Dense Calcification of the Common Femoral Artery Is Protective Against In-Stent Restenosis
Abstract
1. What This Paper Adds
2. Introduction
3. Methods
3.1. Study Design
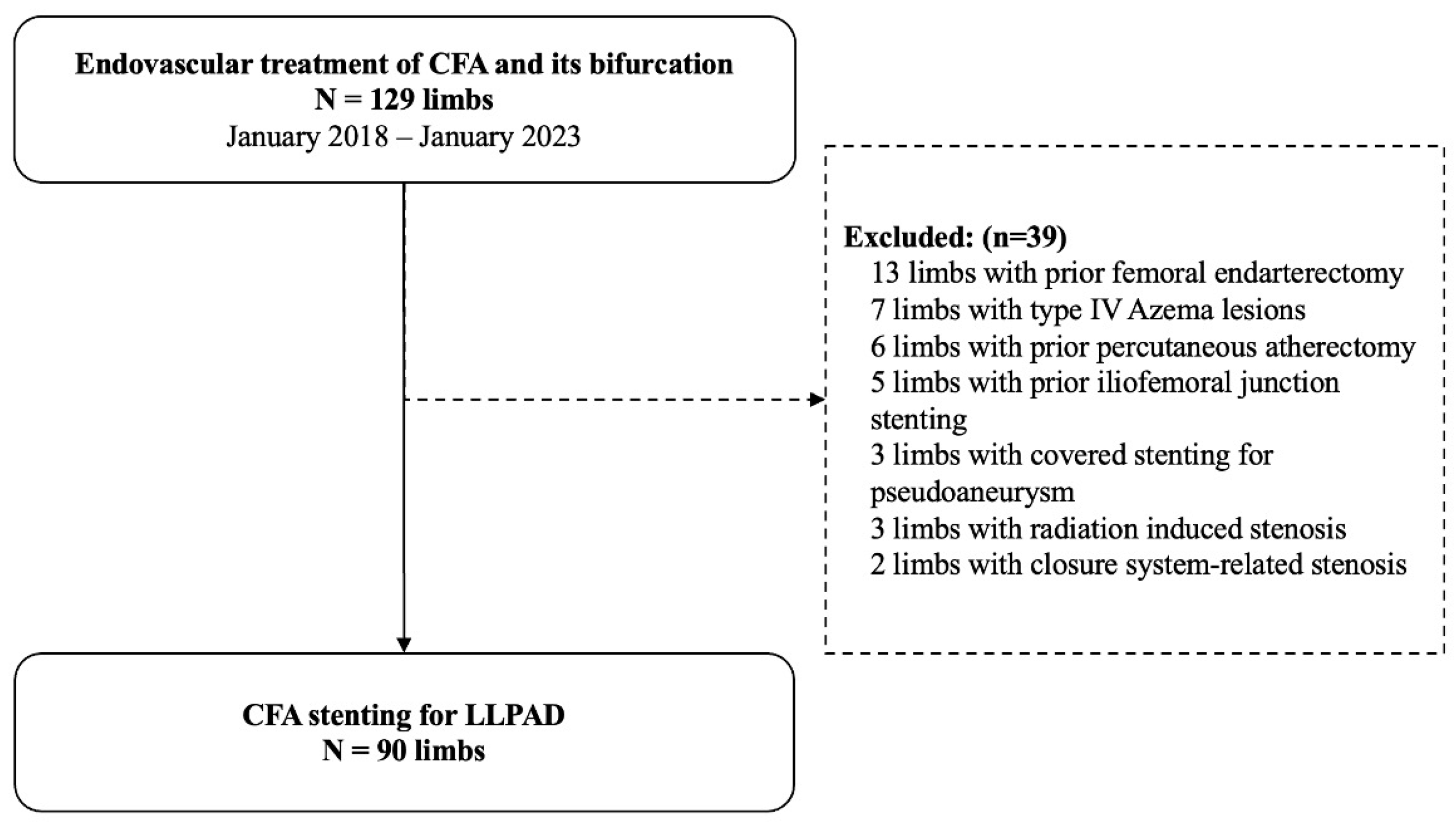
3.2. Patients
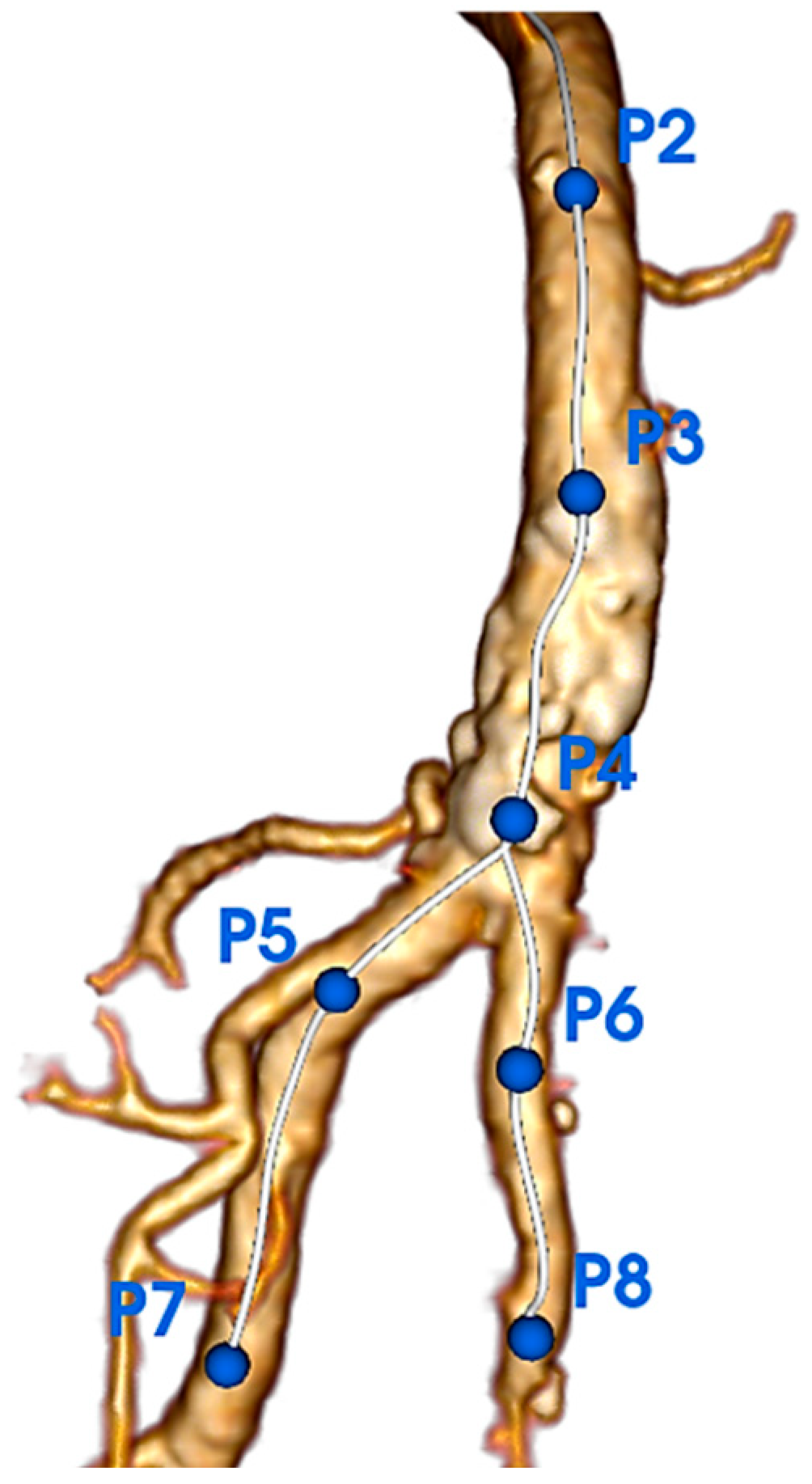
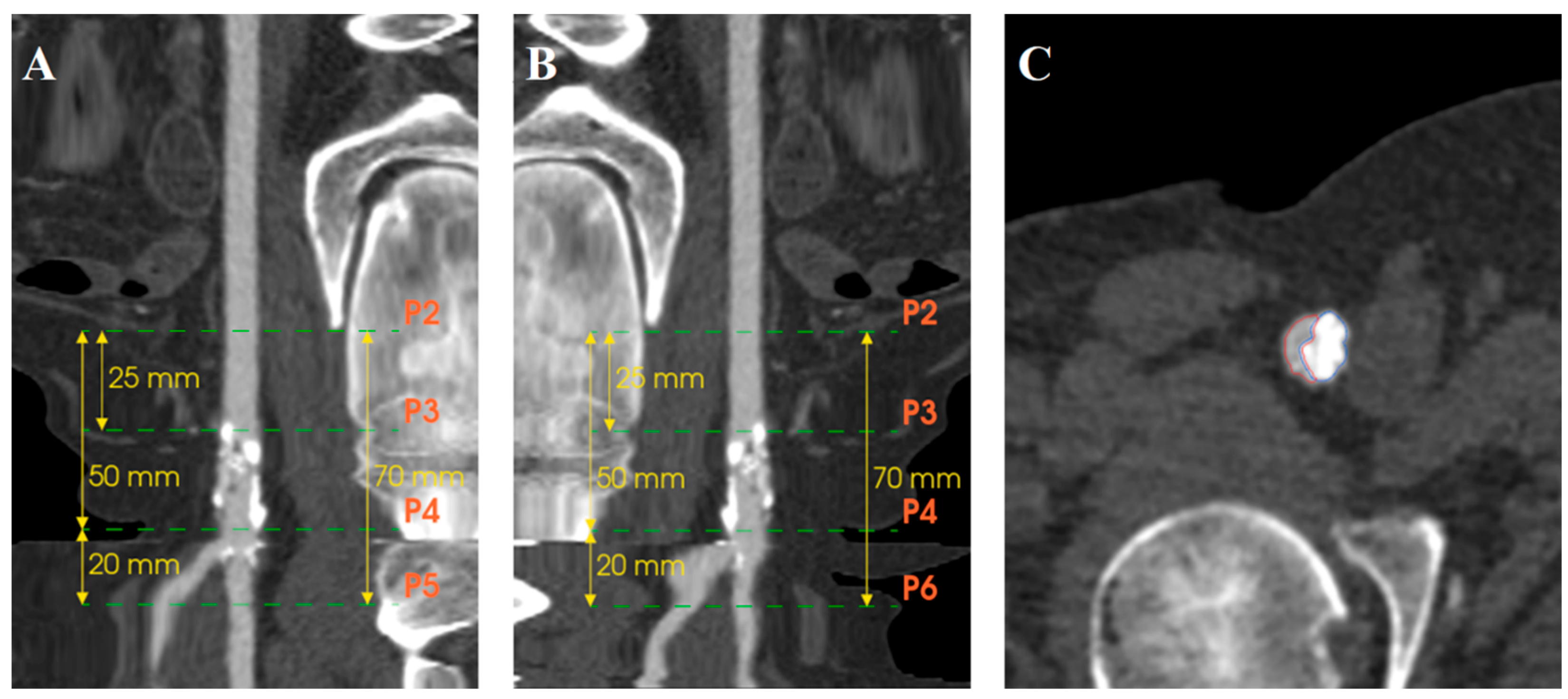
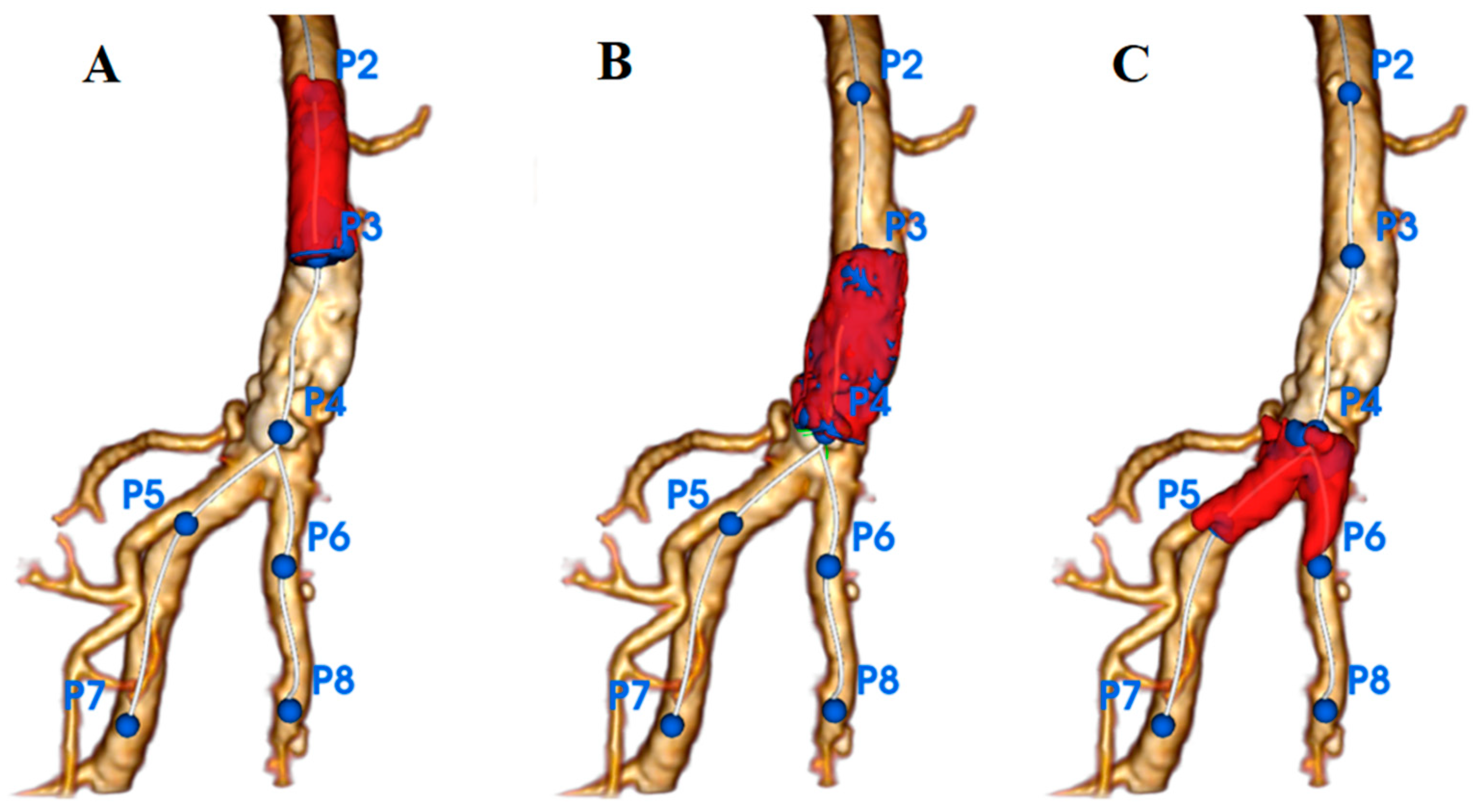
3.3. Quantification of Calcifications
3.4. Follow-Up Protocol
3.5. Endpoints and Definition
3.6. Statistical Analysis
4. Results
4.1. Patient Characteristics
4.2. Primary Outcomes
4.3. Secondary Outcomes
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halpin, D.; Erben, Y.; Jayasuriya, S.; Cua, B.; Jhamnani, S.; Mena-Hurtado, C. Management of Isolated Atherosclerotic Stenosis of the Common Femoral Artery: A Review of the Literature. Vasc. Endovasc. Surg. 2017, 51, 220–227. [Google Scholar] [CrossRef]
- Rassam, S.; Coscas, R. Percutaneous Endovascular Reconstruction of the Common Femoral Artery and Its Bifurcation. J. Clin. Med. 2024, 13, 3169. [Google Scholar] [CrossRef] [PubMed]
- Shammas, N.W.; Doumet, A.A.; Karia, R.; Khalafallah, R. An Overview of the Treatment of Symptomatic Common Femoral Artery Lesions with a Focus on Endovascular Therapy. Vasc. Heal. Risk Manag. 2020, 16, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Siracuse, J.J.; Van Orden, K.; Kalish, J.A.; Eslami, M.H.; Schermerhorn, M.L.; Patel, V.I.; Rybin, D.; Farber, A. Endovascular treatment of the common femoral artery in the Vascular Quality Initiative. J. Vasc. Surg. 2017, 65, 1039–1046. [Google Scholar] [CrossRef]
- Gouëffic, Y.; Della Schiava, N.; Thaveau, F.; Rosset, E.; Favre, J.-P.; du Mont, L.S.; Alsac, J.-M.; Hassen-Khodja, R.; Reix, T.; Allaire, E.; et al. Stenting or Surgery for De Novo Common Femoral Artery Stenosis. JACC Cardiovasc. Interv. 2017, 10, 1344–1354. [Google Scholar] [CrossRef]
- Nordanstig, J.; Behrendt, C.-A.; Baumgartner, I.; Belch, J.; Bäck, M.; Fitridge, R.; Hinchliffe, R.; Lejay, A.; Mills, J.L.; Rother, U.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Asymptomatic Lower Limb Peripheral Arterial Disease and Intermittent Claudication. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 9–96. [Google Scholar] [CrossRef]
- Azéma, L.; Davaine, J.; Guyomarch, B.; Chaillou, P.; Costargent, A.; Patra, P.; Gouëffic, Y. Endovascular Repair of Common Femoral Artery and Concomitant Arterial Lesions. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 787–793. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yamamoto, S.; Kimura, M.; Sano, M.; Sato, O.; Deguchi, J. Long-Term Outcomes following Common Femoral Endarterectomy. J. Clin. Med. 2022, 11, 6873. [Google Scholar] [CrossRef]
- Van den Berg, J.C. Laser debulking and DEB for in-stent restenosis: Technique and review of the literature. J. Cardiovasc. Surg. (Torino) 2014, 55, 351–357. [Google Scholar] [PubMed]
- Böhme, T.; Romano, L.; Macharzina, R.-R.; Noory, E.; Beschorner, U.; Jacques, B.; Bürgelin, K.; Flügel, P.-C.; Zeller, T.; Rastan, A. Outcomes of directional atherectomy for common femoral artery disease. EuroIntervention 2021, 17, 260–266. [Google Scholar] [CrossRef]
- Tepe, G.; Brodmann, M.; Bachinsky, W.; Holden, A.; Zeller, T.; Mangalmurti, S.; Nolte-Ernsting, C.; Virmani, R.; Parikh, S.A.; Gray, W.A. Intravascular Lithotripsy for Peripheral Artery Calcification: 30-Day Outcomes From the Randomized Disrupt PAD III Trial. JACC Cardiovasc. Interv. 2021, 14, 1352–1361. [Google Scholar] [CrossRef]
- Chugh, Y.; Brilakis, E.S. Intravascular lithotripsy for stent under-expansion: Panacea or Pandora’s box? Catheter. Cardiovasc. Interv. Off. J. Soc. Card Angiogr. Interv. 2021, 97, 30–31. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, U.J.; Kim, H.T.; Roh, Y.-N. The Effect of Severe Femoropopliteal Arterial Calcification on the Treatment Outcome of Femoropopliteal Intervention in Patients with Ischemic Tissue Loss. Vasc. Spec. Int. 2020, 36, 96–104. [Google Scholar] [CrossRef]
- Kaladji, A.; Vent, P.-A.; Danvin, A.; Chaillou, P.; Costargent, A.; Guyomarch, B.; Quillard, T.; Gouëffic, Y. Impact of Vascular Calcifications on Long Femoropopliteal Stenting Outcomes. Ann. Vasc. Surg. 2018, 47, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. Vascular Calcification: Key Roles of Phosphate and Pyrophosphate. Int. J. Mol. Sci. 2021, 22, 13536. [Google Scholar] [CrossRef] [PubMed]
- Davaine, J.-M.; Quillard, T.; Brion, R.; Lapérine, O.; Guyomarch, B.; Merlini, T.; Chatelais, M.; Guilbaud, F.; Brennan, M.Á.; Charrier, C.; et al. Osteoprotegerin, pericytes and bone-like vascular calcification are associated with carotid plaque stability. PLoS ONE 2014, 9, e107642. [Google Scholar] [CrossRef] [PubMed]
- Davaine, J.-M.; Quillard, T.; Chatelais, M.; Guilbaud, F.; Brion, R.; Guyomarch, B.; Brennan, M.; Heymann, D.; Heymann, M.-F.; Gouëffic, Y. Bone Like Arterial Calcification in Femoral Atherosclerotic Lesions: Prevalence and Role of Osteoprotegerin and Pericytes. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, R.B.; Baker, J.; Ernst, C.; Johnston, K.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended standards for reports dealing with lower extremity ischemia: Revised version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef]
- A Gray, W.; Keirse, K.; Soga, Y.; Benko, A.; Babaev, A.; Yokoi, Y.; Schroeder, H.; Prem, J.T.; Holden, A.; Popma, J.; et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): A randomised, non-inferiority trial. Lancet 2018, 392, 1541–1551. [Google Scholar] [CrossRef]
- Jaminon, A.; Reesink, K.; Kroon, A.; Schurgers, L. The Role of Vascular Smooth Muscle Cells in Arterial Remodeling: Focus on Calcification-Related Processes. Int. J. Mol. Sci. 2019, 20, 5694. [Google Scholar] [CrossRef]
- Tijani, Y.; Burgaud, M.; Hamel, A.; Raux, M.; Nasr, B.; Gouëffic, Y. The Common Femoral Artery Is a Fixed Arterial Segment. Ann. Vasc. Surg. 2021, 73, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular Calcification: Mechanisms of Vascular Smooth Muscle Cell Calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and growth of extracellular vesi-cle-derived microcalcification in atherosclerotic plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef]
- New, S.E.P.; Aikawa, E. Molecular Imaging Insights Into Early Inflammatory Stages of Arterial and Aortic Valve Calcification. Circ. Res. 2011, 108, 1381–1391. [Google Scholar] [CrossRef]
- Ho, K.J.; Owens, C.D. Diagnosis, classification, and treatment of femoropopliteal artery in-stent restenosis. J. Vasc. Surg. 2017, 65, 545–557. [Google Scholar] [CrossRef]
- Kereiakes, D.J.; Virmani, R.; Hokama, J.Y.; Illindala, U.; Mena-Hurtado, C.; Holden, A.; Hill, J.M.; Lyden, S.P.; Ali, Z.A. Principles of Intravascular Lithotripsy for Calcific Plaque Modification. JACC Cardiovasc. Interv. 2021, 14, 1275–1292. [Google Scholar] [CrossRef]
- Riley, R.F.; Patel, M.P.; Abbott, J.D.; Bangalore, S.; Brilakis, E.S.; Croce, K.J.; Doshi, D.; Kaul, P.; Kearney, K.E.; Kerrigan, J.L.; et al. SCAI Expert Consensus Statement on the Management of Calcified Coronary Lesions. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101259. [Google Scholar] [CrossRef]
| Characteristics | N = 90 (%) |
|---|---|
| Age | 72.0 [66.0; 78.0] |
| Male sex | 73 (81) |
| Hypertension | 83 (92) |
| Dyslipidemia | 78 (87) |
| Diabetes mellitus | 43 (48) |
| Smokers | |
| Current | 19 (21) |
| Former > 6 months | 64 (71) |
| Never | 4 (4) |
| Coronary artery disease | 39 (43) |
| BMI (kg/m2) | 27.3 [24.7; 29.5] |
| eGFR (mL/min/1.73 m2) | 80.5 [61.2; 92.0] |
| Statin | 80 (89) |
| Aspirin | 62 (69) |
| Clopidogrel | 19 (21) |
| Ankle brachial index | 0.640 [0.490; 0.800] |
| Rutherford | |
| 1 | 3 (3) |
| 2 | 25 (28) |
| 3 | 43 (48) |
| 4 | 7 (8) |
| 5 | 10 (11) |
| 6 | 2 (2) |
| Azema Classification | |
| 1 | 1 (1) |
| 2 | 39 (43) |
| 3 | 50 (56) |
| Calcifications | N = 90 |
|---|---|
| Zone 1 | |
| Volume (mm3) | 186 [75.0; 409] |
| Minimum density (HU) | 584 [442; 711] |
| Median density (HU) | 990 [808; 1148] |
| Maximum density (HU) | 1745 [1390; 2008] |
| Calcification volume/arterial lumen volume (%) | 19.1 [8.14; 28.1] |
| Zone 2 | |
| Volume (mm3) | 263 [128; 474] |
| Minimum density (HU) | 640 [529; 773] |
| Median density (HU) | 1025 [884; 1154] |
| Maximum density (HU) | 1896 [1600; 2100] |
| Calcification volume/arterial lumen volume (%) | 20.4 [13.2; 27.6] |
| Zone 3 | |
| Volume (mm3) | 76.0 [22.0; 144] |
| Minimum density (HU) | 637 [510; 742] |
| Median density (HU) | 961 [752; 1094] |
| Maximum density (HU) | 1574 [1210; 1920] |
| Calcification volume/arterial lumen volume (%) | 8.86 [3.82; 13.8] |
| Patency at M12 | PSVR > 2.4 (N = 19) | PSVR < 2.4 (N = 65) | p-Value | Q-Value |
|---|---|---|---|---|
| Zone 1 | ||||
| Volume (mm3) | 121 (75, 238) | 234 (72, 428) | 0.075 | 0.20 |
| Minimum density (HU) | 561 (431, 712) | 588 (452, 708) | 0.92 | 0.92 |
| Median density (HU) | 888 (748, 1124) | 998 (836, 1142) | 0.67 | 0.72 |
| Maximum density (HU) | 1650 (1261, 2092) | 1812 (1469, 1982) | 0.57 | 0.66 |
| Calcification volume/arterial lumen volume (%) | 12.1 [6.25; 17.1] | 19.1 [8.14; 28.1] | 0.096 | 0.91 |
| Zone 2 | ||||
| Volume (mm3) | 190 (86, 335) | 282 (142, 494) | 0.12 | 0.24 |
| Minimum density (HU) | 652 (522, 833) | 627 (537, 723) | 0.46 | 0.60 |
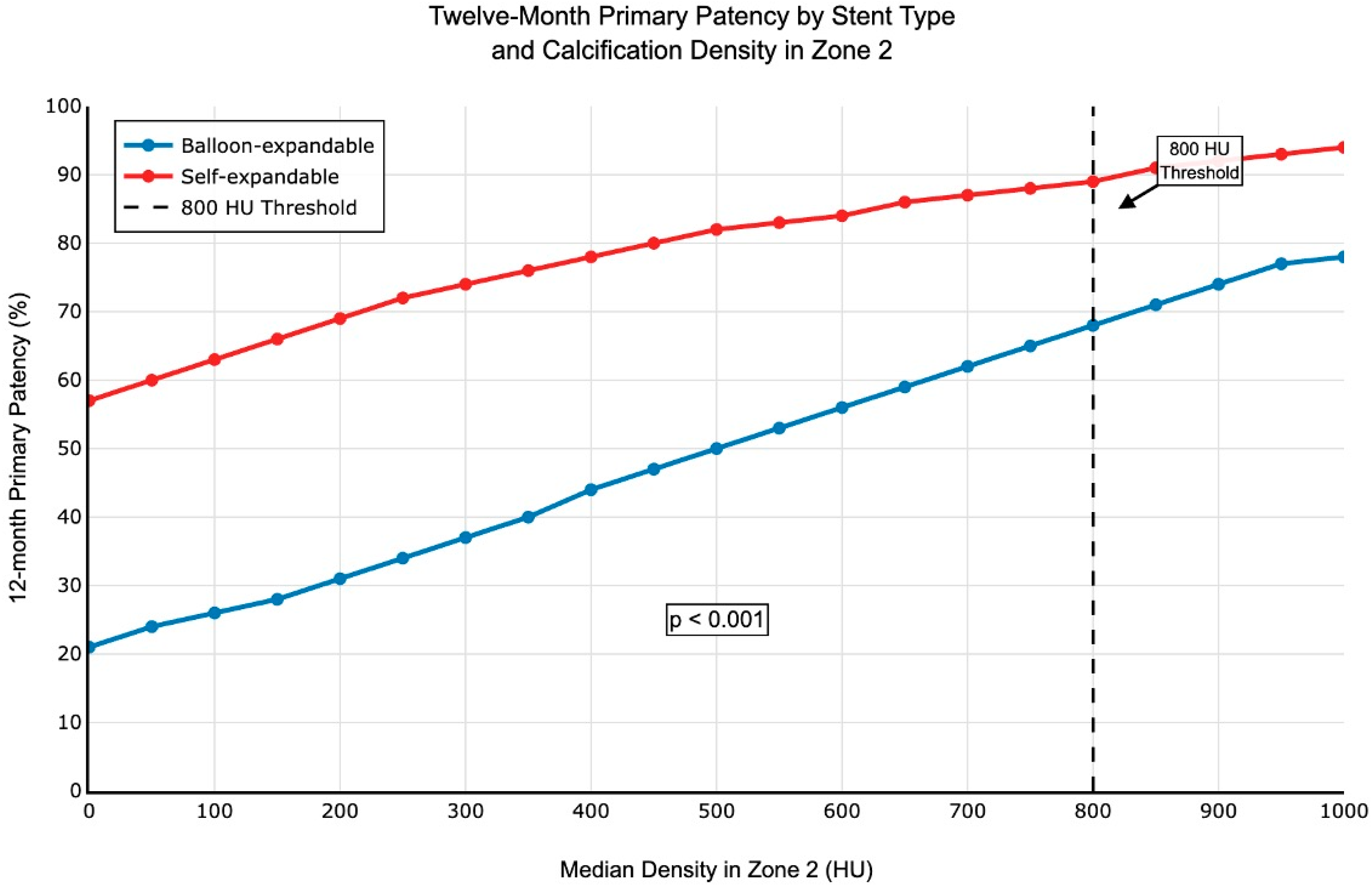
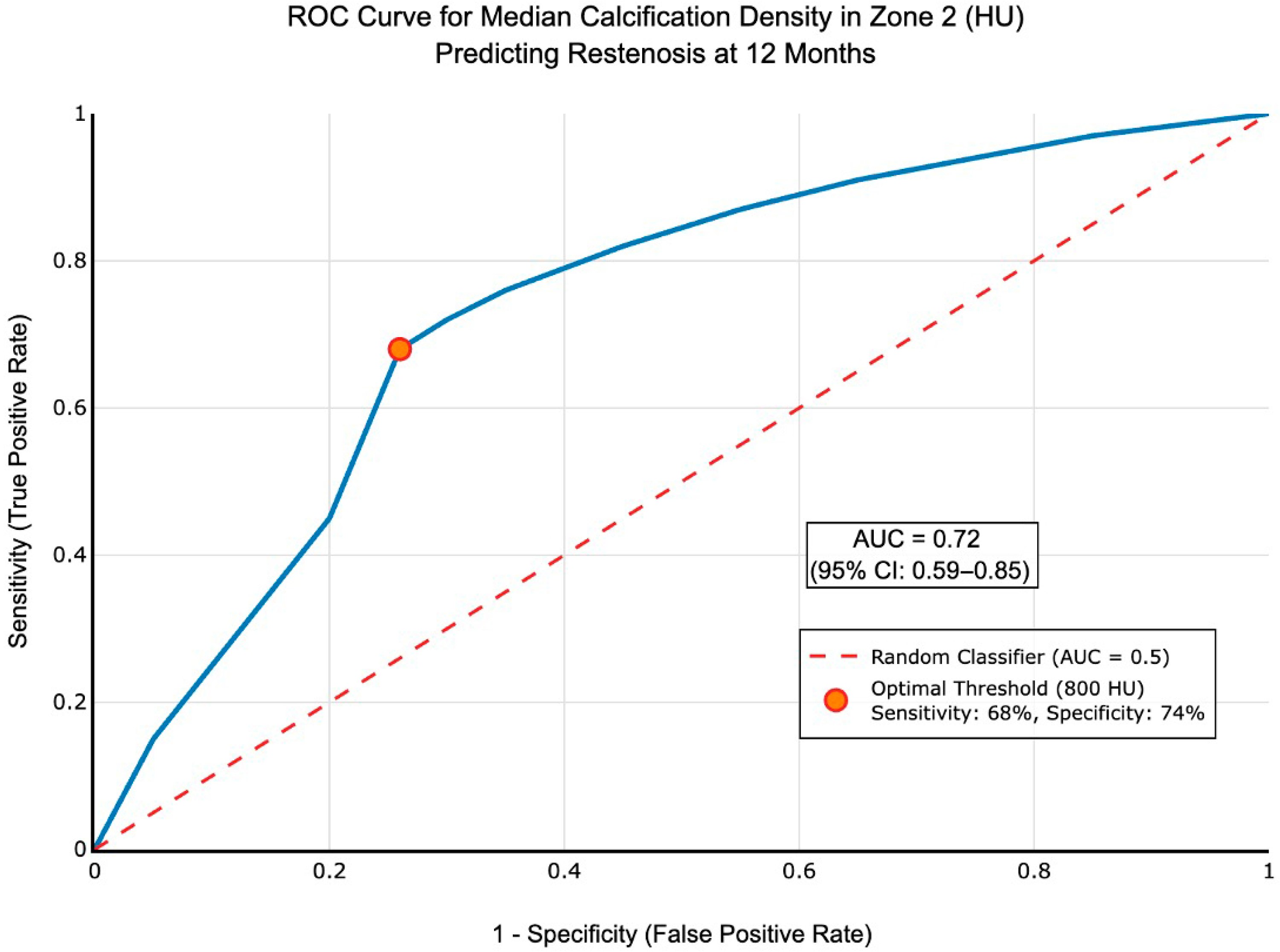
| Median density (HU) | 858 (788, 987) | 1122 (903, 1248) | 0.006 | 0.021 |
| Maximum density (HU) | 1911 (1364, 2008) | 1907 (1682, 2181) | 0.23 | 0.37 |
| Calcification volume/arterial lumen volume (%) | 15.2 [8.7; 21.7] | 20.4 [13.2; 27.6] | 0.055 | 0.20 |
| Zone 3 | ||||
| Volume (mm3) | 48 (8, 100) | 80 (30, 148) | 0.058 | 0.19 |
| Minimum density (HU) | 527 (412, 640) | 648 (537, 752) | 0.041 | 0.17 |
| Median density (HU) | 748 (538, 932) | 999 (821, 1132) | 0.005 | 0.061 |
| Maximum density (HU) | 1202 (824, 1582) | 1631 (1296, 1955) | 0.014 | 0.072 |
| Calcification volume/arterial lumen volume (%) | 6.41 [0.915; 11.4] | 8.86 [3.82; 13.8] | 0.12 | 0.34 |
| Patency at M1 | PSVR > 2.4 (N = 9) | PSVR < 2.4 (N = 80) | p-Value | Q-Value |
|---|---|---|---|---|
| Zone 1 | ||||
| Volume | 227 (149, 293) | 185 (72, 416) | 0.94 | 0.99 |
| Minimum density | 517 (512, 745) | 586 (441, 708) | 0.99 | 0.99 |
| Median density | 796 (693, 1078) | 994 (825, 1150) | 0.49 | 0.65 |
| Maximum density | 1334 (1296, 1776) | 1772 (1416, 2017) | 0.32 | 0.55 |
| Calcification volume/arterial lumen volume (%) | 12.1 [11.6; 18.5] | 15.8 [6.84; 27.0] | 0.41 | 0.63 |
| Zone 2 | ||||
| Volume | 111 (79, 263) | 266 (142, 476) | 0.18 | 0.35 |
| Minimum density | 595 (463, 759) | 644 (537, 778) | 0.65 | 0.76 |
| Median density | 859 (709, 926) | 1030 (890, 1170) | 0.060 | 0.18 |
| Maximum density | 1377 (1351, 1381) | 1914 (1698, 2147) | 0.014 | 0.43 |
| Calcification volume/arterial lumen volume (%) | 15.5 [7.40; 20.0] | 18.3 [11.4; 26.8] | 0.33 | 0.53 |
| Zone 3 | ||||
| Volume | 95 (5, 105) | 75 (23, 148) | 0.42 | 0.63 |
| Minimum density | 514 (435, 541) | 642 (519, 752) | 0.11 | 0.25 |
| Median density | 600 (515, 738) | 983 (788, 1114) | 0.012 | 0.059 |
| Maximum density | 946 (516, 1161) | 1596 (1231, 1948) | 0.005 | 0.36 |
| Calcification volume/arterial lumen volume (%) | 9.12 [3.24; 13.3] | 7.26 [2.48; 13.1] | 0.92 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamdé, C.-C.; Goueffic, Y.; Davaine, J.-M.; Lalande, A.; Guenancia, C.; Steinmetz, E. Dense Calcification of the Common Femoral Artery Is Protective Against In-Stent Restenosis. J. Clin. Med. 2025, 14, 7052. https://doi.org/10.3390/jcm14197052
Bamdé C-C, Goueffic Y, Davaine J-M, Lalande A, Guenancia C, Steinmetz E. Dense Calcification of the Common Femoral Artery Is Protective Against In-Stent Restenosis. Journal of Clinical Medicine. 2025; 14(19):7052. https://doi.org/10.3390/jcm14197052
Chicago/Turabian StyleBamdé, Camil-Cassien, Yann Goueffic, Jean-Michel Davaine, Alain Lalande, Charles Guenancia, and Eric Steinmetz. 2025. "Dense Calcification of the Common Femoral Artery Is Protective Against In-Stent Restenosis" Journal of Clinical Medicine 14, no. 19: 7052. https://doi.org/10.3390/jcm14197052
APA StyleBamdé, C.-C., Goueffic, Y., Davaine, J.-M., Lalande, A., Guenancia, C., & Steinmetz, E. (2025). Dense Calcification of the Common Femoral Artery Is Protective Against In-Stent Restenosis. Journal of Clinical Medicine, 14(19), 7052. https://doi.org/10.3390/jcm14197052






