1. Introduction
Moyamoya disease (MMD) is an idiopathic chronic steno-occlusive cerebrovascular disorder characterized by progressive narrowing of the intracranial internal carotid arteries and their main branches, with development of abnormal basal collateral vessels (“moyamoya vessels”). The primary treatment goals for MMD are to increase cerebral blood flow (CBF), improve cerebrovascular reserve, and prevent future ischemic or hemorrhagic stroke through revascularization. Surgical revascularization, either direct, indirect, or a combination of both, is the mainstay of therapy for achieving these aims [
1,
2]. In particular, direct superficial temporal artery–middle cerebral artery (STA-MCA) bypass has become the gold standard for adult ischemic MMD as it provides immediate augmentation of CBF.
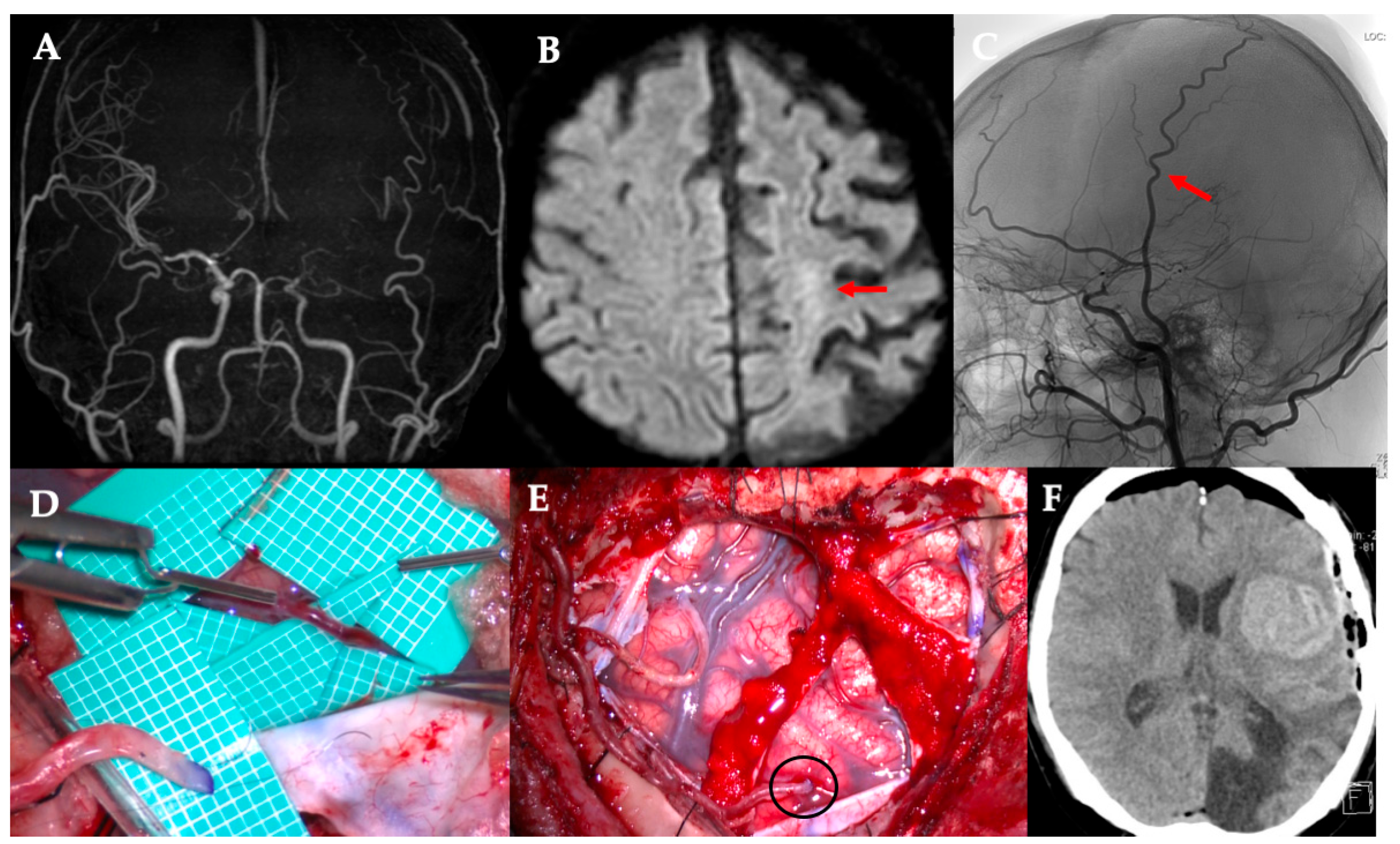
Despite its effectiveness, direct bypass surgery in adult patients with MMD remains challenging. One major concern is that STA donors may not always be optimally suited for anastomosis. The “orthodox” STA-MCA bypass uses one STA branch (frontal or parietal) for a single bypass, or both branches for a double-barrel bypass if broader coverage is needed. However, in some cases, the available cortical recipient artery is very small (diameter < 1 mm), and a larger donor artery can produce a flow surplus and technical difficulty in suturing because of a caliber mismatch. Furthermore, bypass of small recipient vessels is associated with an increased risk of hyperperfusion [
3]. Our institutional experience includes cases of cerebral hemorrhage resulting from hyperperfusion following bypass with a significant donor–recipient caliber mismatch (
Figure 1). In such situations, conventional bypass using the main STA branch may not be ideal.
Various strategies have been explored to address these issues. If an STA is unavailable or unsuitable, alternative donor arteries, such as the occipital artery and the posterior auricular artery (PAA), can be employed. For example, Torazawa et al. reported a successful long-term PAA-MCA bypass in an adult patient with MMD as a rescue procedure when the STA was not available [
4]. Other innovations include the modification of the bypass technique. For instance, Lu et al. recently reported a modified “dual-branch” STA-MCA bypass technique in which both STA branches are utilized (one for direct anastomosis and one for indirect “patch” synangiosis), resulting in lower hyperperfusion rates and improved collateralization compared to conventional surgery [
5]. In a separate approach, Lawton et al. described a side-to-side STA-MCA bypass that preserved the continuity of the STA, thereby maintaining the flow to native collaterals while forming a direct anastomosis [
6]. These modifications have shown promise in reducing complications and improving outcomes in adult patients with MMD; however, further studies are needed.
This study aimed to describe the effect of a modified STA-MCA bypass using only an STA side branch as the donor used in our institution and the initial clinical results in a series of adult patients. The findings of this study could aid in the treatment of MMD.
2. Materials and Methods
2.1. Patient Selection
We retrospectively evaluated 50 consecutive cases of MMD treated by a single surgeon between 2012 and 2025. All patients underwent combined direct and indirect revascularization. From this cohort, we identified patients who underwent a modified direct STA-MCA bypass using an STA side-branch donor. Five cases (five hemispheres in four patients) were included. Patients (aged >18 years) had angiographically confirmed MMD and presented with either ischemic symptoms or hemorrhagic stroke. The modified bypass was selected intraoperatively when the operating surgeon judged that conventional STA-MCA anastomosis using the main STA branch was not ideal (for example, due to a very small recipient artery or suboptimal STA anatomy). Clinical data and operative records were reviewed for all the included cases.
This series comprised five hemispheres in four adult patients. Unless otherwise specified, operative and imaging outcomes are summarized per hemisphere (n = 5); clinical outcomes including modified Rankin Scale (mRS) are summarized per patient (n = 4).
This study was approved by our institutional review board and conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Instead of obtaining written informed consent from all participants, an opt-out notice was posted on our institutional website prior to patient inclusion in this study.
2.2. Surgical Technique
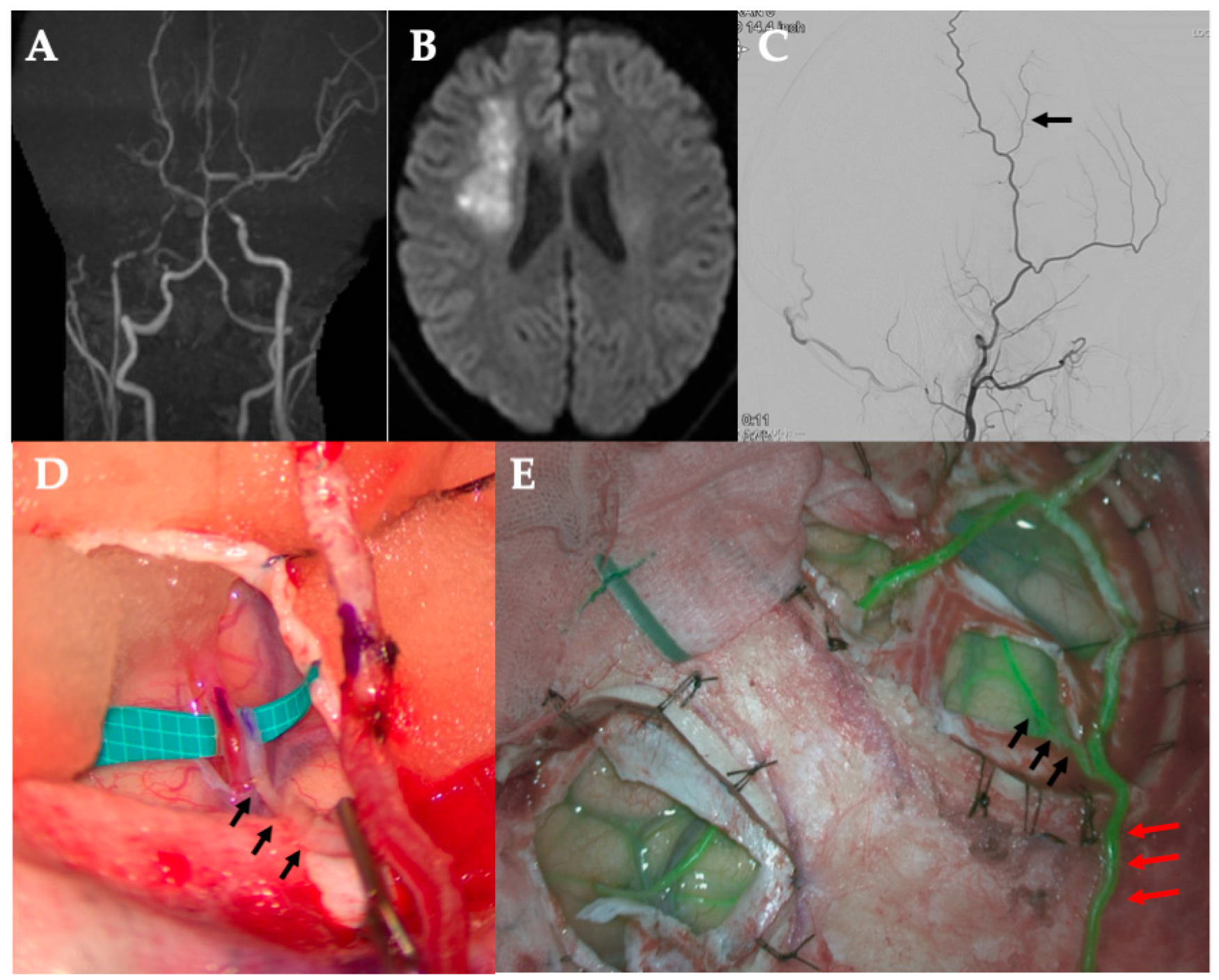
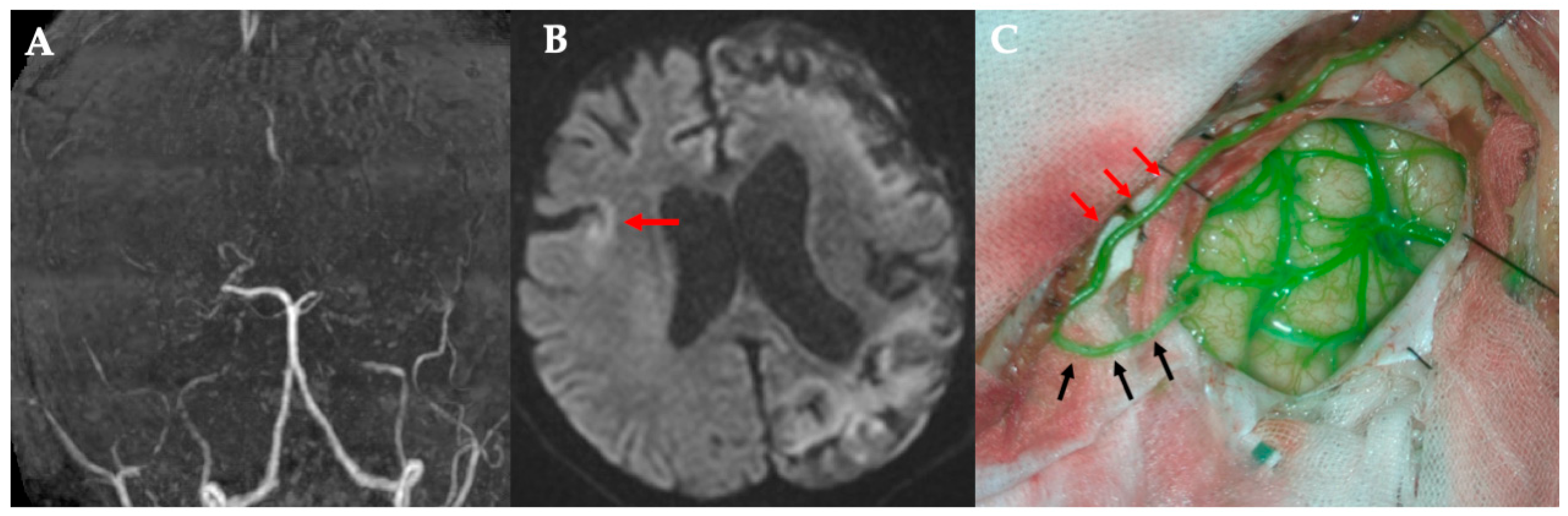
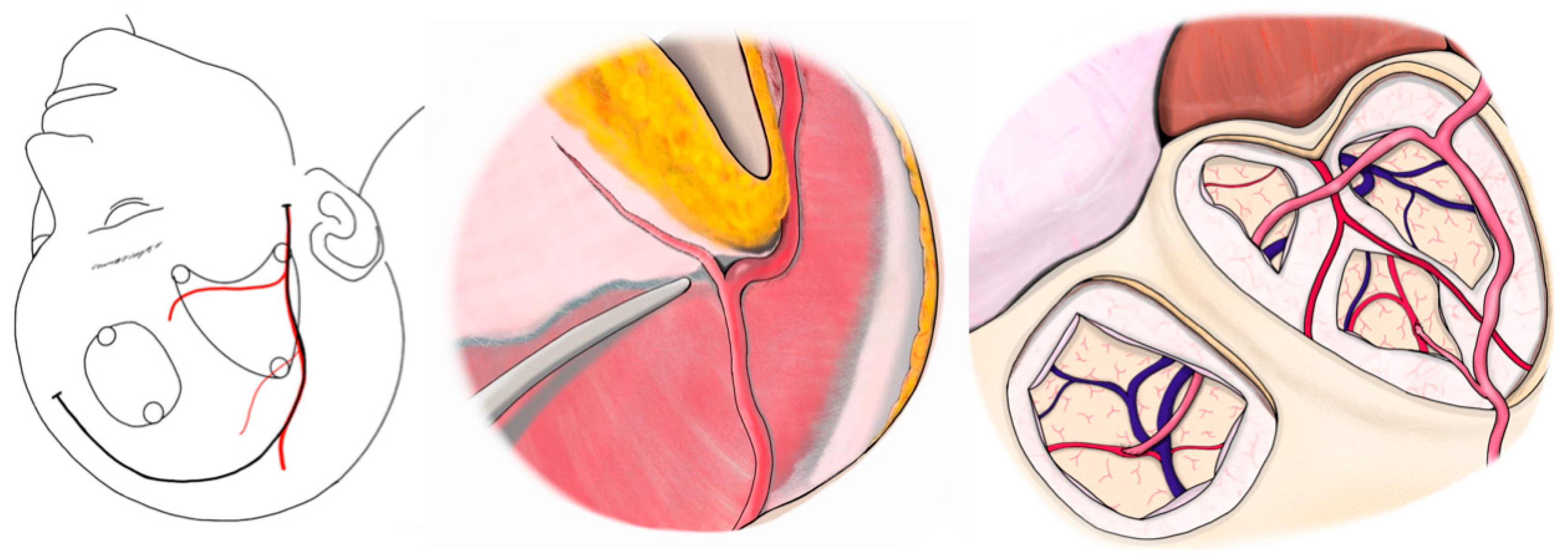
All surgeries were performed under general anesthesia, with the patient’s head fixed in the supine position. A curvilinear frontotemporal skin incision was made to expose the STA and its branches. Preoperative computed tomography angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA) were used to evaluate STA anatomy and identify suitable M4 cortical recipients. Under an operating microscope, the STA—including its trunk and parietal (temporal) branch—was dissected free from the surrounding scalp tissue and preserved in all cases. The frontal branch was dissected and preserved only in cases where bypass was performed at two sites, allowing donor options to be optimized accordingly.
After exposing the recipient artery, the final donor selection (trunk vs. side branch) was based on direct in vivo assessment of vessel caliber and reach without tension. Calibers of the donor side branch and M4 recipient vessels were measured in vivo intraoperatively using a rubber sheet with micro-measurements. Each measurement was performed by a single observer, and values were confirmed by an independent second observer to ensure reproducibility.
In the modified approach, a small-caliber STA side branch—originating from either the frontal or parietal division—was chosen as the donor vessel. It was mobilized sufficiently to reach the recipient site while maintaining its proximal connection to the STA trunk. The STA trunk and other branches were left intact to maintain scalp perfusion.
A standard frontotemporal craniotomy or craniectomy was performed. The dura was opened, preserving meningeal STA branches when present. The M4 cortical branch of the MCA, typically within the ischemic region, was selected as the recipient. After temporary microclip placement, an arteriotomy was performed, and an end-to-side anastomosis between the STA side branch and MCA branch was completed using interrupted 10-0 nylon sutures. Temporary clips were then removed to restore flow.
Bypass patency was confirmed intraoperatively with indocyanine green (ICG) videoangiography in all cases. No revisions were needed. After hemostasis, the dura was loosely closed. The STA and its remaining branches were laid on the brain surface as an indirect bypass. The bone flap was replaced without graft compression, followed by standard closure of the galea and skin.
2.3. Postoperative Management and Follow-Up
All patients were administered a single antiplatelet drug in the preoperative period to promote graft patency and were monitored in an intensive care unit with strict blood pressure control to mitigate the risk of hyperperfusion. Neurological examinations were frequently performed. Signs of cerebral hyperperfusion syndrome (CHS; e.g., headache, seizures, and focal deficits) were specifically observed during the first week after surgery. Single-photon emission computed tomography (SPECT) was performed within 1–2 days after surgery to evaluate hemodynamics. The ratio of the resting blood flow in the bypassed region of interest (A: mL/min/100 g) to that in the contralateral region of interest (B: mL/min/100 g) was calculated (A/B), and the rates of increase in this ratio before and after surgery were measured. Hospital discharge occurred approximately 2 weeks after surgery in each case. Follow-ups included outpatient clinical visits and brain imaging. Follow-up imaging was scheduled with DSA at 1 year in principle. In addition, all patients underwent serial magnetic resonance imaging (MRI) examinations during the follow-up period. Wound events were monitored before discharge and at each subsequent outpatient visit. Scalp trophism was evaluated by visual inspection and palpation.
The clinical outcome measures at the last follow-up included any recurrent ischemic events (transient ischemic attacks [TIAs] or strokes), hemorrhagic events, and the patient’s functional status (mRS). No formal statistical analysis was performed because of the small sample size of this case series. The results were presented descriptively.
4. Discussion
In this case series, we demonstrated that a modified STA-MCA bypass using only an STA side branch as the donor is a feasible and effective surgical option for adult patients with MMD in selected scenarios. All five cases achieved successful revascularization with this technique, as evidenced by 100% bypass patency and the absence of postoperative CHS or other major complications. These initial results suggest that using a smaller-caliber STA branch can provide sufficient cerebral perfusion while potentially mitigating the risks associated with a standard high-flow STA graft in certain patients.
The avoidance of CHS in our study was a notable finding. CHS is a well-recognized complication of direct bypass surgery for MMD, especially in adults with impaired autoregulation. Uchino et al. reported that symptomatic hyperperfusion occurred in 31.5% of adult patients with MMD after direct bypass, compared to only 5% in children [
7]. The risk of CHS correlates with excessive flow through the bypass relative to the capacity of the recipient bed. Using a small side-branch donor, our technique inherently limits the flow introduced into the intracranial circulation. The narrower lumen and lower flow capacity of the side branch act as a natural “flow optimizing,” potentially preventing the sudden overperfusion of the microvasculature that can occur with a larger STA graft. Consistent with this observation, none of our five cases exhibited CHS, although the sample size was too small to draw definitive conclusions. Similarly, Lu et al. found that the modified dual-branch bypass approach was associated with a lower incidence of hyperperfusion (18.2% vs. 23.3%) and improved angiographic collateral grades compared to conventional bypass in adult patients with MMD [
5]. Therefore, these findings support the concept that tailoring bypass flow to patient needs can improve safety.
Another advantage of the side-branch bypass technique is preservation of the scalp blood supply. Because we harvested only a small branch of the STA and left the main STA trunk intact, the overall perfusion of the scalp was less compromised than in a traditional double-barrel bypass (where both STA branches are used). Scalp wound healing complications have been associated with double-barrel bypass procedures that sacrifice both STA branches due to the extensive disruption of scalp circulation. No wound complications were observed in this study. All incisions healed primarily without issues, which is an encouraging outcome, particularly in patients with risk factors for poor wound healing (such as older age and comorbidities). By leaving the main STA in situ, our technique likely helps maintain adequate blood flow to the skin and subcutaneous tissues.
The concept of modifying bypass techniques to optimize outcomes has been gaining attention in the treatment of MMD. Traditionally, a single high-flow STA-MCA bypass has been the standard; however, there is increasing recognition that a more nuanced approach may benefit certain adult patients. Lawton et al. pioneered a side-to-side STA-MCA bypass to preserve native collateral flow and demonstrated that it was technically feasible and provided effective flow augmentation without sacrificing the continuity of the donor STA’s continuity [
6]. Our approach differs in that we still perform an end-to-side anastomosis (sacrificing the side branch’s distal continuity), but we achieve flow moderation by selecting a smaller donor vessel. Both approaches aim to protect the brain from hemodynamic extremes, either by preserving collaterals or reducing flow, and neither approach was associated with CHS in the reported cases.
The efficacy of this side-branch bypass is supported by favorable clinical outcomes. All patients remained stroke-free during follow-up, and their preoperative ischemic symptoms improved or resolved. This suggests that although the bypass flow may be lower than that of a larger-caliber graft, it is sufficient to augment perfusion in chronic ischemic areas. It is well known that even modest increases in CBF can significantly reduce the risk of ischemic events in patients with MMD by improving hemodynamic reserve. Additionally, indirect collateral formation likely continued postoperatively in our patients (typically after revascularization), further augmenting CBF over time. We did not perform quantitative CBF measurements postoperatively in this small series. However, clinically, none of the patients showed signs of inadequate perfusion.
From a technical standpoint, the use of a small side branch as a donor requires careful microsurgery. Anastomosis is performed between two small vessels, which can be more technically demanding than the standard STA-MCA bypass. However, with modern microsurgical instruments and expertise, fine vessel anastomoses are now routinely performed by experienced cerebrovascular surgeons. In our study, all anastomoses were successfully completed without revision or intraoperative bypass failure. We found that ensuring a tension-free graft was critical for adequate mobilization of the side branch, and, if necessary, gentle dissection of the surrounding tissue was performed to maximize the reach. An interposition graft was not required in any case, and the inherent length of the STA side branches was sufficient for a short distance from the cortical surface.
It is important to acknowledge that the side-branch bypass technique is not intended to replace the standard STA-MCA bypass in all cases but rather to serve as an additional option tailored to specific circumstances. In cases in which the STA is large and the recipient artery is of good caliber, conventional direct bypass remains an excellent procedure with proven long-term efficacy. Conversely, in cases where donor–recipient mismatch or hyperperfusion risk is a major concern, our results indicate that opting for a smaller donor can be a prudent strategy. Thus, surgeons should consider the full spectrum of bypass techniques (single, double, side-to-side, and side-branch) and select the one that best fits the patient’s angiographic anatomy and hemodynamic profile.
Our study has some limitations. First, the number of cases was small (
n = 5), limiting the generalizability of the findings. The absence of complications in five cases, although reassuring, could be due to chance, and larger studies are needed to determine complication rates more accurately. Second, this study was retrospective, without a control group. We did not directly compare the outcomes of our side-branch bypass with those of the conventional bypass in similar patients. Thus, we cannot conclusively claim that the modified technique is superior; only that it was successful and safe in this series. Third, we relied on clinical follow-up and non-invasive imaging to infer bypass patency and efficacy; formal angiographic confirmation of long-term patency was not obtained in every case. A future study with routine postoperative angiography at 6–12 months would be valuable for quantifying the extent of revascularization. The Matsushima grading system (Grade A, B, C) is commonly used to categorize the extent of collateral filling after indirect bypass and could be applied to assess our direct/indirect combined results as well [
8]. We anticipate that our bypasses will achieve good collateral grades; however, we defer formal grading until angiographic data become available. Finally, long-term follow-up beyond 3 years is needed to ensure that the benefits of side-branch bypass persist and that no late complications occur.
Therefore, our initial experience suggests that the STA side-branch to MCA bypass is a viable adjunct in the surgical management of adult MMD. This technique provided effective revascularization in five cases who may have been at an elevated risk for hyperperfusion or technical issues with a standard bypass. This modification leverages the fundamental principle of microsurgery, matching the size of the donor and recipient vessels to improve the balance of flow. Although further evaluation in larger cohorts is warranted, STA side-branch bypass offers a promising option for surgeons to add to their armamentarium and tailor revascularization strategies to individual patient needs.
Future studies could incorporate direct intraoperative flow quantification to more precisely evaluate the hemodynamic impact of the side branch STA–MCA bypass. Such measurements would allow a more detailed comparison between the conventional STA–MCA bypass and the modified approach described in this study, potentially clarifying the theoretical benefits in hyperperfusion risk mitigation.