Clinical and Demographic Characteristics of Oral Sarcoidosis: A Systematic Review of Case Reports and Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol & Registration
2.2. Search Strategy and Information Sources
2.3. Eligibility
2.4. Data Extraction
2.5. Study Quality Assessment
2.6. Statistical Analysis
3. Results
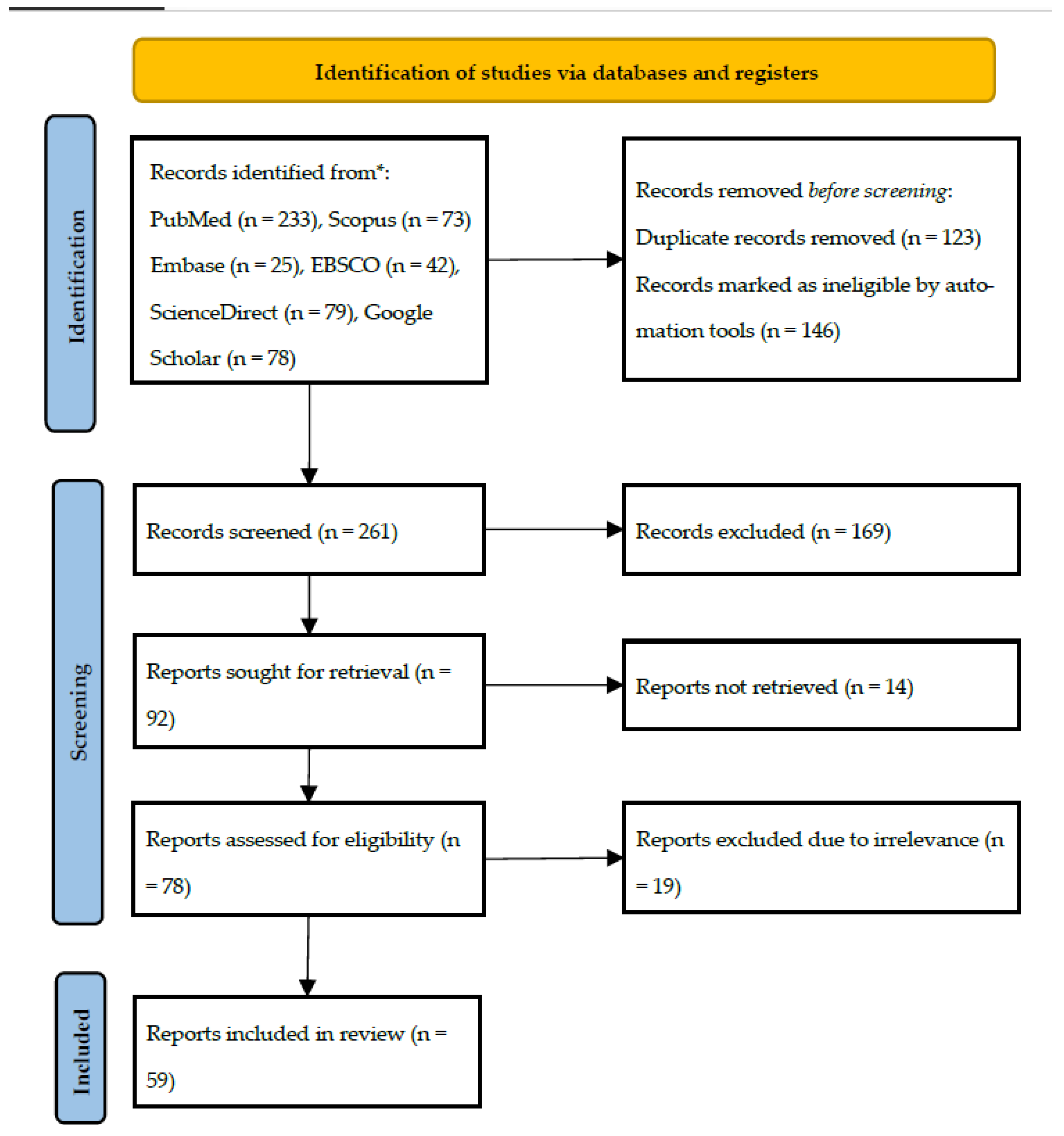
3.1. Literature Search
3.2. Study Quality Assessment
- Patient demographics clearly described
- Patient history and presenting symptoms adequately described
- Current clinical condition clearly described
- Diagnostic tests or methods and findings clearly reported
- Intervention or treatment procedure clearly described
- Post-intervention condition described
- Adverse events or unanticipated outcomes reported
- Case report provides takeaway lessons
3.3. Study Characteristics
3.3.1. Sarcoidosis with Jaw Bones Involvement (Table S1)
3.3.2. Sarcoidosis Without Jaw Bones Involvement (Table S2)
3.3.3. Comparison Between Sarcoidosis with and Without Bone Involvement (Table 2)
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, O.P. Definition and history of sarcoidosis. Eur. Respir. Monogr. 2005, 32, 1–13. [Google Scholar]
- Baughman, R.P.; Lower, E.E.; du Bois, R.M. Sarcoidosis. Lancet 2003, 361, 1111–1118. [Google Scholar] [CrossRef]
- Patterson, K.C.; Strek, M.E. Pulmonary fibrosis in sarcoidosis: Clinical features and outcomes. Ann. Am. Thorac. Soc. 2013, 10, 362–370. [Google Scholar] [CrossRef]
- Tana, C.; Donatiello, I.; Caputo, A.; Tana, M.; Naccarelli, T.; Mantini, C.; Giamberardino, M.A. Clinical features, histopathology and differential diagnosis of sarcoidosis. Cells 2021, 11, 59. [Google Scholar] [CrossRef]
- Bernardinello, N.; Petrarulo, S.; Balestro, E.; Cocconcelli, E.; Veltkamp, M.; Spagnolo, P. Pulmonary sarcoidosis: Diagnosis and differential diagnosis. Diagnostics 2021, 11, 1558. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Whaley, P.; Thayer, K.A.; Schünemann, H.J. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 2018, 121, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Poe, D.L. Sarcoidosis of the jaw: A new disease of the mandible. Am. J. Orthod. Oral Surg. 1943, 29, C52–C56. [Google Scholar] [CrossRef]
- Van Swol, R.L. Periodontosis in a patient with previously diagnosed sarcoidosis. J. Periodontol. 1973, 44, 697–704. [Google Scholar] [CrossRef]
- Hillerup, S. Diagnosis of sarcoidosis from oral manifestation. Int. J. Oral Surg. 1976, 5, 95–99. [Google Scholar] [CrossRef]
- Betten, B.; Koppang, H.S. Sarcoidosis with mandibular involvement: Report of a case. Oral Surg. Oral Med. Oral Pathol. 1976, 42, 731–737. [Google Scholar] [CrossRef]
- Schwartz, H.C. Sarcoid temporomandibular arthritis. Oral Surg. Oral Med. Oral Pathol. 1981, 52, 588–590. [Google Scholar] [CrossRef]
- Cohen, D.M.; Reinhardt, R.A. Systemic sarcoidosis presenting with Horner’s syndrome and mandibular paresthesia. Oral Surg. Oral Med. Oral Pathol. 1982, 53, 577–581. [Google Scholar] [CrossRef]
- Verheijen-Breemhaar, L.; de Man, K.; Zondervan, P.E.; Hilvering, C. Sarcoidosis with maxillary involvement. Int. J. Oral Maxillofac. Surg. 1987, 16, 104–107. [Google Scholar] [CrossRef]
- Hildebrand, J.; Plezia, R.A.; Rao, S.B. Sarcoidosis: Report of two cases with oral involvement. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 217–222. [Google Scholar] [CrossRef]
- Rubin, M.M.; Sanfilippo, R.J.; Pliskin, A. Maxillary alveolar bone loss in a patient with sarcoidosis. J. Oral Maxillofac. Surg. 1991, 49, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Clayman, L.; MacLennan, M.; Dolan, R.L. Nonpainful swelling of the palate and loosening of the maxillary incisors. J. Oral Maxillofac. Surg. 1998, 56, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Farish, S.E. Intraosseous Sarcoidosis of the Maxilla: Case Report. J. Oral Maxillofac. Surg. 2000, 58, 435–439. [Google Scholar] [CrossRef] [PubMed]
- White, R.D.; Crocker, D.J. Persistent Anterior Maxillary Bone Loss. J. Oral Maxillofac. Surg. 2000, 58, 1145–1149. [Google Scholar] [CrossRef]
- Suresh, L.; Calixto, L.E.M.; Aguirre, A.; Fischman, S.L. Mid-Line Swelling of the Palate. J. Laryngol. Otol. 2004, 118, 385–387. [Google Scholar] [CrossRef]
- Suresh, L.; Aguirre, A.; Buhite, R.J.; Radfar, L. Intraosseous Sarcoidosis of the Jaws Mimicking Aggressive Periodontitis: A Case Report and Literature Review. J. Periodontol. 2004, 75, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, L.; de Santis, R.; Brandi, C.; D’Aniello, C. Mandibular Intra-Bony Lesion as First Sign of Sarcoidosis: Case Report. Int. J. Oral Maxillofac. Surg. 2004, 33, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.J.; Fiocchi, M.F.; Flaitz, C.M. Sarcoidosis Affecting the Periodontium: A Long-Term Follow-Up Case. J. Periodontol. 2007, 78, 1179–1183. [Google Scholar] [CrossRef]
- Wright, R.J.; Ambro, B.T. Osteolytic Lesions in the Craniofacial Skeleton of a Patient with Extrapulmonary Sarcoidosis. Laryngoscope 2009, 119, S130. [Google Scholar] [CrossRef]
- Cain, R.B.; Tamura, T.K.; Elhosseiny, A.A.; Vanisky, E.J.; Brundage, W.J. Sarcoidosis Presenting as a Lytic Lesion of the Mandible. J. Oral Maxillofac. Surg. 2012, 70, 2823–2828. [Google Scholar] [CrossRef]
- Wiesli, M.G.; Hostettler, K.E.; Tamm, M.; Jaquiéry, C. Osteolysis of Unknown Origin: A Case Report. BMC Oral Health 2015, 15, 168. [Google Scholar] [CrossRef]
- Gupta, S.; Tripathi, A.K.; Kumar, V.; Saimbi, C.S. Sarcoidosis: Oral and Extra-Oral Manifestation. J. Indian Soc. Periodontol. 2015, 19, 582–585. [Google Scholar] [CrossRef]
- Hori, S.; Tomihara, K.; Sekido, K.; Tachinami, H.; Imaue, S.; Fujiwara, K.; Noguchi, M. Sarcoidosis of the Mandibular Condyle Manifesting as a Temporomandibular Joint Arthrosis: A Rare Case Report. Oral Sci. Int. 2020, 18, 229–232. [Google Scholar] [CrossRef]
- Hosni, I.U.; Karbhari, B.; Orr, R.; Opie, N. Extensive Bony Sarcoidosis of the Head and Neck Region: A Rare Presentation. BMJ Case Rep. 2021, 14, e237105. [Google Scholar] [CrossRef]
- Cheema, A.W.; Buckey, J.C., Jr.; Holmgren, E.P. A Rare Presentation of Intraosseous Sarcoidosis of the Mandible Presenting as Peri-Implantitis: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2022, 80, 728–735.e2. [Google Scholar] [CrossRef]
- Koutrakis, N.E.; Sahu, A.; Vasilyeva, D.; Peters, S.M. Orofacial Sarcoidosis: Report of Three Cases. J. Oral Med. Oral Surg. 2022, 28, 9. [Google Scholar] [CrossRef]
- Mittal, P.; Gupta, B.; Kumar, S.; Rawat, A.; Singh, A. Beyond the Usual Suspects: Primary Premaxilla Sarcoidosis. Iran. J. Otorhinolaryngol. 2024, 36, 365–369. [Google Scholar] [CrossRef]
- Patel, J.; Walker, E.; Clarkson, R.; Bhakta, S. Maxillary Sarcoidosis: A Case Report. Dent. Update 2024, 51, 418–420. [Google Scholar] [CrossRef]
- Hoggins, G.S.; Allan, D. Sarcoidosis of the Maxillary Region. Oral Surg. Oral Med. Oral Pathol. 1969, 27, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Van Maarsseveen, A.C.; van der Waal, I.; Stam, J.; Veldhuizen, R.W.; van der Kwast, W.A. Oral Involvement in Sarcoidosis. Int. J. Oral Surg. 1982, 11, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Sloan, P.J.; O’Neil, T.C.; Smith, C.J.; Holdsworth, C.D. Multisystem Sarcoid Presenting with Gingival Hyperplasia. Br. J. Oral Surg. 1983, 21, 31–35. [Google Scholar] [CrossRef]
- DeLuke, D.M.; Sciubba, J.J. Oral Manifestations of Sarcoidosis: Report of a Case Masquerading as a Neoplasm. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 223–227. [Google Scholar] [CrossRef]
- Macleod, R.I.; Snow, M.H.; Hawkesford, J.E. Sarcoidosis of the Tongue—A Case Report. Br. J. Oral Maxillofac. Surg. 1985, 23, 243–246. [Google Scholar] [CrossRef]
- Mendelsohn, S.S.; Field, E.A.; Woolgar, J. Sarcoidosis of the Tongue. Clin. Exp. Dermatol. 1992, 17, 47–48. [Google Scholar] [CrossRef]
- Soto, A.S.; Valentín, P.L.; González, L.M.R.; Santa Cruz, C.S.; Hernández, A.V. Oral Sarcoidosis with Tongue Involvement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 594–597. [Google Scholar] [CrossRef]
- Piattelli, A.; Favia, G.F.; Di Alberti, L. Oral Ulceration as a Presenting Sign of Unknown Sarcoidosis Mimicking a Tumour: Report of 2 Cases. Oral Oncol. 1998, 34, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Kanekura, T.; Kawabata, H.; Shimomai, K.; Higashi, Y.; Setoyama, M.; Kanzaki, T. A Case of Sarcoidosis Involving the Tongue. J. Dermatol. 1999, 26, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.C.; Blair, F.M. Sarcoidosis of the Gingiva—A Case Report. Dent. Update 2003, 30, 264–268. [Google Scholar] [CrossRef]
- Armstrong, C.; Napier, S.; Linden, G.J. Sarcoidosis with Gingival Involvement: A Case Report. J. Periodontol. 2004, 75, 608–612. [Google Scholar] [CrossRef]
- Kasamatsu, A.; Kanazawa, H.; Watanabe, T.; Matsuzaki, O. Oral Sarcoidosis: Report of a Case and Review of Literature. J. Oral Maxillofac. Surg. 2007, 65, 1256–1259. [Google Scholar] [CrossRef]
- Koike, K.; Ide, K.; Shiratsuchi, H.; Nakashima, T.; Umezaki, T.; Komune, S. Sarcoidosis of the Tongue: A Case Report. Auris Nasus Larynx 2007, 34, 131–133. [Google Scholar] [CrossRef]
- Poate, T.W.; Sharma, R.; Moutasim, K.A.; Escudier, M.P.; Warnakulasuriya, S. Orofacial Presentations of Sarcoidosis—A Case Series and Review of the Literature. Br. Dent. J. 2008, 205, 437–442. [Google Scholar] [CrossRef]
- Antunes, K.B.; Miranda, Á.M.M.A.; Carvalho, S.R.D.S.; Azevedo, A.L.D.R.; Tatakis, D.N.; Pires, F.R. Sarcoidosis Presenting as Gingival Erosion in a Patient under Long-Term Clinical Control. J. Periodontol. 2008, 79, 1327–1331. [Google Scholar] [CrossRef]
- Kolokotronis, A.E.; Belazi, M.A.; Haidemenos, G.; Zaraboukas, T.K.; Antoniades, D.Z. Sarcoidosis: Oral and Perioral Manifestations. Hippokratia 2009, 13, 119–121. [Google Scholar]
- Bouaziz, A.; Le Scanff, J.; Chapelon-Abric, C.; Varron, L.; Khenifer, S.; Gleizal, A.; Bentz, M.H.; Barthel, A.; Valeyre, D.; Seve, P.; et al. Oral Involvement in Sarcoidosis: Report of 12 Cases. QJM Int. J. Med. 2012, 105, 755–767. [Google Scholar] [CrossRef]
- Kadiwala, S.A.; Dixit, M.B. Gingival Enlargement Unveiling Sarcoidosis: Report of a Rare Case. Contemp. Clin. Dent. 2013, 4, 551–555. [Google Scholar] [CrossRef]
- Motswaledi, M.H.; Khammissa, R.A.; Jadwat, Y.; Lemmer, J.; Feller, L. Oral Sarcoidosis: A Case Report and Review of the Literature. Aust. Dent. J. 2014, 59, 389–394. [Google Scholar] [CrossRef]
- Tripathi, P.; Aggarwal, J.; Chopra, D.; Bagga, S.; Sethi, K. Sarcoidosis Presenting as Isolated Gingival Enlargement: A Rare Case Entity. J. Clin. Diagn. Res. 2014, 8, ZD25–ZD26. [Google Scholar] [CrossRef]
- Kalsi, H.; McParland, H.; Cook, R.J. Spontaneous Oral Mucosal Bleeding Unmasking Undiagnosed Sarcoidosis: A Case Report. Dent. Update 2016, 43, 353–356. [Google Scholar] [CrossRef]
- Radochová, V.; Radocha, J.; Laco, J.; Slezák, R. Oral Manifestation of Sarcoidosis: A Case Report and Review of the Literature. J. Indian Soc. Periodontol. 2016, 20, 627–629. [Google Scholar] [CrossRef]
- Gill, I.; Siddiqi, J. An Oral Lesion as the Primary Clinical Manifestation of Sarcoidosis. Ann. R. Coll. Surg. Engl. 2017, 99, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.; Adegun, O.; Hodgson, T. A Lump on the Palate. Clin. Exp. Dermatol. 2019, 44, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Shah, N.; Sheikh, M.A.; Chatterjee, R.P. Oral Sarcoidosis Aiding in Diagnosis of Underlying Systemic Disease. BMJ Case Rep. 2019, 12, e232093. [Google Scholar] [CrossRef] [PubMed]
- Gulseren, D.; Elçin, G. Images of the Month: Demonstrative Oral Mucosal Sarcoidosis in a Patient with Pulmonary Disease. Clin. Med. 2020, 20, e127–e128. [Google Scholar] [CrossRef]
- Shahabinejad, M.; Delavarian, Z.; Zamani, T.; Fallah Toosi, F. Sarcoidosis and Its Oral Manifestations: A Case Report Study. Clin. Case Rep. 2023, 11, e6923. [Google Scholar] [CrossRef]
- Galohda, A.; Shreehari, A.K. Orofacial Granulomatosis as a Manifestation of Sarcoidosis: A Rare Case Report. J. Oral Maxillofac. Pathol. 2023, 27, 543–547. [Google Scholar] [CrossRef]
- Khongsit, A.K.; Kumar, S.; Gupta, B.; Kumar, S. An Unusual Case of Oral Sarcoidosis: A Diagnostic Dilemma. J. Oral Maxillofac. Pathol. 2023, 27, 607. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.S.V.; Ferreira, G.T.; Henrique, P.R.; de Araujo, M.S.; Silva Servato, J.P. Upper Lip Nodule as the First Manifestation of Sarcoidosis: A Case Report. J. Skin Stem Cell 2023, 10, e139072. [Google Scholar] [CrossRef]
- Medeiros, Y.L.; Mota, M.E.; Pinto, C.A.L.; Moreira, M.S.; Alves, F.A.; Jaguar, G.C. A Hard Lobulated Submucosal Nodule on the Lower Lip. Oral Dis. 2024, 30, 4811–4814. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, G.; Bhandary, R.; Ramesh, A. Gingival Hyperplasia and Conjunctival Inflammatory Nodule: A Diagnostic Pathway to Sarcoidosis? Sarcoidosis Vasc. Diffuse Lung Dis. 2024, 41, e2024027. [Google Scholar] [CrossRef]
- Swain, W.; Castrichini, M.; Siontis, K.; Hasan, F.; Arment, C. Oral Sarcoidosis Preceding Sudden Cardiac Arrest: A Case Report. Eur. Heart J. Case Rep. 2024, 8, ytae557. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: North Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Polverino, F.; Balestro, E.; Spagnolo, P. Clinical presentations, pathogenesis, and therapy of sarcoidosis: State of the art. J. Clin. Med. 2020, 9, 2363. [Google Scholar] [CrossRef]
- Trivieri, M.G.; Spagnolo, P.; Birnie, D.; Liu, P.; Drake, W.; Kovacic, J.C.; Baughman, R.; Fayad, Z.A.; Judson, M.A. Challenges in cardiac and pulmonary sarcoidosis: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020, 76, 1878–1901. [Google Scholar] [CrossRef]
- Tana, C.; Drent, M.; Nunes, H.; Kouranos, V.; Cinetto, F.; Jessurun, N.T.; Spagnolo, P. Comorbidities of sarcoidosis. Ann. Med. 2022, 54, 1014–1035. [Google Scholar] [CrossRef]
- Sreeja, C.; Priyadarshini, A.; Premika; Nachiammai, N. Sarcoidosis—A Review Article. J. Oral Maxillofac. Pathol. 2022, 26, 242–253. [Google Scholar] [CrossRef]
- Rossides, M.; Darlington, P.; Kullberg, S.; Arkema, E.V. Sarcoidosis: Epidemiology and clinical insights. J. Intern. Med. 2023, 293, 668–680. [Google Scholar] [CrossRef]
- Hena, K.M. Sarcoidosis Epidemiology: Race Matters. Front. Immunol. 2020, 11, 537382. [Google Scholar] [CrossRef]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Baughman, R.P. Diagnosis and detection of sarcoidosis: An official American Thoracic Society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef]
- Statement on Sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS), and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG). Am. J. Respir. Crit. Care Med. 1999, 160, 736–755. [Google Scholar] [CrossRef]
- Jain, R.; Yadav, D.; Puranik, N.; Guleria, R.; Jin, J.O. Sarcoidosis: Causes, diagnosis, clinical features, and treatments. J. Clin. Med. 2020, 9, 1081. [Google Scholar] [CrossRef]
| Parameter | Inclusion | Exclusion |
|---|---|---|
| Population | Adult & young-adult patients diagnosed with sarcoidosis | Patients diagnosed with other granulomatous diseases |
| Exposure | Sarcoidosis presenting in the oral cavity | Sarcoidosis in other regions of the body |
| Comparison | N/A | N/A |
| Outcome | Clinical features Diagnostic methods Treatment and outcome | Incomplete demographic, histopathological, and clinical details. |
| Study design | Case reports & case series Full-text availability English language only | Review articles, editorials, animal studies and conference abstracts Non-English language articles Duplicates that may introduce bias |
| Clinical Characteristics | With Bone Involvement (n = 26) | Without Bone Involvement (n = 51) | |||
|---|---|---|---|---|---|
| Average age | 42.3 | 44.3 | |||
| M:F ratio | 5:8 | 11:25 | |||
| Duration | 1 mos–3 yrs | 4 days–10 yrs | |||
| Symptoms * | n | % | n | % | p value |
| Swelling | 4 | 15.4 | 19 | 38 | 0.040 |
| Nodule | 4 | 15.4 | 18 | 36 | 0.056 |
| Bone loss | 18 | 69.2 | 5 | 10 | <0.001 |
| Asymptomatic | 4 | 15.4 | 5 | 10 | 0.355 |
| Ulcerations | 2 | 7.7 | 11 | 22 | 0.109 |
| Erythema | 4 | 15.4 | 7 | 14 | 0.547 |
| Pain | 7 | 26.9 | 6 | 12 | 0.089 |
| Painless | 5 | 19.2 | 9 | 18 | 0.547 |
| Discomfort | 1 | 3.8 | 2 | 4 | 0.738 |
| Mobile teeth | 9 | 34.6 | 1 | 2 | <0.001 |
| Gingival recession | 3 | 11.5 | 1 | 2 | 0.109 |
| Periodontitis | 1 | 3.8 | 1 | 2 | 0.564 |
| Papular lesion | 1 | 3.8 | 2 | 4 | 0.738 |
| Nasal obstruction | 4 | 15.4 | 0 | 0 | 0.011 |
| Gingival hyperplasia | 0 | 0 | 6 | 12 | 0.076 |
| Asymmetry | 0 | 0 | 3 | 6 | 0.285 |
| Failing implants | 2 | 7.7 | 0 | 0 | 0.111 |
| Bleeding | 0 | 0 | 7 | 14 | 0.048 |
| Reduction in tongue mobility | 0 | 0 | 2 | 4 | 0.436 |
| Location | |||||
| Mandible | 12 | 46.2 | 20 | 39.2 | 0.116 |
| Maxilla | 10 | 38.5 | 12 | 23.5 | |
| Generalized | 4 | 15.4 | 19 | 37.3 | |
| Diagnosis | |||||
| New | 12 | 46.2 | 36 | 70.6 | 0.033 |
| Pre-existing | 14 | 53.8 | 15 | 29.4 | |
| Treatment | |||||
| Combination | 9 | 34.6 | 9 | 17.6 | 0.080 |
| Surgical | 7 | 26.9 | 6 | 11.8 | |
| Steroids | 5 | 19.2 | 14 | 27.5 | |
| Non-surgical | 2 | 7.7 | 12 | 23.5 | |
| None | 1 | 3.8 | 8 | 15.7 | |
| N/A | 2 | 7.7 | 2 | 3.9 | |
| Outcome | |||||
| Resolved | 20 | 76.9 | 36 | 70.6 | 0.218 |
| Worsened | 1 | 3.8 | 0 | 0 | |
| Spontaneous remission | 0 | 0 | 2 | 3.9 | |
| No change | 0 | 0 | 5 | 9.8 | |
| N/A | 5 | 19.2 | 8 | 15.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaber, M.; Abouseif, N.; Abdelmagied, M.; El-Ameen, E.M. Clinical and Demographic Characteristics of Oral Sarcoidosis: A Systematic Review of Case Reports and Case Series. J. Clin. Med. 2025, 14, 7006. https://doi.org/10.3390/jcm14197006
Jaber M, Abouseif N, Abdelmagied M, El-Ameen EM. Clinical and Demographic Characteristics of Oral Sarcoidosis: A Systematic Review of Case Reports and Case Series. Journal of Clinical Medicine. 2025; 14(19):7006. https://doi.org/10.3390/jcm14197006
Chicago/Turabian StyleJaber, Mohamed, Nadin Abouseif, Mawada Abdelmagied, and Essra Mohamed El-Ameen. 2025. "Clinical and Demographic Characteristics of Oral Sarcoidosis: A Systematic Review of Case Reports and Case Series" Journal of Clinical Medicine 14, no. 19: 7006. https://doi.org/10.3390/jcm14197006
APA StyleJaber, M., Abouseif, N., Abdelmagied, M., & El-Ameen, E. M. (2025). Clinical and Demographic Characteristics of Oral Sarcoidosis: A Systematic Review of Case Reports and Case Series. Journal of Clinical Medicine, 14(19), 7006. https://doi.org/10.3390/jcm14197006






