Advancing Non-Invasive Ophthalmic Imaging in Sturge–Weber Syndrome: Clinical Guidelines Towards Early Choroidal Hemangioma Detection
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Comi, A.M. Presentation, Diagnosis, Pathophysiology, and Treatment of the Neurological Features of Sturge-Weber Syndrome. Neurologist 2011, 17, 179–184. [Google Scholar] [CrossRef]
- Shirley, M.D.; Tang, H.; Gallione, C.J.; Baugher, J.D.; Frelin, L.P.; Cohen, B.; North, P.E.; Marchuk, D.A.; Comi, A.M.; Pevsner, J. Sturge-Weber Syndrome and Port-Wine Stains Caused by Somatic Mutation in GNAQ. N. Engl. J. Med. 2013, 368, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- El Hachem, M.; Diociaiuti, A.; Galeotti, A.; Grussu, F.; Gusson, E.; Ferretti, A.; Marras, C.E.; Vecchio, D.; Cappelletti, S.; Severino, M.; et al. Multidisciplinary, Multicenter Consensus for the Care of Patients Affected with Sturge-Weber Syndrome. Orphanet J. Rare Dis. 2025, 20, 28. [Google Scholar] [CrossRef]
- Waelchli, R.; Aylett, S.E.; Robinson, K.; Chong, W.K.; Martinez, A.E.; Kinsler, V.A. New Vascular Classification of Port-Wine Stains: Improving Prediction of Sturge-Weber Risk. Br. J. Dermatol. 2014, 171, 861–867. [Google Scholar] [CrossRef]
- Lo, W.; Marchuk, D.A.; Ball, K.L.; Juhász, C.; Jordan, L.C.; Ewen, J.B.; Comi, A. Brain Vascular Malformation Consortium National Sturge-Weber Syndrome Workgroup Updates and Future Horizons on the Understanding, Diagnosis, and Treatment of Sturge-Weber Syndrome Brain Involvement. Dev. Med. Child. Neurol. 2012, 54, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A.; Muscianese, M.; Fanfoni, C.; Bellone, G.; Mennini, M.; Di Nardo, G.; Abdolrahimzadeh, S.; De Marco, G.; Orsini, A.; Foiadelli, T.; et al. Headache in Sturge-Weber Syndrome: A Systematic Review. Cephalalgia 2024, 44, 03331024241265881. [Google Scholar] [CrossRef] [PubMed]
- Sabeti, S.; Ball, K.L.; Bhattacharya, S.K.; Bitrian, E.; Blieden, L.S.; Brandt, J.D.; Burkhart, C.; Chugani, H.T.; Falchek, S.J.; Jain, B.G.; et al. Consensus Statement for the Management and Treatment of Sturge-Weber Syndrome: Neurology, Neuroimaging, and Ophthalmology Recommendations. Pediatr. Neurol. 2021, 121, 59–66. [Google Scholar] [CrossRef]
- Bichsel, C.A.; Goss, J.; Alomari, M.; Alexandrescu, S.; Robb, R.; Smith, L.E.; Hochman, M.; Greene, A.K.; Bischoff, J. Association of Somatic GNAQ Mutation with Capillary Malformations in a Case of Choroidal Hemangioma. JAMA Ophthalmol. 2019, 137, 91–95. [Google Scholar] [CrossRef]
- Francis, J.H.; Milman, T.; Grossniklaus, H.; Albert, D.; Folberg, R.; Levitin, G.; Coupland, S.; Catalanotti, F.; Rabady, D.; Kandoth, C.; et al. GNAQ Mutations in Diffuse and Solitary Choroidal Hemangiomas. Ophthalmology 2019, 126, 759–763. [Google Scholar] [CrossRef]
- Surve, A.; Azad, S.; Venkatesh, P.; Kumar, V.; Chawla, R.; Gupta, V.; Vohra, R. Choroidal Vascular Pattern in Cases of Sturge-Weber Syndrome. Ophthalmol. Retin. 2019, 3, 1091–1097. [Google Scholar] [CrossRef]
- Arora, K.S.; Quigley, H.A.; Comi, A.M.; Miller, R.B.; Jampel, H.D. Increased Choroidal Thickness in Patients with Sturge-Weber Syndrome. JAMA Ophthalmol. 2013, 131, 1216–1219. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Scavella, V.; Battaglia, D.; Recupero, S.M. Spectral Domain Optical Coherence Tomography of Choroidal and Outer Retinal Layer Thickness in the Sturge Weber Syndrome. Curr. Eye Res. 2016, 41, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, R.-J.; Chen, D.; Xu, L.; Li, M.; Zhu, L.; Guo, C.-Y.; Guo, W.-Y. Glaucoma in Patients with Eyes Close to Areas Affected by Port-Wine Stain Has Lateral and Gender Predilection. Chin. Med. J. 2017, 130, 2922–2926. [Google Scholar] [CrossRef]
- Singh, A.D.; Rundle, P.A.; Vardy, S.J.; Rennie, I.G. Photodynamic Therapy of Choroidal Haemangioma Associated with Sturge-Weber Syndrome. Eye 2005, 19, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.M.; Michels, S.; Kusserow, C.; Jurklies, B.; Augustin, A.J. Photodynamic Therapy for Symptomatic Choroidal Hemangioma: Visual and Anatomic Results. Ophthalmology 2002, 109, 2284–2294. [Google Scholar] [CrossRef]
- Arepalli, S.; Shields, C.L.; Kaliki, S.; Emrich, J.; Komarnicky, L.; Shields, J.A. Diffuse Choroidal Hemangioma Management with Plaque Radiotherapy in 5 Cases. Ophthalmology 2013, 120, 2358–2359.e2. [Google Scholar] [CrossRef] [PubMed]
- Kubicka-Trząska, A.; Karska-Basta, I.; Oleksy, P.; Romanowska-Dixon, B. Management of Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome with Ruthenium-106 Plaque Radiotherapy. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 2015–2019. [Google Scholar] [CrossRef]
- Azarcon, C.P.; Qiu, R.L.J.; Sobol, E.K.; Hubbard, G.B.; Craven, C.M.; Bergstrom, C.S.; Wells, J.R. Iodine-125 Plaque Brachytherapy for Diffuse Choroidal Hemangioma. Retin. Cases Brief Rep. 2024, 18, 51–58. [Google Scholar] [CrossRef]
- Puthussery, J.C.; Ufondu, A.; Cherian, S.; Singh, A.D. Diffuse Choroidal Hemangioma: Ophthalmic Outcomes Following Intensity-Modulated Radiation Therapy. Taiwan. J. Ophthalmol. 2025, 15, 103–108. [Google Scholar] [CrossRef]
- Thareja, S.; Lucero, E.; Ramasubramanian, A. Choroidal Hemangioma Treatment with Propranolol—A Case Study in Sturge-Weber Syndrome and Systematic Literature Review. Semin. Ophthalmol. 2025, 40, 674–682. [Google Scholar] [CrossRef]
- Thapa, R.; Shields, C.L. Oral Propranolol Therapy for Management of Exudative Retinal Detachment from Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome. Eur. J. Ophthalmol. 2013, 23, 922–924. [Google Scholar] [CrossRef]
- El Mollayess, G.; Sleiman, K.; Ibrahim, R.; Bleik, J. Intravitreal Aflibercept for Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome. Middle East Afr. J. Ophthalmol. 2023, 30, 270–273. [Google Scholar] [CrossRef]
- Anaya-Pava, E.J.; Saenz-Bocanegra, C.H.; Flores-Trejo, A.; Castro-Santana, N.A. Diffuse Choroidal Hemangioma Associated with Exudative Retinal Detachment in a Sturge-Weber Syndrome Case: Photodynamic Therapy and Intravitreous Bevacizumab. Photodiagnosis Photodyn. Ther. 2015, 12, 136–139. [Google Scholar] [CrossRef]
- Huang, Y.; Gangaputra, S.; Lee, K.E.; Narkar, A.R.; Klein, R.; Klein, B.E.K.; Meuer, S.M.; Danis, R.P. Signal Quality Assessment of Retinal Optical Coherence Tomography Images. Invest. Ophthalmol. Vis. Sci. 2012, 53, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Gershoni, A.; Barayev, E.; Vainer, I.; Allon, R.; Yavnieli, R.; Shapira, Y.; Mimouni, M.; Geffen, N.; Nemet, A.Y.; Segal, O. Thickness Measurements Taken with the Spectralis OCT Increase with Decreasing Signal Strength. BMC Ophthalmol. 2022, 22, 148. [Google Scholar] [CrossRef] [PubMed]
- García Caride, S.; Fernández-Vigo, J.I.; Valverde-Megías, A. Update on the Diagnosis and Treatment of Choroidal Hemangioma. Arch. Soc. Esp. Oftalmol. 2023, 98, 281–291. [Google Scholar] [CrossRef]
- Shields, C.L.; Honavar, S.G.; Shields, J.A.; Cater, J.; Demirci, H. Circumscribed Choroidal Hemangioma: Clinical Manifestations and Factors Predictive of Visual Outcome in 200 Consecutive Cases. Ophthalmology 2001, 108, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Atalay, H.T.; Wuthisiri, W.; Levin, A.V.; Lally, S.E.; Shields, J.A. Sector Iris Hemangioma in Association with Diffuse Choroidal Hemangioma. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2015, 19, 83–86. [Google Scholar] [CrossRef]
- Helmi, H.A.; Alkatan, H.M.; Al-Essa, R.S.; Aljudi, T.W.; Maktabi, A.M.Y.; Eberhart, C.G. Choroidal Hemangioma in Sturge Weber Syndrome: Case Series with Confirmed Tissue Diagnosis. Int. J. Surg. Case Rep. 2021, 89, 106626. [Google Scholar] [CrossRef]
- Formisano, M.; di Pippo, M.C.; Scuderi, L.; Abdolrahimzadeh, S. Current Concepts on Diffuse Choroidal Hemangioma in Sturge Weber Syndrome. Ophthalmic Genet. 2021, 42, 375–382. [Google Scholar] [CrossRef]
- Ciancimino, C.; Di Pippo, M.; Rullo, D.; Ruggeri, F.; Grassi, F.; Scuderi, G.; Abdolrahimzadeh, S. An Update on Multimodal Ophthalmological Imaging of Diffuse Choroidal Hemangioma in Sturge–Weber Syndrome. Vision 2023, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Y.; Li, X.-X.; Liang, J.-H. Ruthenium-106 Plaque Brachytherapy for the Treatment of Diffuse Choroidal Hemangioma in Sturge-Weber Syndrome. Int. J. Ophthalmol. 2020, 13, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Callaway, N.F.; Mruthyunjaya, P. Widefield Imaging of Retinal and Choroidal Tumors. Int. J. Retin. Vitr. 2019, 5, 49. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A.; Potter, P.D. Patterns of Indocyanine Green Videoangiography of Choroidal Tumours. Br. J. Ophthalmol. 1995, 79, 237–245. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Parisi, F.; Mantelli, F.; Perdicchi, A.; Scuderi, G. Retinal Pigment Epithelium-Photoreceptor Layer Alterations in a Patient with Sturge-Weber Syndrome with Diffuse Choroidal Hemangioma. Ophthalmic Genet. 2017, 38, 567–569. [Google Scholar] [CrossRef]
- Tan, C.S.; Ouyang, Y.; Ruiz, H.; Sadda, S.R. Diurnal Variation of Choroidal Thickness in Normal, Healthy Subjects Measured by Spectral Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 261–266. [Google Scholar] [CrossRef]
- Moderiano, D.; Do, M.; Hobbs, S.; Lam, V.; Sarin, S.; Alonso-Caneiro, D.; Chakraborty, R. Influence of the Time of Day on Axial Length and Choroidal Thickness Changes to Hyperopic and Myopic Defocus in Human Eyes. Exp. Eye Res. 2019, 182, 125–136. [Google Scholar] [CrossRef]
- Abdolrahimzadeh, S.; Ciancimino, C.; Grassi, F.; Sordi, E.; Fragiotta, S.; Scuderi, G. Near-Infrared Reflectance Imaging in Retinal Diseases Affecting Young Patients. J. Ophthalmol. 2021, 2021, 5581851. [Google Scholar] [CrossRef]
- Amirikia, A.; Scott, I.U.; Murray, T.G. Bilateral Diffuse Choroidal Hemangiomas with Unilateral Facial Nevus Flammeus in Sturge–Weber Syndrome. Am. J. Ophthalmol. 2000, 130, 362–364. [Google Scholar] [CrossRef]
- Venkataraman, A.; Al-Gilgawi, A.; Stoker, I.; Reddy, M.A.; Sagoo, M.S. Ultrasound Guided Ru106 Plaque Brachytherapy for Treatment of Exudative Retinal Detachment in Children with Diffuse Choroidal Haemangioma. Eye 2025, 39, 533–537. [Google Scholar] [CrossRef] [PubMed]
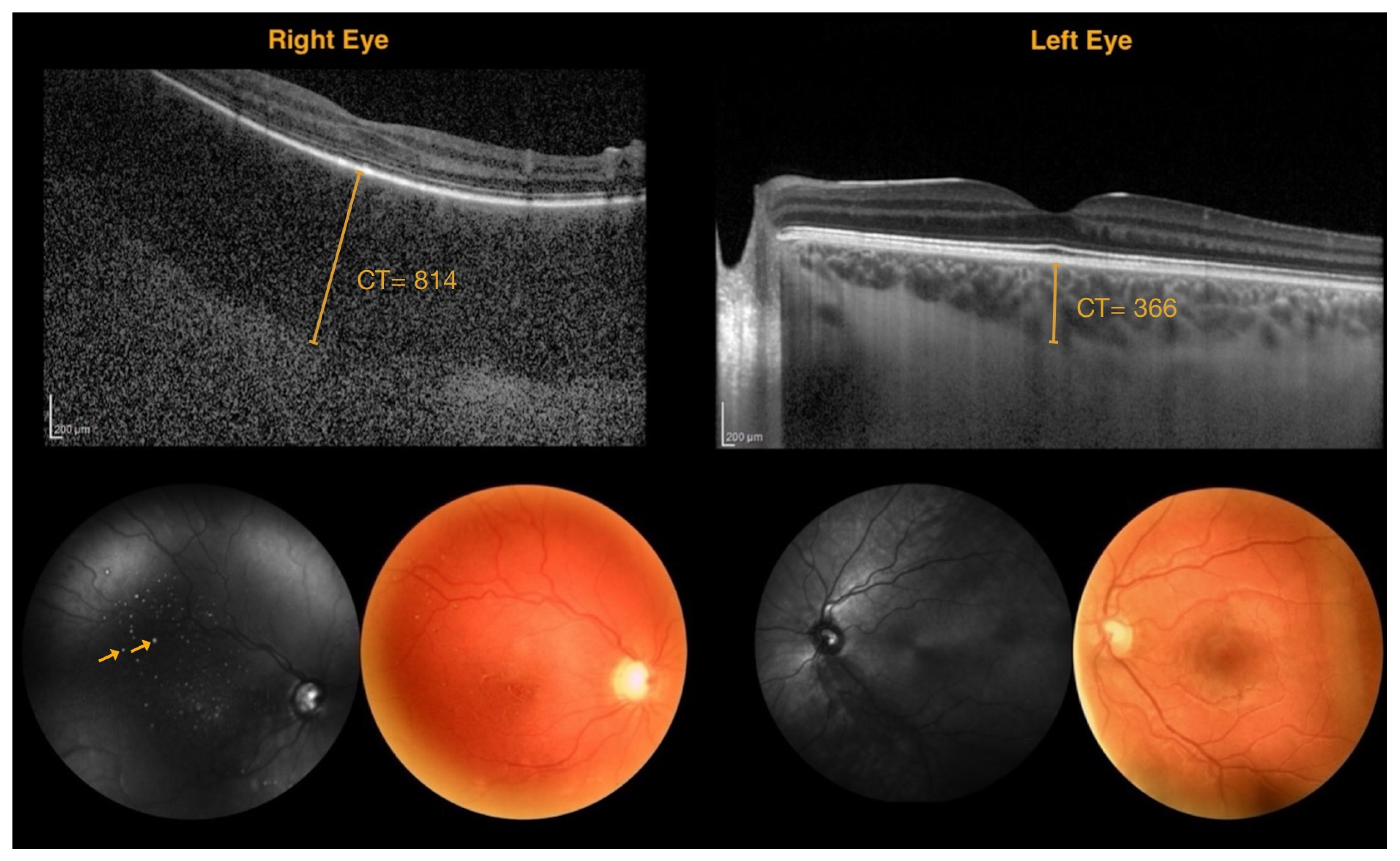
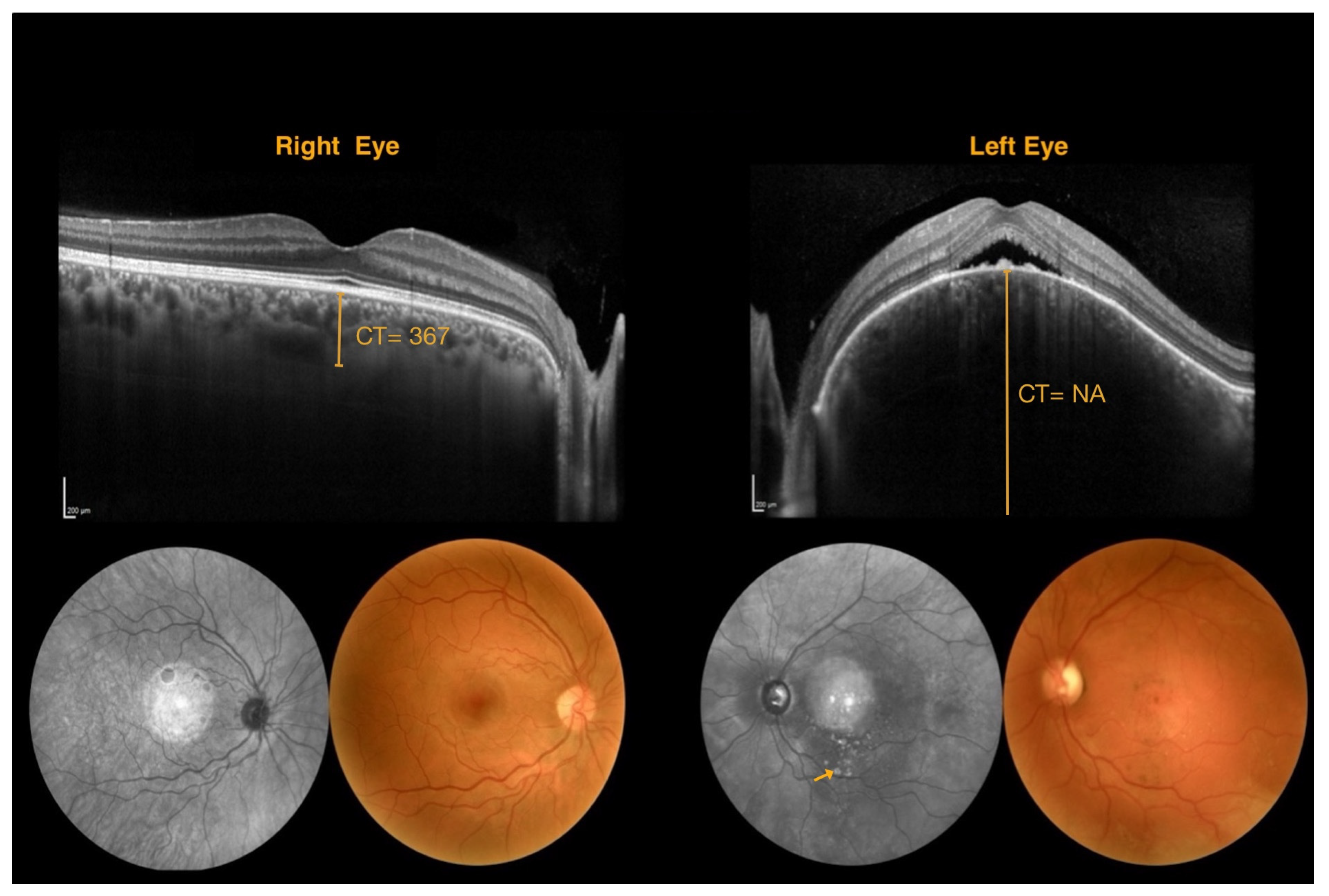
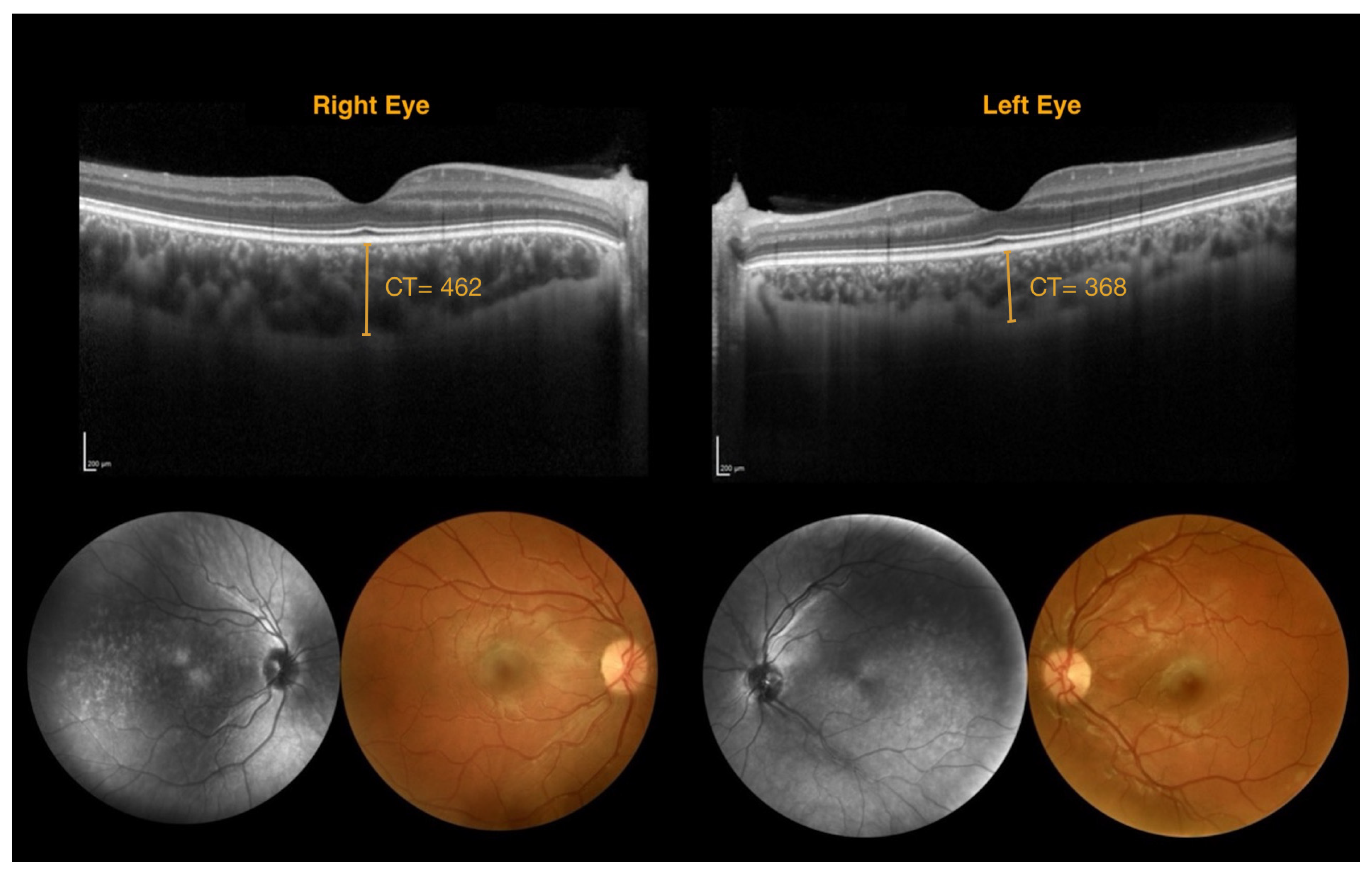
| Patient Number | Age | Sex | Leptomeningeal Angioma | Nevus Flammeus | Glaucoma | BCVA (ETDRS Charts) | IOP RE/LE | Enrolled | |
|---|---|---|---|---|---|---|---|---|---|
| RE | LE | ||||||||
| 1 | 27 | F | YES | YES (LE) | YES (LE) | 25 | 0 | 12/13 | YES |
| 2 | 16 | M | YES | YES (LE) | YES (LE) | 47 | 48 | 14/10 | YES |
| 3 | 42 | F | NO | YES (RE) | YES (RE) | 0 | 57 | 20/12 | YES |
| 4 | 20 | M | NO | YES (RE) | YES (RE) | 27 | 51 | 18/16 | YES |
| 5 | 48 | F | YES | YES (RE) | YES (RE) | 52 | 55 | 17/14 | YES |
| 6 | 13 | F | YES | YES (RE) | YES (RE) | NA | NA | NA | NO |
| 7 | 19 | M | NO | YES (RE) | YES (RE) | 48 | 51 | 20/16 | YES |
| 8 | 4 | M | YES | YES (RE) | NO | 55 | 55 | 12/12 | YES |
| 9 | 7 | M | YES | YES (RE) | YES (RE) | 0 | 35 | 14/16 | YES |
| 10 | 17 | F | YES | YES (RE) | YES (RE) | 0 | 35 | 14/16 | YES |
| 11 | 38 | F | YES | YES (LE) | NO | 55 | 50 | 10/17 | YES |
| 12 | 20 | F | NO | YES | YES | 5 | 53 | 18/14 | YES |
| 13 | 68 | M | NO | YES (LE) | YES (LE) | 55 | 0 | 14/20 | YES |
| 14 | 4 | F | YES | YES (RE) | NO | NA | NA | 12/12 | YES |
| 15 | 57 | F | NO | YES (LE) | NO | 55 | 55 | 14/14 | YES |
| 16 | 62 | F | NO | YES (RE) | NO | 55 | 55 | 14/14 | YES |
| 17 | 11 | M | YES | YES (RE) | NO | 53 | 55 | 18/18 | YES |
| Patient Number | Diagnosis Through Fundus Photography | Fundus Photography Findings | Diagnosis Through SDOCT | SDOCT Findings | Diagnosis Through NIR | NIR Findings |
|---|---|---|---|---|---|---|
| 1 | NO, poor quality imaging | NA | YES | LE: hyporreflective choroidal lesion associated with paralesional choroidal folds | NO, poor quality imaging | demarcation line |
| 2 | YES | chorioretinal folds, no tessellation, arteriovenous crossing | YES | LE: hyporreflective choroidal lesion, inner retina folding, paralesional choroidal fold | NO | hyperreflective area/dots (temporal), vessel alterations |
| 3 | NO | RE chorioretinal folds | YES | RE: hyporreflective choroidal lesion, neuroepithelium detachment with associated subretinal fluid | YES | lacquer cracks, hypo-hyperreflectivity |
| 4 | NO | RE chorioretinal folds | YES | RE: hyporreflective choroidal lesion | NO | folds, diffuse hypo-hyperreflectivity |
| 5 | YES | no tessellation | YES | RE: choroidal thickening | NO | hyperreflective area/dots (temporal) |
| 6 | NA | NA | NA | NA | NA | NA |
| 7 | YES | no tessellation, vascular tortuosity, chorioretinal folds | YES | RE: hyporreflective choroidal lesion | YES | hyperreflective area/dots (temporal), vessel alterations |
| 8 | NO | NA | YES | RE: diffuse choroidal thickening | NO | hyperreflective area/dots (temporal) |
| 9 | YES | no tessellation, vascular tortuosity | YES | RE: diffuse choroidal thickening | NO | NA |
| 10 | YES | no tessellation, vascular tortuosity, drusen-like lesions, macular hyperpigmentation | YES | RE: diffuse choroidal thickening, pseudodrusen-like lesions. | YES | hyperreflective dots, vessel alterations |
| 11 | NO | normal | YES | LE: diffuse choroidal thickening | NO | normal |
| 12 | NO | vascular tortuosity | YES | LE: diffuse choroidal thickening | NO | hyperreflective dots |
| 13 | NO | no tessellation | NO | normal | NO | normal |
| 14 | NO | no tessellation | YES | RE: diffuse choroidal thickening | NO | normal |
| 15 | NO | no tessellation | YES | LE: diffuse choroidal thickening | NO | hyperreflective dots |
| 16 | NO | drusen-like lesion | YES | Bilateral: choroidal thickening | NO | hyperreflective dots |
| 17 | NO | no tessellation | YES | RE: diffuse choroidal thickening | NO | hyperreflective dots |
| Choroidal Thickness (μm) in the DCH Affected Eye | Choroidal Thickness (μm) in the Fellow Eye | Choroidal Thickness (μm) Inter Eye Difference |
|---|---|---|
| NA | 341 | NA |
| 315 | 256 | 59 |
| NA | 202 | NA |
| 709 | 452 | 257 |
| 517 | 135 | 382 |
| NA | NA | NA |
| NA | 367 | NA |
| 454 | 233 | 221 |
| 520 | 270 | 250 |
| 814 | 366 | 448 |
| 1060 | 520 | 540 |
| 472 | 359 | 113 |
| NA | 345 | NA |
| 460 | 330 | 130 |
| 421 | 418 | 3 |
| 433 | 447 | −14 |
| 462 | 368 | 94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pippo, M.; Rullo, D.; Ciancimino, C.; Grassi, F.; Ferretti, A.; Parisi, P.; Di Nardo, G.; Orsini, A.; Perulli, M.; Battaglia, D.I.; et al. Advancing Non-Invasive Ophthalmic Imaging in Sturge–Weber Syndrome: Clinical Guidelines Towards Early Choroidal Hemangioma Detection. J. Clin. Med. 2025, 14, 7012. https://doi.org/10.3390/jcm14197012
Di Pippo M, Rullo D, Ciancimino C, Grassi F, Ferretti A, Parisi P, Di Nardo G, Orsini A, Perulli M, Battaglia DI, et al. Advancing Non-Invasive Ophthalmic Imaging in Sturge–Weber Syndrome: Clinical Guidelines Towards Early Choroidal Hemangioma Detection. Journal of Clinical Medicine. 2025; 14(19):7012. https://doi.org/10.3390/jcm14197012
Chicago/Turabian StyleDi Pippo, Mariachiara, Daria Rullo, Chiara Ciancimino, Flaminia Grassi, Alessandro Ferretti, Pasquale Parisi, Giovanni Di Nardo, Alessandro Orsini, Marco Perulli, Domenica Immacolata Battaglia, and et al. 2025. "Advancing Non-Invasive Ophthalmic Imaging in Sturge–Weber Syndrome: Clinical Guidelines Towards Early Choroidal Hemangioma Detection" Journal of Clinical Medicine 14, no. 19: 7012. https://doi.org/10.3390/jcm14197012
APA StyleDi Pippo, M., Rullo, D., Ciancimino, C., Grassi, F., Ferretti, A., Parisi, P., Di Nardo, G., Orsini, A., Perulli, M., Battaglia, D. I., Nicodemi, E. M., & Abdolrahimzadeh, S. (2025). Advancing Non-Invasive Ophthalmic Imaging in Sturge–Weber Syndrome: Clinical Guidelines Towards Early Choroidal Hemangioma Detection. Journal of Clinical Medicine, 14(19), 7012. https://doi.org/10.3390/jcm14197012






