Prevalence of Arterial Stiffness Determined by Cardio-Ankle Vascular Index in Myeloproliferative Neoplasms †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Outcomes
2.3. Procedures
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018, 8, 15. [Google Scholar] [CrossRef]
- De Stefano, V.; Za, T.; Rossi, E.; Vannucchi, A.M.; Ruggeri, M.; Elli, E.; Micò, C.; Tieghi, A.; Cacciola, R.R.; Santoro, C.; et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: Incidence, risk factors, and effect of treatments. Haematologica 2008, 93, 372–380. [Google Scholar] [CrossRef]
- Landolfi, R.; Di Gennaro, L. Pathophysiology of thrombosis in myeloproliferative neoplasms. Haematologica 2011, 96, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Carobbio, A.; Finazzi, G.; Guerini, V.; Spinelli, O.; Delaini, F.; Marchioli, R.; Borrelli, G.; Rambaldi, A.; Barbui, T. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: Interaction with treatment, standard risk factors, and JAK2 mutation status. Blood 2007, 109, 2310–2313. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, R.; Finazzi, G.; Landolfi, R.; Kutti, J.; Gisslinger, H.; Patrono, C.; Marilus, R.; Villegas, A.; Tognoni, G.; Barbui, T. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J. Clin. Oncol. 2005, 23, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Besses, C.; Cervantes, F.; Pereira, A.; Florensa, L.; Sole, F.; Hernandez-Boluda, J.C.; Woessner, S.; Sans-Sabrafen, J.; Rozman, C.; Montserrat, E. Major vascular complications in essential thrombocythemia: A study of the predictive factors in a series of 148 patients. Leukemia 1999, 13, 150–154. [Google Scholar] [CrossRef][Green Version]
- Barbui, T.; Tefferi, A.; Vannucchi, A.M.; Passamonti, F.; Silver, R.T.; Hoffman, R.; Verstovsek, S.; Mesa, R.; Kiladjian, J.-J.; Hehlmann, R.; et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: Revised management recommendations from European LeukemiaNet. Leukemia 2018, 32, 1057–1069. [Google Scholar] [CrossRef]
- Saiki, A.; Sato, Y.; Watanabe, R.; Watanabe, Y.; Imamura, H.; Yamaguchi, T.; Ban, N.; Kawana, H.; Nagumo, A.; Nagayama, D.; et al. The role of a novel arterial stiffness parameter, cardio-ankle vascular index (CAVI), as a surrogate marker for cardiovascular diseases. J. Atheroscler. Thromb. 2016, 23, 155–168. [Google Scholar] [CrossRef]
- Oliver, J.J.; Webb, D.J. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 554–566. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, H. Assessment of arterial stiffness using the cardio-ankle vascular index. Pulse 2016, 4, 11–23. [Google Scholar] [CrossRef]
- Saiki, A.; Ohira, M.; Yamaguchi, T.; Nagayama, D.; Shimizu, N.; Shirai, K.; Tatsuno, I. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J. Atheroscler. Thromb. 2010, 27, 732–748. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Ding, N.; Kim, E.D.; Budoff, M.; Chirinos, J.A.; Fernhall, B.; Hamburg, N.M.; Kario, K.; Miyoshi, T.; Tanaka, H.; et al. Cardio-ankle vascular index and cardiovascular disease: Systematic review and meta-analysis of prospective and cross-sectional studies. J. Clin. Hypertens. 2019, 21, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Park, H.E.; Choi, S.Y.; Kim, M.K.; Oh, B.H. Cardio-ankle vascular index reflects coronary atherosclerosis in patients with abnormal glucose metabolism: Assessment with 256 slice multi-detector computed tomography. J. Cardiol. 2012, 60, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, T.; Limpijankit, T.; Jongjirasiri, S.; Laothamatas, J.; Yamwong, S.; Sritara, P. Arterial stiffness contributes to coronary artery disease risk prediction beyond the traditional risk score (RAMA-EGAT score). Heart Asia 2012, 4, 77–82. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- Mozos, I.; Borzak, G.; Caraba, A.; Mihaescu, R. Arterial stiffness in hematologic malignancies. Oncol. Target. Ther. 2017, 10, 1381–1388. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Muenkaew, M.; Chiewvit, P.; Ratanasit, N.; Kaolawanich, Y.; Phrommintikul, A. Electrocardiographic predictors of cardiovascular events in patients at high cardiovascular risk: A multicenter study. J. Geriatr. Cardiol. 2019, 16, 630–638. [Google Scholar]
- Barosi, G.; Birgegard, G.; Finazzi, G.; Griesshammer, M.; Harrison, C.; Hasselbalch, H.C.; Kiladjian, J.-J.; Lengfelder, E.; McMullin, M.F.; Passamonti, F.; et al. Response criteria for essential thrombocythemia and polycythemia vera: Result of a European LeukemiaNet consensus conference. Blood 2009, 113, 4829–4833. [Google Scholar] [CrossRef]
- Tefferi, A.; Barosi, G.; Mesa, R.A.; Cervantes, F.; Deeg, H.J.; Reilly, J.T.; Verstovsek, S.; Dupriez, B.; Silver, R.T.; Odenike, O.; et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood 2006, 108, 1497–1503. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Sritara, P. Cardio-ankle vascular index in a Thai population. Pulse 2017, 4 (Suppl. S1), 8–10. [Google Scholar] [CrossRef]
- Accurso, V.; Santoro, M.; Mancuso, S.; Contrino, A.D.; Casimio, P.; Sardo, M.; Raso, S.; Di Piazza, F.; Perez, A.; Bono, M.; et al. Cardiovascular risk in essential thrombocythemia and polycythemia vera: Thrombotic risk and survival. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020008. [Google Scholar] [CrossRef]
- Barbui, T.; Carobbio, A.; Cervantes, F.; Vannucchi, A.M.; Guglielmelli, P.; Antonioli, E.; Alvarez-Larrán, A.; Rambaldi, A.; Finazzi, G.; Barosi, G. Thrombosis in primary myelofibrosis: Incidence and risk factors. Blood 2010, 115, 778–782. [Google Scholar] [CrossRef]
- Rungjirajittranon, T.; Owattanapanich, W.; Ungprasert, P.; Siritanaratkul, N.; Ruchutrakool, T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer 2019, 19, 184. [Google Scholar] [CrossRef]
- Frederiksen, H.; Szépligeti, S.; Bak, M.; Ghanima, W.; Hasselbalch, H.C.; Christiansen, C.F. Vascular diseases in patients with chronic myeloproliferative neoplasms—Impact of comorbidity. Clin. Epidemiol. 2019, 11, 955–967. [Google Scholar] [CrossRef]
- Hasselbalch, H.C. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: Is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 2012, 119, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- van Popele, N.M.; Grobbee, D.E.; Bots, M.L.; Asmar, R.; Topouchian, J.; Reneman, R.S.; Hoeks, A.P.G.; van der Kuip, D.A.M.; Hofman, A.; Witteman, J.C.M. Association between arterial stiffness and atherosclerosis: The Rotterdam Study. Stroke 2001, 32, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.; Feely, J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 2005, 46, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Hasselbalch, H.C.; Bjørn, M.E. MPNs as inflammatory diseases: The evidence, consequences, and perspectives. Mediat. Inflamm. 2015, 2015, 102476. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, H. Arterial stiffness in health and disease: The role of cardio–ankle vascular index. J. Cardiol. 2021, 78, 493–501. [Google Scholar] [CrossRef]
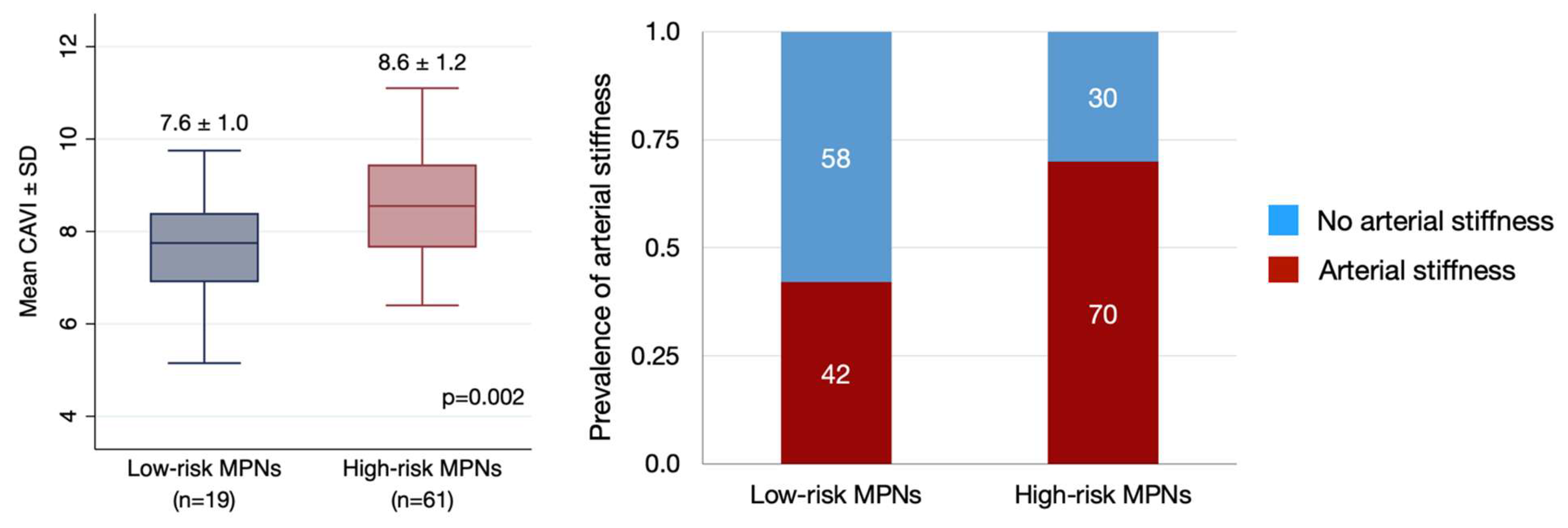
| Characteristics | Total (n = 80) | ET (n = 24) | PV (n = 50) | PMF (n = 6) |
|---|---|---|---|---|
| Age (mean ± SD), years | 63.5 ±12.6 | 60.9 ±16.2 | 64.0 ± 11.0 | 70.3 ± 5.1 |
| Sex, n (%) | ||||
| Male | 37 (46.3) | 9 (37.5) | 26 (52.0) | 2 (33.3) |
| Female | 43 (53.8) | 15 (62.5) | 24 (48.0) | 4 (66.7) |
| Mutation, n (%) | ||||
| JAK2 V617F | 68 (85.0) | 15 (62.5) | 49 (98.0) | 4 (66.7) |
| CALR | 5 (6.3) | 4 (16.7) | 0 | 1 (16.7) |
| Non-JAK2 V617F and CALR | 7 (8.8) | 5 (20.8) | 1 (2.0) | 1 (16.7) |
| History of smoking, n (%) | 20 (25.0) | 7 (29.2) | 13 (26.0) | 0 |
| Comorbidity, n (%) | ||||
| Hypertension | 33 (41.3) | 7 (29.2) | 23 (46.0) | 3 (50.0) |
| Diabetes | 11 (13.8) | 7 (29.2) | 4 (8.0) | 0 |
| Treatment, n (%) | ||||
| Hydroxyurea | 65 (81.3) | 18 (75.0) | 45 (90.0) | 2 (33.3) |
| Aspirin | 67 (83.8) | 20 (83.3) | 47 (94.0) | 0 |
| Anticoagulant | 3 (3.8) | 1 (4.2) | 2 (4.0) | 0 |
| Anagrelide | 4 (5.0) | 2 (8.3) | 2 (4.0) | 0 |
| Ruxolitinib | 1 (1.3) | 0 | 0 | 1 (16.7) |
| Clopidogrel | 3 (3.8) | 1 (4.2) | 2 (4.0) | 0 |
| History of thrombosis +, n (%) | 17 (21.3) | 5 (20.8) | 12 (24.0) | 1 (16.7) |
| Venous DVT or PE | 0 | 0 | 0 | 0 |
| Portal vein thrombosis | 1 (1.3) | 0 | 1 (2.0) | 0 |
| Arterial MI | 5 (6.3) | 2 (8.3) | 3 (6.0) | 0 |
| Stroke | 11 (13.8) | 2 (8.3) | 8 (16.0) | 1 (16.7) |
| PAD | 1 (1.3) | 1 (4.2) | 0 | 0 |
| Time from diagnosis, median (range), years | 5.5 (0.1–17.4) | 4.0 (0.2–13.6) | 6.0 (0.1–17.4) | 3.0 (0.4–6.3) |
| Waist circumference * (mean ± SD), inches | 31.9 ± 4.4 | 31.9 ± 5.5 | 31.9 ± 4.0 | 30.8 ± 3.7 |
| C-reactive protein level #, median (range), mg/L | 3.1 (0.6–21.3) | 3.0 (1.0–12.4) | 3.1 (0.6–17.7) | 3.0 (2.9–21.3) |
| Total Cholesterol (mean ± SD), mg/dL | 152.8 ± 35.6 | 155.0 ± 37.3 | 154.4 ± 32.9 | 130.7 ± 49.4 |
| LDL (mean ± SD), mg/dL | 85.0 ± 29.2 | 83.1 ± 34.4 | 87.7 ± 24.2 | 69.7 ± 43.4 |
| HDL (mean ± SD), mg/dL | 44.8 ± 13.2 | 49.1 ± 15.4 | 44.0 ± 10.7 | 34.2 ± 16.4 |
| CBC at date of diagnosis (mean ± SD) | ||||
| Hemoglobin, g/dL | 15.9 ± 3.6 | 12.6 ± 2.0 | 18.1 ± 2.1 | 10.3 ± 2.3 |
| Hematocrit, % | 49.7 ± 6.9 | 38.9 ± 5.7 | 57.0 ± 6.9 | 32.7 ± 6.8 |
| WBC count, × 109/L | 17.0 ± 8.8 | 12.5 ± 4.1 | 17.6 ± 8.4 | 29.9 ± 12.7 |
| Platelet count, × 109/L | 836.1 ± 433.1 | 119.2 ± 406.1 | 723.7 ± 340.2 | 349.3 ± 227.1 |
| CBC at date of enrollment (mean ± SD) | ||||
| Hemoglobin, g/dL | 12.6 ± 2.3 | 12.4 ± 1.8 | 13.2 ± 2.1 | 8.7 ± 1.7 |
| Hematocrit, % | 38.3 ± 6.4 | 37.5 ± 5.3 | 40.0 ± 5.9 | 28.1 ± 5.0 |
| WBC count, × 109/L, median (range) | 7.9 (3.0–62.0) | 6.0 (3.0–30.1) | 9.5 (3.4–53.6) | 18.7 (5.6–62.0) |
| Platelet count, × 109/L | 488.1 ± 271.8 | 589.4 ± 310.1 | 473.0 ± 239.0 | 208.3 ± 141.0 |
| Results | Total (n = 80) | ET (n = 24) | PV (n = 50) | PMF (n = 6) | p Value |
|---|---|---|---|---|---|
| Prevalence of arterial stiffness, n (%) | 51 (63.8) | 17 (70.8) | 30 (60.0) | 4 (66.7) | 0.655 |
| CAVI (mean ± SD) | 8.4 ± 8.3 | 8.5 ± 1.3 | 8.3 ± 1.2 | 8.7 ± 1.7 | 0.459 |
| 10-yr CV risk by Thai CV risk score, %, median (range) | 13.6 (0.7–30.0) | 10.9 (0.7–30.0) | 15.1 (0.9–30.0) | 11.00 (8.7–23.2) | 0.844 |
| MPNs Patients (n = 66) | Matched Control (n = 197) | p Value | |
|---|---|---|---|
| Age, median (IQR), years | 64.3 (59–71) | 63.4 (56–71) | 0.574 |
| Male, n (%) | 34 (51.5) | 106 (53.8) | 0.747 |
| 10-year CV risk by Thai CV risk score, median (IQR) | 16.0 (9.6–23.9) | 14.7 (8.2–21.0) | 0.960 |
| CAVI, mean ± SD | 8.5 ± 1.3 | 8.4 ± 1.4 | 0.487 |
| Presence of arterial stiffness, n (%) | 43 (65.2) | 120 (60.9) | 0.539 |
| Results | N (%) | CAVI (Mean ± SD) | p Value |
|---|---|---|---|
| Myeloproliferative neoplasms | 0.459 | ||
| ET | 24 (30.0) | 8.5 ± 1.3 | |
| PV | 50 (62.5) | 8.3 ± 1.2 | |
| PMF | 6 (7.5) | 8.7 ± 1.7 | |
| Mutation status | 0.474 | ||
| JAK2 V617F | 68 (81.3) | 8.3 ± 1.3 | |
| CALR | 5 (6.3) | 8.4 ± 0.9 | |
| Non-JAK2 or CALR | 7 (8.8) | 8.6 ± 1.6 | |
| Time from diagnosis | 0.253 | ||
| Less than 1 year | 12 (15.0) | 8.2 ± 1.5 | |
| 2–5 years | 27 (33.7) | 8.5 ± 1.4 | |
| 6–10 years | 28 (35.0) | 8.1 ± 0.9 | |
| More than 11 years | 13 (16.3) | 8.8 ± 1.3 | |
| Age | <0.005 | ||
| ≥60 years | 53 (66.3) | 8.8 ± 0.2 | |
| <60 years | 27 (33.7) | 7.6 ± 0.2 | |
| History of thrombosis | 0.055 | ||
| Thrombosis | 17 (21.3) | 7.9 ± 0.9 | |
| No thrombosis | 58 (78.7) | 8.5 ± 1.3 | |
| WBC counts at diagnosis | 0.978 | ||
| <10 × 109/L | 12 (15.0) | 8.4 ± 1.1 | |
| ≥10 × 109/L | 68 (85.0) | 8.3 ± 1.3 | |
| WBC counts at CAVI performed | 0.121 | ||
| <10 × 109/L | 51 (63.8) | 8.2 ± 1.2 | |
| ≥10 × 109/L | 29 (36.3) | 8.7 ± 1.3 | |
| Platelet counts at diagnosis | 0.844 | ||
| <450 × 109/L | 17 (21.3) | 8.4 ± 1.4 | |
| ≥450 × 109/L | 63 (78.8) | 8.4 ± 1.2 | |
| Platelet counts at CAVI performed | 0.844 | ||
| <450 × 109/L | 44 (55.0) | 8.4 ± 1.4 | |
| ≥450 × 109/L | 36 (45.0) | 8.4 ± 1.2 | |
| Response of treatment ET * | 0.965 | ||
| Complete remission | 7 (29.2) | 8.4 ± 1.7 | |
| Partial remission | 6 (25.0) | 8.6 ± 1.2 | |
| No response | 11 (45.8) | 8.5 ± 1.2 | |
| Response of treatment PV * | 0.344 | ||
| Complete remission | 19 (38.0) | 8.1 ± 1.5 | |
| Partial remission | 14 (28.0) | 8.1 ± 1.2 | |
| No response | 17 (34.0) | 8.6 ± 1.2 | |
| Response of treatment PMF * | 0.672 | ||
| Clinical improvement | 1 (16.7) | 7.1 | |
| Stable disease | 2 (33.3) | 9.3 ± 1.5 | |
| Symptom response | 3 (50.0) | 8.8 ± 2.1 | |
| Treatment with aspirin | 0.103 | ||
| Yes | 67 (83.7) | 8.5 ± 1.2 | |
| No | 13 (16.3) | 7.9 ± 1.4 | |
| Treatment with hydroxyurea | 0.579 | ||
| Yes | 65 (81.2) | 8.3 ± 1.2 | |
| No | 15 (18.3) | 8.5 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindaluang, T.; Rattarittamrong, E.; Chai-Adisaksopha, C.; Piriyakhuntorn, P.; Norasetthada, L.; Tantiworawit, A.; Rattanathammethee, T.; Hantrakool, S.; Hantrakun, N.; Punnachet, T.; et al. Prevalence of Arterial Stiffness Determined by Cardio-Ankle Vascular Index in Myeloproliferative Neoplasms. J. Clin. Med. 2025, 14, 6944. https://doi.org/10.3390/jcm14196944
Jindaluang T, Rattarittamrong E, Chai-Adisaksopha C, Piriyakhuntorn P, Norasetthada L, Tantiworawit A, Rattanathammethee T, Hantrakool S, Hantrakun N, Punnachet T, et al. Prevalence of Arterial Stiffness Determined by Cardio-Ankle Vascular Index in Myeloproliferative Neoplasms. Journal of Clinical Medicine. 2025; 14(19):6944. https://doi.org/10.3390/jcm14196944
Chicago/Turabian StyleJindaluang, Thanakharn, Ekarat Rattarittamrong, Chatree Chai-Adisaksopha, Pokpong Piriyakhuntorn, Lalita Norasetthada, Adisak Tantiworawit, Thanawat Rattanathammethee, Sasinee Hantrakool, Nonthakorn Hantrakun, Teerachat Punnachet, and et al. 2025. "Prevalence of Arterial Stiffness Determined by Cardio-Ankle Vascular Index in Myeloproliferative Neoplasms" Journal of Clinical Medicine 14, no. 19: 6944. https://doi.org/10.3390/jcm14196944
APA StyleJindaluang, T., Rattarittamrong, E., Chai-Adisaksopha, C., Piriyakhuntorn, P., Norasetthada, L., Tantiworawit, A., Rattanathammethee, T., Hantrakool, S., Hantrakun, N., Punnachet, T., Niprapan, P., Gunaparn, S., & Phrommintikul, A. (2025). Prevalence of Arterial Stiffness Determined by Cardio-Ankle Vascular Index in Myeloproliferative Neoplasms. Journal of Clinical Medicine, 14(19), 6944. https://doi.org/10.3390/jcm14196944







