of recurrence of retinopathy of prematurity after initial intravitreal ranibizumab therapy. Sci. Rep. 2016, 6, 27082. [Google Scholar] [CrossRef]
- Cao, J.K.; Han, T.; Tang, H.Y.; Zhang, S.; Wang, Z.H.; Feng, Z.C.; Li, Q.P. Comparison of post-treatment recurrence between ranibizumab injection and laser photocoagulation for type 1 retinopathy of prematurity. BMC Ophthalmol. 2023, 23, 137. [Google Scholar] [CrossRef]
- Huang, C.; Zou, W.; Ma, W.; Li, J.; Bai, Y.; Wu, R.; Li, Q.; Fang, Q.; Chen, W.; Lu, X.; et al. Effect and factors associated with reactivation after intravitreal conbercept or aflibercept in retinopathy of prematurity. Eur. J. Med. Res. 2025, 28, 55. [Google Scholar] [CrossRef] [PubMed]
- Gangwe, A.B.; Ekumankama, C.B.; Singh, A.; Parchand, S.M.; Agrawal, D.; Azad, R.V. Comparison of reactivation between ranibizumab and bevacizumab in aggressive retinopathy of prematurity: A retrospective case series. Indian J. Ophthalmol. 2025, 73 (Suppl. S1), S119–S125. [Google Scholar] [CrossRef] [PubMed]
- Maitra, P.; Jaju, S.; Agrawal, K.U.; Das, A.; Subramaniam, P.; Venkatapathy, N.; Shah, P.K. Comparing safety and efficacy of Bevacizumab, Ranibizumab and Ranibizumab biosimilar in Retinopathy of prematurity. Eye 2025, 39, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Takano, F.; Ueda, K.; Yamada-Nakanishi, Y.; Nakamura, M. Comparison of Single-Treatment Efficacy of Bevacizumab and Ranibizumab for Retinopathy of Prematurity. Children 2024, 11, 927. [Google Scholar] [CrossRef]
- Sukgen, E.A.; Koçluk, Y. Comparison of clinical outcomes of intravitreal ranibizumab and aflibercept treatment for retinopathy of prematurity. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 49–55. [Google Scholar] [CrossRef]
- Branisteanu, D.C.; Branisteanu, D.E.; Feraru, C.I.; Branisteanu, C.I.; Moraru, A.; Zemba, M.; Balta, F. Influence of unilateral intravitreal bevacizumab injection on the incidence of symptomatic choroidal neovascularization in the fellow eye in patients with neovascular age-related macular degeneration (Review). Exp. Ther. Med. 2020, 20, 182. [Google Scholar] [CrossRef]
- Muether, P.S.; Hermann, M.M.; Dröge, K.; Kirchhof, B.; Fauser, S. Long-term stability of vascular endothelial growth factor suppression time under ranibizumab treatment in age-related macular degeneration. Am. J. Ophthalmol. 2013, 156, 989–993.e2. [Google Scholar] [CrossRef]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Maia, M.; et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Yoon, H.H.; de Regnier, R.O.; Arzu, J.; Rahmani, S. Postnatal Growth Trajectories and Neurodevelopmental Outcomes Following Bevacizumab Treatment for Retinopathy of Prematurity. Clin. Ophthalmol. 2022, 16, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Baiad, A.A.; Kherani, I.Z.; Popovic, M.M.; Katsnelson, G.; Muni, R.H.; Mireskandari, K.; Tehrani, N.N.; Zhou, T.E.; Kertes, P.J. A Meta-Analysis of Neurodevelopmental Outcomes following Intravitreal Bevacizumab for the Treatment of Retinopathy of Prematurity. Neonatology 2023, 120, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1616–1624. [Google Scholar] [CrossRef]
- Hu, J.; Blair, M.P.; Shapiro, M.J.; Lichtenstein, S.J.; Galasso, J.M.; Kapur, R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch. Ophthalmol. 2012, 130, 1000–1006. [Google Scholar] [CrossRef]
- Padhi, T.R.; Bhunia, S.; Das, T.; Nayak, S.; Jalan, M.; Rath, S.; Barik, B.; Ali, H.; Rani, P.K.; Routray, D.; et al. Outcome of real-time telescreening for retinopathy of prematurity using videoconferencing in a community setting in Eastern India. Indian J. Ophthalmol. 2024, 72, 697–703. [Google Scholar] [CrossRef]
- Tawfik, S.; Mansour, A.; Selim, N.L.; Habib, A.M.; Fouad, Y.A.; Tawfik, M.A.; Al-Feky, M. Analysis of a two-year independent screening effort for retinopathy of prematurity in rural Egypt. BMC Ophthalmol. 2021, 21, 445. [Google Scholar] [CrossRef]
- Bhat, V.; Patil, S.H. Role of deferred retinal laser following intravitreal injection of bevacizumab in treatment of severe retinopathy of prematurity. Oman J. Ophthalmol. 2023, 16, 233–236. [Google Scholar] [CrossRef]
- Gangwe, A.B.; Agrawal, D.; Gangrade, A.K.; Parchand, S.M.; Agrawal, D.; Azad, R.V. Outcomes of early versus deferred laser after intravitreal ranibizumab in aggressive posterior retinopathy of prematurity. Indian J. Ophthalmol. 2021, 69, 2171–2176. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, Y.C. Retinal vascular development in an immature retina at 33–34 weeks postmenstrual age predicts retinopathy of prematurity. Sci. Rep. 2020, 10, 18111. [Google Scholar] [CrossRef]
- Quinn, G.E.; Ying, G.S.; Bell, E.F.; Donohue, P.K.; Morrison, D.; Tomlinson, L.A.; Binenbaum, G. G-ROP Study Group. Incidence and Early Course of Retinopathy of Prematurity: Secondary Analysis of the Postnatal Growth and Retinopathy of Prematurity (G-ROP) Study. JAMA Ophthalmol. 2018, 136, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Larsson, E.; Carle-Petrelius, B.; Cernerud, G.; Ots, L.; Wallin, A.; Holmström, G. Incidence of ROP in two consecutive Swedish population based studies. Br. J. Ophthalmol. 2002, 86, 1122–1126. [Google Scholar] [CrossRef]
- Austeng, D.; Källen, K.B.; Ewald, U.W.; Jakobsson, P.G.; Holmström, G.E. Incidence of retinopathy of prematurity in infants born before 27 weeks’ gestation in Sweden. Arch. Ophthalmol. 2009, 127, 1315–1319. [Google Scholar] [CrossRef]
- Tamura, N.; Hanaoka, T.; Ito, K.; Araki, A.; Miyashita, C.; Ito, S.; Minakami, H.; Cho, K.; Endo, T.; Sengoku, K.; et al. Different Risk Factors for Very Low Birth Weight, Term-Small-for-Gestational-Age, or Preterm Birth in Japan. Int. J. Environ. Res. Public Health 2018, 15, 369. [Google Scholar] [CrossRef]
| Total | Group | A vs. B p-Value | ||||
|---|---|---|---|---|---|---|
| A | B | B1 | B2 | |||
| Number of eyes | 142 | 98 | 44 | 28 | 16 | |
| Birth weight (g) | 699.2 ± 175.02 | 720.3 ± 188.39 | 652.3 ± 133.49 | 652.9 ± 146.2 | 651.1 ± 112.3 | 0.0504 |
| Gestational age (weeks) | 25.6 ± 1.69 | 26.0 ± 1.67 | 25.0 ± 1.62 | 24.9 ± 1.23 | 25.3 ± 2.17 | 0.0023 |
| Postmenstrual age (weeks) | 33.5 ± 2.15 | 33.5 ± 2.09 | 33.4 ± 2.11 | 32.8 ± 1.11 | 34.6 ± 5.65 | 0.96 |
| ROP stage | ||||||
| Zone 2, Stage 2+ | 78 | 64 | 14 | 6 | 8 | 0.0001 |
| Zone 2, Stage 3+ | 4 | 3 | 0 | 0 | 0 | |
| Zone 1, Stage 3 | 2 | 1 | 1 | 1 | 0 | |
| Zone 1, Stage 1+ | 15 | 8 | 7 | 7 | 0 | |
| Zone 1, Stage 2+ | 26 | 8 | 19 | 12 | 7 | |
| Zone 1, Stage 3+ | 17 | 14 | 3 | 2 | 1 | |
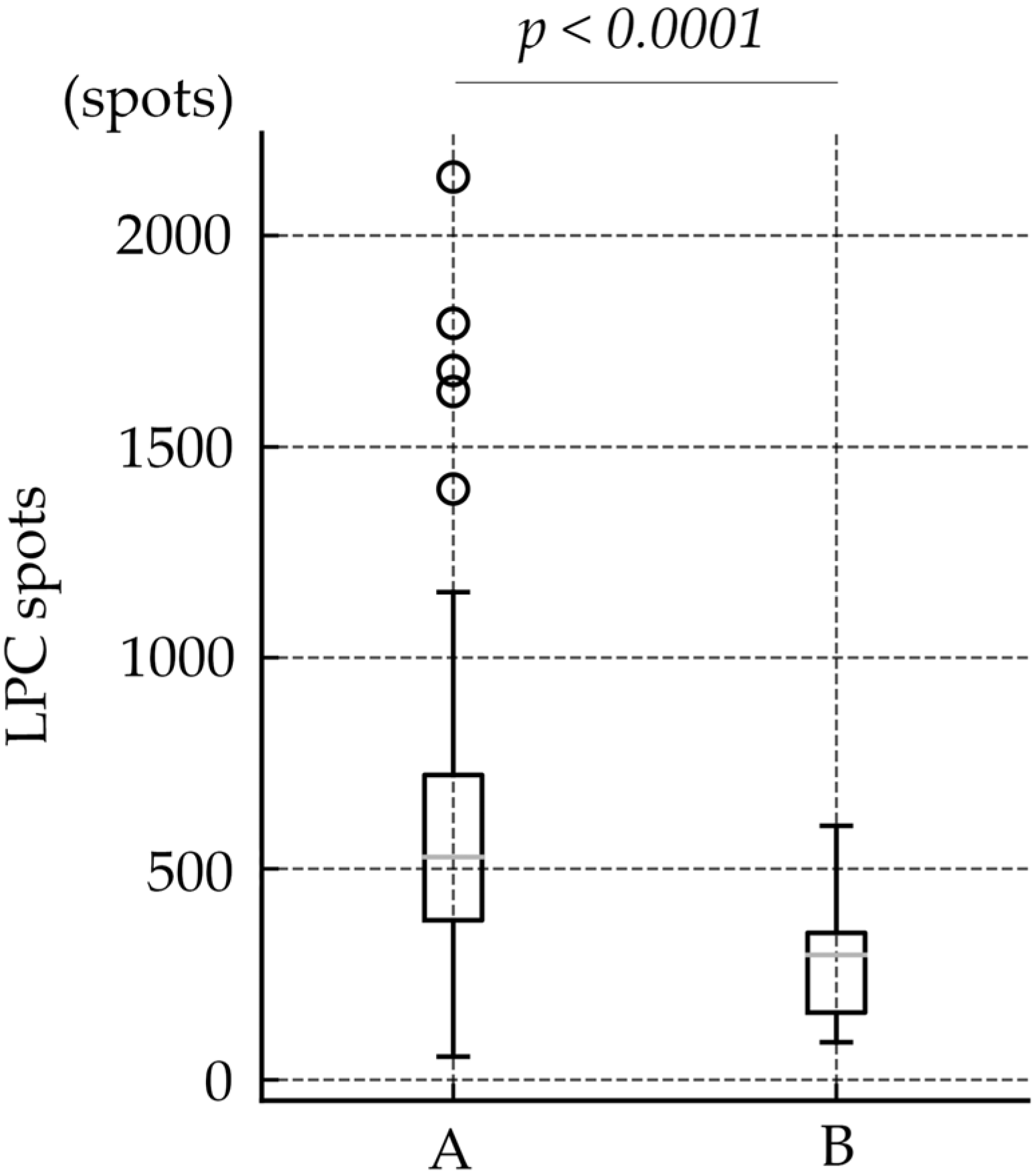
| Number of LPC spots | 487.6 ± 330.20 | 583.0 ± 350.72 | 274.9 ± 124.77 | 300.9 ± 115.5 | 229.5± 130.9 | <0.0001 |
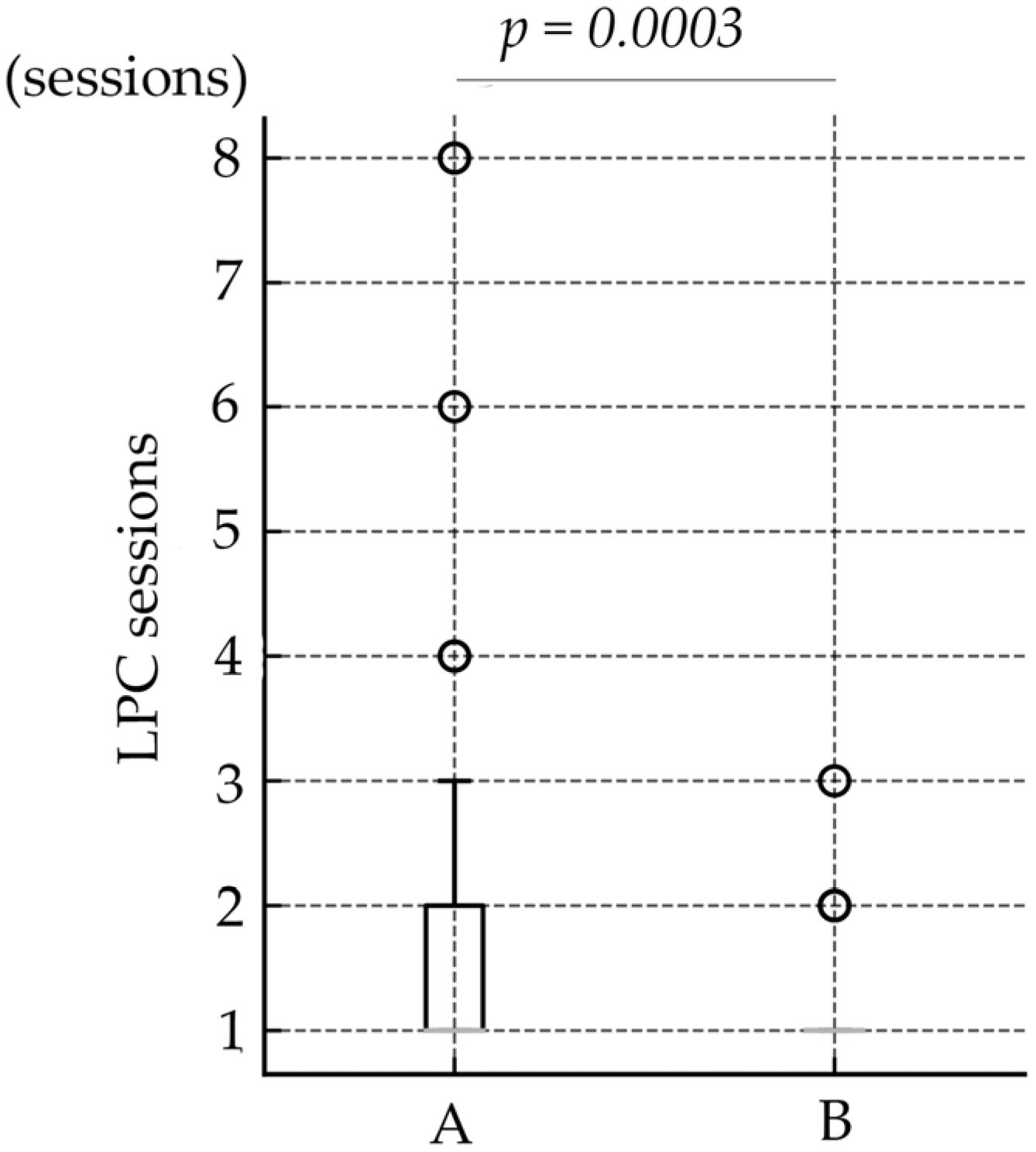
| Number of LPC sessions | 1.6 ± 1.12 | 1.8 ± 1.28 | 1.2 ± 0.45 | 1.1 ± 0.31 | 1.3 ± 0.60 | 0.0003 |
| Vitrectomy, yes/no (eyes) | 3/139 | 3/95 | 0/44 | 0/28 | 0/16 | 0.59 |
| Variable | Estimate | Standard Error | t Value | p Value |
|---|---|---|---|---|
| Intercept | 1928.8795 | 421.9832 | 4.57 | <0.0001 * |
| Birth weight | −0.5483 | 0.1709 | −3.21 | 0.0017 * |
| Gestational age | −4.9197 | 2.8072 | −1.75 | 0.082 |
| Postmenstrual age | −1.0427 | 1.7557 | −0.59 | 0.5536 |
| Zone (1 vs. 2) | 53.4438 | 25.4448 | 2.10 | 0.0376 * |
| Treatment group (A vs. B) | −206.0733 | 25.4162 | −8.11 | <0.0001 * |
| Group A | Group B | p-Value | |
|---|---|---|---|
| Zone 1 | 31 | 30 | <0.0001 |
| Zone 2 | 67 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oba, S.; Kiriishi, T.; Omi, M.; Hattori, Y.; Mori, H.; Ohnaka, M.; Hoshino, T.; Yamada, H.; Imai, H. Short-Term Efficacy of Two-Step Treatment of Retinopathy of Prematurity in a Japanese Cohort: Anti-VEGF Therapy Followed by Routine Laser Photocoagulation. J. Clin. Med. 2025, 14, 7094. https://doi.org/10.3390/jcm14197094
Oba S, Kiriishi T, Omi M, Hattori Y, Mori H, Ohnaka M, Hoshino T, Yamada H, Imai H. Short-Term Efficacy of Two-Step Treatment of Retinopathy of Prematurity in a Japanese Cohort: Anti-VEGF Therapy Followed by Routine Laser Photocoagulation. Journal of Clinical Medicine. 2025; 14(19):7094. https://doi.org/10.3390/jcm14197094
Chicago/Turabian StyleOba, Shimpei, Tatsunori Kiriishi, Masatoshi Omi, Yuki Hattori, Hidetsugu Mori, Masayuki Ohnaka, Takeshi Hoshino, Haruhiko Yamada, and Hisanori Imai. 2025. "Short-Term Efficacy of Two-Step Treatment of Retinopathy of Prematurity in a Japanese Cohort: Anti-VEGF Therapy Followed by Routine Laser Photocoagulation" Journal of Clinical Medicine 14, no. 19: 7094. https://doi.org/10.3390/jcm14197094
APA StyleOba, S., Kiriishi, T., Omi, M., Hattori, Y., Mori, H., Ohnaka, M., Hoshino, T., Yamada, H., & Imai, H. (2025). Short-Term Efficacy of Two-Step Treatment of Retinopathy of Prematurity in a Japanese Cohort: Anti-VEGF Therapy Followed by Routine Laser Photocoagulation. Journal of Clinical Medicine, 14(19), 7094. https://doi.org/10.3390/jcm14197094





