Metabolic and Endocrine Alterations in Underweight and Normal-Weight Women with Functional Hypothalamic Amenorrhea
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Patients
2.3. Measurements
2.4. Biochemical Analysis
2.5. Statistical Analysis
3. Results
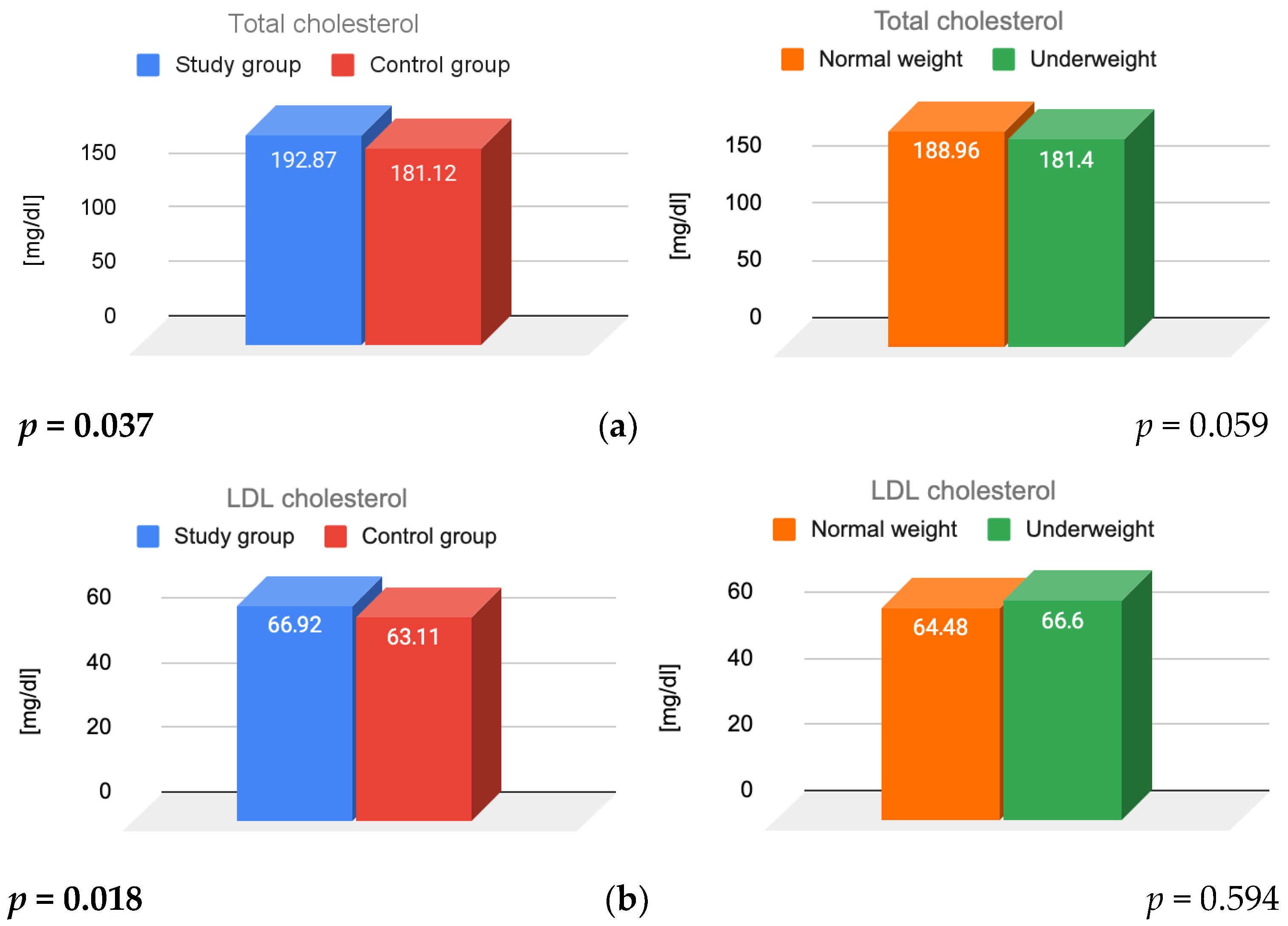
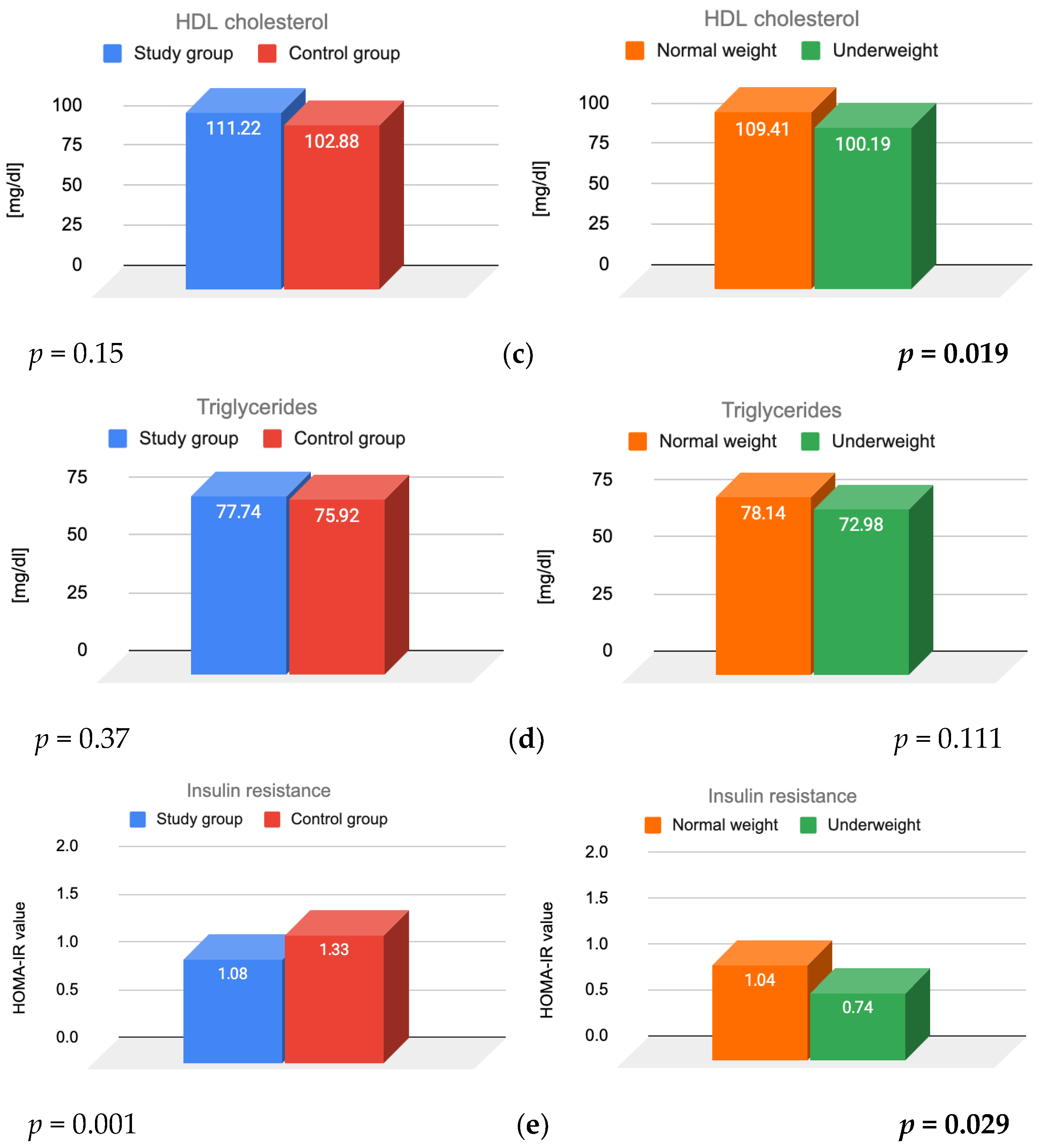
3.1. Lipid Parameters and Insulin Resistance
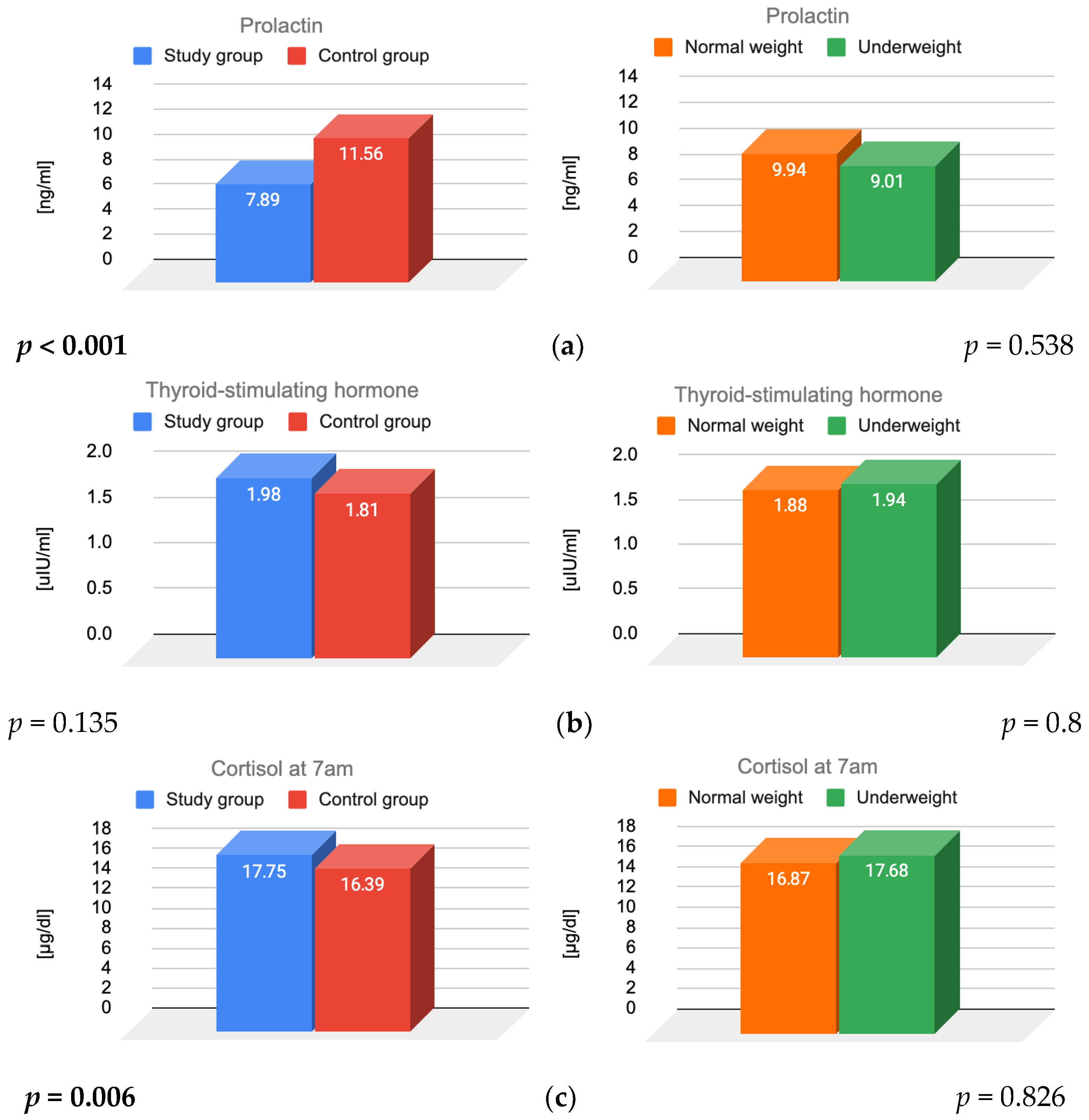
3.2. Prolactin, TSH and Cortisol
4. Discussion
4.1. Lipid Parameters
4.2. Insulin
4.3. Prolactin
4.4. TSH
4.5. Cortisol
4.6. Strengths and Weaknesses of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FHA | Functional Hypothalamic Amenorrhea |
| BMI | Body Mass Index |
| LDL | Low-Density Lipoprotein |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| PRL | Prolactin |
| HPA axis | Hypothalamic–Pituitary–Adrenal Axis |
| TSST | Trier Social Stress Test |
| CMS | Chronic Mild Stress |
| TSH | Thyroid-Stimulating Hormone |
| FT3 | Free Triiodothyronine |
| FT4 | Free Thyroxine |
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| rT3 | Reverse Triiodothyronine |
| TBG | Thyroid Binding Globulin |
| CRH | Corticotropin-Releasing Hormone |
| ACTH | Adrenocorticotropic Hormone |
| HPO | Hypothalamic–Pituitary–Ovarian axis |
| HPT | Hypothalamic–Pituitary–Thyroid axis |
| OC | Oral Contraceptives |
| PCOM | Polycystic Ovarian Morphology |
| PCSK9 | Proprotein Convertase Subtilisin/Kexin Type 9 |
| CMIA | Chemiluminescent Microparticle Immunoassay |
| hsCRP | High-sensitivity C-Reactive Protein |
| IGF | Insulin-like Growth Factor |
| IGFBP-1 | Insulin-like Growth Factor Binding Protein 1 |
| ECLIA | Electrochemiluminescence Immunoassay |
| ERα | Estrogen Receptor alpha |
| FMD | Flow-Mediated Dilation |
References
- Meczekalski, B.; Niwczyk, O.; Bala, G.; Szeliga, A. Stress, Kisspeptin, and Functional Hypothalamic Amenorrhea. Curr. Opin. Pharmacol. 2022, 67, 102288. [Google Scholar] [CrossRef]
- Ryterska, K.; Kordek, A.; Załęska, P. Has Menstruation Disappeared? Functional Hypothalamic Amenorrhea—What Is This Story About? Nutrients 2021, 13, 2827. [Google Scholar] [CrossRef] [PubMed]
- Sophie Gibson, M.E.; Fleming, N.; Zuijdwijk, C.; Dumont, T. Where Have the Periods Gone? The Evaluation and Management of Functional Hypothalamic Amenorrhea. J. Clin. Res. Pediatr. Endocrinol. 2020, 12 (Suppl. S1), 18–27. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Bosoni, D.; Perone, V.; Cucinella, L.; Dealberti, D.; Cannarella, R.; Calogero, A.E.; Nappi, R.E. Gene-Environment Interaction in Functional Hypothalamic Amenorrhea. Front. Endocrinol. 2024, 15, 1423898. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.; Zhao, Y.; Wang, Y.; Chen, H.; Zhang, F.; Li, X. Analysis of the Effects of Eating and Emotions on Reproductive Axis Function in Patients with Functional Hypothalamic Amenorrhea. J. Psychosom. Obstet. Gynaecol. 2024, 45, 2375718. [Google Scholar] [CrossRef]
- Saadedine, M.; Kapoor, E.; Shufelt, C. Functional Hypothalamic Amenorrhea: Recognition and Management of a Challenging Diagnosis. Mayo Clin. Proc. 2023, 98, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Rickenlund, A.; Eriksson, M.J.; Schenck-Gustafsson, K.; Hirschberg, A.L. Amenorrhea in Female Athletes Is Associated with Endothelial Dysfunction and Unfavorable Lipid Profile. J. Clin. Endocrinol. Metab. 2005, 90, 1354–1359. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Saadedine, M.; Cook-Wiens, G.; Pisarska, M.D.; Manson, J.E.; Berga, S.L.; Arditi, M.; Shah, P.K.; Bairey Merz, C.N. Functional Hypothalamic Amenorrhea and Preclinical Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2023, 109, e51–e57. [Google Scholar] [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef]
- Dansinger, M.; Williams, P.T.; Superko, H.R.; Asztalos, B.F.; Schaefer, E.J. Effects of Weight Change on HDL-Cholesterol and Its Subfractions in over 28,000 Men and Women. J. Clin. Lipidol. 2019, 13, 308–316. [Google Scholar] [CrossRef]
- Podfigurna, A.; Meczekalski, B. Functional Hypothalamic Amenorrhea: A Stress-Based Disease. Endocrines 2021, 2, 203–211. [Google Scholar] [CrossRef]
- van Oortmerssen, J.A.E.; Mulder, J.W.C.M.; Kavousi, M.; Roeters van Lennep, J.E. Lipid Metabolism in Women: A Review. Atherosclerosis 2025, 405, 119213. [Google Scholar] [CrossRef]
- Derby, C.A.; Crawford, S.L.; Pasternak, R.C.; Sowers, M.; Sternfeld, B.; Matthews, K.A. Lipid Changes during the Menopause Transition in Relation to Age and Weight: The Study of Women’s Health Across the Nation. Am. J. Epidemiol. 2009, 169, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Gao, X.-P.; Zhang, S.; Dai, Y.-P.; Zou, W.-J.; Yue, L.-M. 17β-Estradiol Inhibits PCSK9-Mediated LDLR Degradation Through GPER/PLC Activation in HepG2 Cells. Front. Endocrinol. 2019, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Lakoski, S.G.; Lagace, T.A.; Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Genetic and Metabolic Determinants of Plasma PCSK9 Levels. J. Clin. Endocrinol. Metab. 2009, 94, 2537–2543. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Gälman, C.; Rudling, M.; Angelin, B. Influence of Physiological Changes in Endogenous Estrogen on Circulating PCSK9 and LDL Cholesterol. J. Lipid Res. 2015, 56, 463–469. [Google Scholar] [CrossRef]
- Rickenlund, A.; Eriksson, M.J.; Schenck-Gustafsson, K.; Hirschberg, A.L. Oral Contraceptives Improve Endothelial Function in Amenorrheic Athletes. J. Clin. Endocrinol. Metab. 2005, 90, 3162–3167. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Johnson, B.D.; Sharaf, B.L.; Bittner, V.; Berga, S.L.; Braunstein, G.D.; Hodgson, T.K.; Matthews, K.A.; Pepine, C.J.; Reis, S.E.; et al. Hypoestrogenemia of Hypothalamic Origin and Coronary Artery Disease in Premenopausal Women: A Report from the NHLBI-Sponsored WISE Study. J. Am. Coll. Cardiol. 2003, 41, 413–419. [Google Scholar] [CrossRef]
- Andrico, S.; Gambera, A.; Specchia, C.; Pellegrini, C.; Falsetti, L.; Sartori, E. Leptin in Functional Hypothalamic Amenorrhoea. Hum. Reprod. 2002, 17, 2043–2048. [Google Scholar] [CrossRef]
- Mayrhofer, D.; Dewailly, D.; Hager, M.; Marculescu, R.; Beitl, K.; Ott, J. Functional Hypothalamic Amenorrhea with or without Polycystic Ovarian Morphology: A Retrospective Cohort Study about Insulin Resistance. Fertil. Steril. 2022, 118, 1183–1185. [Google Scholar] [CrossRef]
- Hong, S.-H.; Sung, Y.-A.; Hong, Y.S.; Jeong, K.; Chung, H.; Lee, H. Polycystic Ovary Morphology Is Associated with Insulin Resistance in Women with Polycystic Ovary Syndrome. Clin. Endocrinol. 2017, 87, 375–380. [Google Scholar] [CrossRef]
- Hager, M.; Dewailly, D.; Marculescu, R.; Ghobrial, S.; Parry, J.P.; Ott, J. Stress and Polycystic Ovarian Morphology in Functional Hypothalamic Amenorrhea: A Retrospective Cohort Study. Reprod. Biol. Endocrinol. 2023, 21, 42. [Google Scholar] [CrossRef]
- Chen, X.; Wan, Y.; Xie, L. Insulin Resistance in PCOS: Pathophysiological Mechanisms of Menstrual Dysfunction and Evidence-Based Treatment Strategies. Biol. Reprod. 2025, ioaf197. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Dominguez, C.E.; Yen, S.S. Nutritional and Endocrine-Metabolic Aberrations in Women with Functional Hypothalamic Amenorrhea. J. Clin. Endocrinol. Metab. 1998, 83, 25–32. [Google Scholar] [CrossRef]
- Berga, S.L.; Mortola, J.F.; Girton, L.; Suh, B.; Laughlin, G.; Pham, P.; Yen, S.S. Neuroendocrine Aberrations in Women with Functional Hypothalamic Amenorrhea. J. Clin. Endocrinol. Metab. 1989, 68, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Divall, S.A.; Williams, T.R.; Carver, S.E.; Koch, L.; Brüning, J.C.; Kahn, C.R.; Wondisford, F.; Radovick, S.; Wolfe, A. Divergent Roles of Growth Factors in the GnRH Regulation of Puberty in Mice. J. Clin. Investig. 2010, 120, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Męczekalski, B.; Niwczyk, O.; Battipaglia, C.; Troia, L.; Kostrzak, A.; Bala, G.; Maciejewska-Jeske, M.; Genazzani, A.D.; Luisi, S. Neuroendocrine Disturbances in Women with Functional Hypothalamic Amenorrhea: An Update and Future Directions. Endocrine 2024, 84, 769–785. [Google Scholar] [CrossRef]
- Johnson, J.D.; Bernal-Mizrachi, E.; Alejandro, E.U.; Han, Z.; Kalynyak, T.B.; Li, H.; Beith, J.L.; Gross, J.; Warnock, G.L.; Townsend, R.R.; et al. Insulin Protects Islets from Apoptosis via Pdx1 and Specific Changes in the Human Islet Proteome. Proc. Natl. Acad. Sci. USA 2006, 103, 19575–19580. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Liu, X.; Li, M.; Wu, B.; Li, Y.; Liang, Y.; Shao, X.; Holthöfer, H.; Zou, H. Insulin Resistance and Metabolic Syndrome in Normal-Weight Individuals. Endocrine 2014, 46, 496–504. [Google Scholar] [CrossRef]
- Tanti, J.-F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of Inflammatory Signaling Pathways in Obesity-Induced Insulin Resistance. Front. Endocrinol. 2012, 3, 181. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Blüher, M. Adipocyte Dysfunction, Inflammation and Metabolic Syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Sato, M.; Tamura, Y.; Kaga, H.; Yamasaki, N.; Kadowaki, S.; Sugimoto, D.; Nakagata, T.; Someya, Y.; Nishida, Y.; Kawamori, R.; et al. Adipose Tissue Insulin Resistance in Young Japanese Women Is Associated with Metabolic Abnormalities and Dehydroepiandrosterone-Sulfate. Front. Endocrinol. 2024, 15, 1390778. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of Leptin and Adiponectin in Insulin Resistance. Clin. Chim. Acta 2013, 417, 80–84. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Torbati, T.; Dutra, E. Hypothalamic Amenorrhea and the Long-Term Health Consequences. Semin. Reprod. Med. 2017, 35, 256–262. [Google Scholar] [CrossRef]
- Schneider, L.F.; Warren, M.P. Functional Hypothalamic Amenorrhea Is Associated with Elevated Ghrelin and Disordered Eating. Fertil. Steril. 2006, 86, 1744–1749. [Google Scholar] [CrossRef]
- Warren, M.P.; Voussoughian, F.; Geer, E.B.; Hyle, E.P.; Adberg, C.L.; Ramos, R.H. Functional Hypothalamic Amenorrhea: Hypoleptinemia and Disordered Eating. J. Clin. Endocrinol. Metab. 1999, 84, 873–877. [Google Scholar] [CrossRef]
- Podfigurna-Stopa, A.; Pludowski, P.; Jaworski, M.; Lorenc, R.; Genazzani, A.R.; Meczekalski, B. Skeletal Status and Body Composition in Young Women with Functional Hypothalamic Amenorrhea. Gynecol. Endocrinol. 2012, 28, 299–304. [Google Scholar] [CrossRef]
- Christian, L.M.; Glaser, R.; Porter, K.; Malarkey, W.B.; Beversdorf, D.; Kiecolt-Glaser, J.K. Poorer Self-Rated Health Is Associated with Elevated Inflammatory Markers among Older Adults. Psychoneuroendocrinology 2011, 36, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- Meites, J. Neuroendocrine Control of Prolactin in Experimental Animals. Clin. Endocrinol. 1977, 6 (Suppl. S1), 9S–18S. [Google Scholar] [CrossRef] [PubMed]
- Pirchio, R.; Graziadio, C.; Colao, A.; Pivonello, R.; Auriemma, R.S. Metabolic Effects of Prolactin. Front. Endocrinol. 2022, 13, 1015520. [Google Scholar] [CrossRef]
- Selzer, C.; Ott, J.; Dewailly, D.; Marculescu, R.; Steininger, J.; Hager, M. Prolactin levels in functional hypothalamic amenorrhea: A retrospective case-control study. Arch. Gynecol. Obstet. 2024, 309, 651–658. [Google Scholar] [CrossRef]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, V.; Mancini, F.; Loucks, T.L.; Berga, S.L. Neuroendocrine Recovery Initiated by Cognitive Behavioral Therapy in Women with Functional Hypothalamic Amenorrhea: A Randomized, Controlled Trial. Fertil. Steril. 2013, 99, 2084–2091.e1. [Google Scholar] [CrossRef]
- Gordon, C.M. Clinical Practice. Functional Hypothalamic Amenorrhea. N. Engl. J. Med. 2010, 363, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, C.E.; Laughlin, G.A.; Nelson, J.C.; Yen, S.S. Altered Binding of Serum Thyroid Hormone to Thyroxine-Binding Globulin in Women with Functional Hypothalamic Amenorrhea. Fertil. Steril. 1997, 68, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Sowińska-Przepiera, E.; Andrysiak-Mamos, E.; Jarząbek-Bielecka, G.; Walkowiak, A.; Osowicz-Korolonek, L.; Syrenicz, M.; Kędzia, W.; Syrenicz, A. Functional Hypothalamic Amenorrhoea—Diagnostic Challenges, Monitoring, and Treatment. Endokrynol. Pol. 2015, 66, 252–268. [Google Scholar] [CrossRef]
- Kyriakidis, M.; Caetano, L.; Anastasiadou, N.; Karasu, T.; Lashen, H. Functional Hypothalamic Amenorrhoea: Leptin Treatment, Dietary Intervention and Counselling as Alternatives to Traditional Practice—Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 198, 131–137. [Google Scholar] [CrossRef]
- Meczekalski, B.; Katulski, K.; Czyzyk, A.; Podfigurna-Stopa, A.; Maciejewska-Jeske, M. Functional Hypothalamic Amenorrhea and Its Influence on Women’s Health. J. Endocrinol. Investig. 2014, 37, 1049–1056. [Google Scholar] [CrossRef]
- Morrison, A.E.; Fleming, S.; Levy, M.J. A Review of the Pathophysiology of Functional Hypothalamic Amenorrhoea in Women Subject to Psychological Stress, Disordered Eating, Excessive Exercise or a Combination of These Factors. Clin. Endocrinol. 2021, 95, 229–238. [Google Scholar] [CrossRef]
- Ackerman, K.E.; Patel, K.T.; Guereca, G.; Pierce, L.; Herzog, D.B.; Misra, M. Cortisol Secretory Parameters in Young Exercisers in Relation to LH Secretion and Bone Parameters. Clin. Endocrinol. 2013, 78, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.Y.; Liu, J.H.; Berga, S.L.; Quigley, M.E.; Laughlin, G.A.; Yen, S.S. Hypercortisolism in Patients with Functional Hypothalamic-Amenorrhea. J. Clin. Endocrinol. Metab. 1988, 66, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Kraeuter, A.-K.; McDermott, B.; Lupien, S.; Sarnyai, Z. Human Nail Cortisol as a Retrospective Biomarker of Chronic Stress: A Systematic Review. Psychoneuroendocrinology 2021, 123, 104903. [Google Scholar] [CrossRef]
- Dobranowska, K.; Plińska, S.; Dobosz, A. Dietary and Lifestyle Management of Functional Hypothalamic Amenorrhea: A Comprehensive Review. Nutrients 2024, 16, 2967. [Google Scholar] [CrossRef] [PubMed]
| Variable (Mean Value) | Study Group N = 166 | Control Group N = 163 | p Value |
|---|---|---|---|
| Age [years] | 29.83 ± 6.35 | 25.65 ± 5.55 | <0.001 |
| BMI [kg/m2] | 19.74 ± 2.29 | 20.62 ± 1.9 | <0.001 |
| Variable (Mean Value) | Study Group | Control Group | ||
|---|---|---|---|---|
| Normal Weight | Underweight | Normal Weight | Underweight | |
| Age [years] | 26.12 ± 5.7 | 24.10 ± 5.08 | 29.42 ± 5.56 | 26.88 ± 5.11 |
| BMI [kg/m2] | 21.02 ± 1.64 | 17.24 ± 0.9 | 21.17 ± 1.53 | 17.72 ± 0.65 |
| Parameter | Study Group N = 166 | Control Group N = 163 | p Value |
|---|---|---|---|
| Total cholesterol [mg/dL] (<200) | 193 ± 41.96 | 181 ± 28.23 | 0.037 |
| HDL cholesterol [mg/dL] (>40) | 111 ± 13.99 | 103 ± 12.44 | 0.15 |
| LDL cholesterol [mg/dL] (<130) | 67 ± 34.89 | 63 ± 24.78 | 0.018 |
| Triglycerides [mg/dL] (<150) | 78 ± 33.61 | 76 ± 40.78 | 0.37 |
| Fasting glucose [mg/dL] (74–106) | 83.13 ± 8.59 | 87.78 ± 7.15 | <0.001 |
| Fasting insulin [µIU/mL] (<24) | 5.3 ± 3.1 | 6.11 ± 2.79 | 0.014 |
| HOMA-IR [units] (<2.5) | 1.08 ± 0.68 | 1.33 ± 0.64 | 0.001 |
| Prolactin [ng/mL] (4.79–23.3) | 8 ± 11.1 | 12 ± 7.62 | <0.001 |
| TSH [µIU/mL] (0.27–4.2) | 2 ± 2.11 | 2 ± 0.86 | 0.135 |
| Morning cortisol [µg/dL] (4.82–19.5) | 18 ± 177.81 | 16 ± 5.21 | 0.006 |
| Parameter | Study Group N = 166 | Control Group N = 163 | ||
|---|---|---|---|---|
| Normal Weight N = 109 | Underweight N = 57 | Normal Weight N = 137 | Underweight N = 26 | |
| Total cholesterol [mg/dL] | 198.3 ± 44.34 | 182.49 ± 35.82 | 181.53 ± 27.94 | 179 ± 34.29 |
| HDL cholesterol [mg/dL] | 117.44 ± 35.95 | 99.32 ± 30.27 | 103.02 ± 24.82 | 102.12 ± 24.98 |
| LDL cholesterol [mg/dL] | 66.2 ± 14.24 | 68.28 ± 13.52 | 63.11 ± 12.2 | 62.92 ± 12.88 |
| Triglycerides [mg/dL] | 79.5 ± 33.5 | 74.37 ± 23.44 | 77.06 ± 27.82 | 69.92 ± 16.69 |
| Fasting glucose [mg/dL] | 84.25 ± 7.69 | 87.70 ± 7.49 | 80.91 ± 9.82 | 88.19 ± 5.16 |
| Fasting insulin [µIU/mL] | 5.68 ± 3.12 | 6.17 ± 2.78 | 4.57 ± 2.94 | 5.78 ± 2.86 |
| HOMA-IR [units] | 1.04 ± 0.7 | 0.74 ± 0.6 | 1.23 ± 0.65 | 1.14 ± 0.58 |
| Prolactin [ng/mL] | 7.57 ± 3.75 | 8.48 ± 4.68 | 11.83 ± 4.98 | 10.17 ± 4.69 |
| TSH [µIU/mL] | 1.97 ± 1 | 2.01 ± 0.86 | 1.82 ± 0.81 | 1.8 ± 0.84 |
| Morning cortisol [µg/dL] | 17.23 ± 5.35 | 18.73 ± 6.16 | 16.57 ± 5.07 | 15.4 ± 5.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, K.; Szymańska, I.; Zawistowska, O.; Bieńkowska, J.; Drosdzol-Cop, A.; Madej, P. Metabolic and Endocrine Alterations in Underweight and Normal-Weight Women with Functional Hypothalamic Amenorrhea. J. Clin. Med. 2025, 14, 7082. https://doi.org/10.3390/jcm14197082
Kowalczyk K, Szymańska I, Zawistowska O, Bieńkowska J, Drosdzol-Cop A, Madej P. Metabolic and Endocrine Alterations in Underweight and Normal-Weight Women with Functional Hypothalamic Amenorrhea. Journal of Clinical Medicine. 2025; 14(19):7082. https://doi.org/10.3390/jcm14197082
Chicago/Turabian StyleKowalczyk, Karolina, Iga Szymańska, Olga Zawistowska, Julia Bieńkowska, Agnieszka Drosdzol-Cop, and Paweł Madej. 2025. "Metabolic and Endocrine Alterations in Underweight and Normal-Weight Women with Functional Hypothalamic Amenorrhea" Journal of Clinical Medicine 14, no. 19: 7082. https://doi.org/10.3390/jcm14197082
APA StyleKowalczyk, K., Szymańska, I., Zawistowska, O., Bieńkowska, J., Drosdzol-Cop, A., & Madej, P. (2025). Metabolic and Endocrine Alterations in Underweight and Normal-Weight Women with Functional Hypothalamic Amenorrhea. Journal of Clinical Medicine, 14(19), 7082. https://doi.org/10.3390/jcm14197082






