Predicting Acute Kidney Injury in Acute Rhabdomyolysis
Abstract
1. Introduction
2. Acute Kidney Injury
3. Traditional Biomarkers in AKI Prediction
3.1. Creatine Kinase
3.2. Myoglobin
3.3. Alanine Aminotransferase and Aspartate Aminotransferase
3.4. Lactate Dehydrogenase, Aldolase, Carbonic Anhydrase III
3.5. Metabolic Acidosis and Lactate
3.6. Calcium-Phosphate
3.7. Potassium
3.8. Uric Acid
3.9. Markers of Inflammation and Coagulation Cascade
4. Novel Biomarkers in AKI Prediction
4.1. Muscle Related Markers
4.2. Kidney Related Markers
4.3. Biomarkers and Chronic Kidney Disease Risk
5. Other Considerations in AKI Prediction
5.1. Age and Sex
5.2. Etiology of Rhabdomyolysis
5.3. Concurrent Sepsis
5.4. Chronic Kidney Disease
6. Risk Prediction Models
6.1. Artificial Intelligence Models
6.2. Examples of Risk Prediction Models
7. Limitations of Prediction Models
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | Acute kidney injury |
| ALT | Alanine aminotransferase |
| AI | Artificial intelligence |
| AST | Aspartate aminotransferase |
| AUC | Area under receiver operating curve |
| Ca2+ | Calcium |
| CK | Creatine kinase (creatine phosphokinase) |
| CKD | Chronic kidney disease |
| DIC | Disseminated intravascular coagulation |
| DL | Deep learning |
| eGFR | Estimated glomerular filtration rate |
| GDF-15 | Growth differentiation factor-15 |
| HCO3− | Bicarbonate |
| ICU | Intensive care unit |
| K+ | Potassium |
| KIM-1 | Kidney injury molecule-1 |
| KDIGO | Kidney Disease Global Outcomes |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| LDH | Lactate dehydrogenase |
| LFT | Liver function test |
| ML | Machine learning |
| MAKE | Major adverse kidney events |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NGAL | Neutrophil gelatinase-associated lipocalin |
| OR | Odds ratio |
| PO4− | Phosphate (phosphorus) |
| RRT | Renal replacement therapy |
| SOFA | Sequential Organ Failure Assessment |
| TNFR | Tumor necrosis factor receptor |
References
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef]
- Better, O.S.; Abassi, Z.A. Early fluid resuscitation in patients with rhabdomyolysis. Nat. Rev. Nephrol. 2011, 7, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.F.; Burfeind, K.G.; Malinoski, D.; Hutchens, M.P. Molecular Mechanisms of Rhabdomyolysis-Induced Kidney Injury: From Bench to Bedside. Kidney Int. Rep. 2023, 8, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol. 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Yang, C.W.; Li, S.; Dong, Y.; Paliwal, N.; Wang, Y. Epidemiology and the Impact of Acute Kidney Injury on Outcomes in Patients with Rhabdomyolysis. J. Clin. Med. 2021, 10, 1950. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Parikh, C.R.; Himmelfarb, J.; Chinchilli, V.M.; Liu, K.D.; Coca, S.G.; Garg, A.X.; Hsu, C.Y.; Siew, E.D.; Wurfel, M.M.; et al. A prospective cohort study of acute kidney injury and kidney outcomes, cardiovascular events, and death. Kidney Int. 2021, 99, 456–465. [Google Scholar] [CrossRef]
- Gunal, A.I.; Celiker, H.; Dogukan, A.; Ozalp, G.; Kirciman, E.; Simsekli, H.; Gunay, I.; Demircin, M.; Belhan, O.; Yildirim, M.A.; et al. Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J. Am. Soc. Nephrol. 2004, 15, 1862–1867. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Siew, E.D.; Davenport, A. The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int. 2015, 87, 46–61. [Google Scholar] [CrossRef]
- Pan, N.; Wu, Y.; Yang, B.; Zhang, M.; He, Y.; Wang, Z.; Tan, L.; Zhang, L. The liver and blood cells are responsible for creatine kinase clearance in blood Circulation: A retrospective study among different human diseases. Clin. Chim. Acta 2023, 544, 117335. [Google Scholar] [CrossRef]
- Simpson, J.P.; Taylor, A.; Sudhan, N.; Menon, D.K.; Lavinio, A. Rhabdomyolysis and acute kidney injury: Creatine kinase as a prognostic marker and validation of the McMahon Score in a 10-year cohort: A retrospective observational evaluation. Eur. J. Anaesthesiol. 2016, 33, 906–912. [Google Scholar] [CrossRef]
- Nielsen, F.E.; Cordtz, J.J.; Rasmussen, T.B.; Christiansen, C.F. The Association Between Rhabdomyolysis, Acute Kidney Injury, Renal Replacement Therapy, and Mortality. Clin. Epidemiol. 2020, 12, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Lau Hing Yim, C.; Wong, E.W.W.; Jellie, L.J.; Lim, A.K.H. Illicit drug use and acute kidney injury in patients admitted to hospital with rhabdomyolysis. Intern. Med. J. 2019, 49, 1285–1292. [Google Scholar] [CrossRef]
- El-Abdellati, E.; Eyselbergs, M.; Sirimsi, H.; Hoof, V.V.; Wouters, K.; Verbrugghe, W.; Jorens, P.G. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: Acute kidney injury. Ann. Intensive Care 2013, 3, 8. [Google Scholar] [CrossRef]
- McMahon, G.M.; Zeng, X.; Waikar, S.S. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern. Med. 2013, 173, 1821–1828. [Google Scholar] [CrossRef]
- Candela, N.; Silva, S.; Georges, B.; Cartery, C.; Robert, T.; Moussi-Frances, J.; Rondeau, E.; Rebibou, J.M.; Lavayssiere, L.; Belliere, J.; et al. Short- and long-term renal outcomes following severe rhabdomyolysis: A French multicenter retrospective study of 387 patients. Ann. Intensive Care 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Safari, S.; Yousefifard, M.; Hashemi, B.; Baratloo, A.; Forouzanfar, M.M.; Rahmati, F.; Motamedi, M.; Najafi, I. The value of serum creatine kinase in predicting the risk of rhabdomyolysis-induced acute kidney injury: A systematic review and meta-analysis. Clin. Exp. Nephrol. 2016, 20, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, V.; Figueiredo, S.; Hamada, S.; Pochard, J.; Haines, R.W.; Prowle, J.R.; Duranteau, J.; Vigue, B.; Harrois, A. Admission serum myoglobin and the development of acute kidney injury after major trauma. Ann. Intensive Care 2021, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.A.; Rao, S.V.; Joel, J.C.; Jacob, G.G.; Anil, A.K.; Gowri, S.M.; Kandasamy, S. Predictive Value of Serum Myoglobin and Creatine Phosphokinase for Development of Acute Kidney Injury in Traumatic Rhabdomyolysis. Indian. J. Crit. Care Med. 2017, 21, 852–856. [Google Scholar] [CrossRef]
- Wu, M.; Wang, C.; Zhong, L.; Liu, Z. Serum myoglobin as predictor of acute kidney injury and 90-day mortality in patients with rhabdomyolysis after exertional heatstroke: An over 10-year intensive care survey. Int. J. Hyperth. 2022, 39, 446–454. [Google Scholar] [CrossRef]
- Vangstad, M.; Bjornaas, M.A.; Jacobsen, D. Rhabdomyolysis: A 10-year retrospective study of patients treated in a medical department. Eur. J. Emerg. Med. 2019, 26, 199–204. [Google Scholar] [CrossRef]
- Rodriguez-Capote, K.; Balion, C.M.; Hill, S.A.; Cleve, R.; Yang, L.; El Sharif, A. Utility of urine myoglobin for the prediction of acute renal failure in patients with suspected rhabdomyolysis: A systematic review. Clin. Chem. 2009, 55, 2190–2197. [Google Scholar] [CrossRef]
- Lim, A.K. Abnormal liver function tests associated with severe rhabdomyolysis. World J. Gastroenterol. 2020, 26, 1020–1028. [Google Scholar] [CrossRef]
- Weibrecht, K.; Dayno, M.; Darling, C.; Bird, S.B. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. J. Med. Toxicol. 2010, 6, 294–300. [Google Scholar] [CrossRef]
- Lim, A.K.H.; Arumugananthan, C.; Lau Hing Yim, C.; Jellie, L.J.; Wong, E.W.W.; Junckerstorff, R.K. A Cross-Sectional Study of the Relationship between Serum Creatine Kinase and Liver Biochemistry in Patients with Rhabdomyolysis. J. Clin. Med. 2019, 9, 81. [Google Scholar] [CrossRef]
- Chandel, A.; Brusher, K.; Hall, V.; Howard, R.S.; Clark, P.A. Diagnosis and Management of Rhabdomyolysis in the Absence of Creatine Phosphokinase: A Medical Record Review. Mil. Med. 2019, 184, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.; Massry, S.G. Reversible hepatic dysfunction associated with rhabdomyolysis. Am. J. Nephrol. 1990, 10, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yuan, Q.; Mao, Z.; Hu, P.; Wu, R.; Liu, X.; Hong, Q.; Chi, K.; Geng, X.; Sun, X. Development and validation of a model for the early prediction of the RRT requirement in patients with rhabdomyolysis. Am. J. Emerg. Med. 2021, 46, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Sietsema, K.E.; Meng, F.; Yates, N.A.; Hendrickson, R.C.; Liaw, A.; Song, Q.; Brass, E.P.; Ulrich, R.G. Potential biomarkers of muscle injury after eccentric exercise. Biomarkers 2010, 15, 249–258. [Google Scholar] [CrossRef]
- Lippi, G.; Schena, F.; Ceriotti, F. Diagnostic biomarkers of muscle injury and exertional rhabdomyolysis. Clin. Chem. Lab. Med. 2018, 57, 175–182. [Google Scholar] [CrossRef]
- Beuerle, J.R.; Azzazy, H.M.; Styba, G.; Duh, S.H.; Christenson, R.H. Characteristics of myoglobin, carbonic anhydrase III and the myoglobin/carbonic anhydrase III ratio in trauma, exercise, and myocardial infarction patients. Clin. Chim. Acta 2000, 294, 115–128. [Google Scholar] [CrossRef]
- Yin, X.; Wang, W. Predictive value of serum myoglobin and lactate dehydrogenase in rhabdomyolysis-induced acute kidney injury from severe heatstroke. Am. J. Transl. Res. 2024, 16, 1477–1483. [Google Scholar] [CrossRef]
- Rodriguez, E.; Soler, M.J.; Rap, O.; Barrios, C.; Orfila, M.A.; Pascual, J. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS ONE 2013, 8, e82992. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Yeo, I.; Kim, C.; Kim, D.; Lim, J.H.; Park, K.; Jeong, J.; Kwon, H.; Cho, Y.; Park, S. Factors Associated with Acute Kidney Injury Occurrence and Prognosis in Rhabdomyolysis at the Emergency Department. Medicina 2024, 60, 105. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Y.; Shen, X.; Dou, A.; Xie, H.; Zhang, Y.; Xie, K. Utilization of lactate trajectory models for predicting acute kidney injury and mortality in patients with hyperlactatemia: Insights across three independent cohorts. Ren. Fail. 2025, 47, 2474205. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.C.; Hsu, C.W. Septic acute kidney injury patients in emergency department: The risk factors and its correlation to serum lactate. Am. J. Emerg. Med. 2019, 37, 204–208. [Google Scholar] [CrossRef]
- Hohenegger, M. Drug induced rhabdomyolysis. Curr. Opin. Pharmacol. 2012, 12, 335–339. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Ryu, H.Y.; Cha, K.S.; Sung, D.J. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2016, 5, 324–333. [Google Scholar] [CrossRef]
- Desmeules, S.; Bergeron, M.J.; Isenring, P. Acute phosphate nephropathy and renal failure. N. Engl. J. Med. 2003, 349, 1006–1007. [Google Scholar] [CrossRef]
- Jetanapirom, R.; Boonsrirat, U.; Geater, S.L.; Leelawattana, R.; Phongphithakchai, A. Impact of Calcium Phosphate Product on Acute Kidney Injury and Mortality: A Retrospective Cohort Study. Cureus 2024, 16, e64861. [Google Scholar] [CrossRef]
- Safari, S.; Ghasemi, M.; Yousefifard, M.; Ghasemi, A.; Najafi, I. Uric acid in predicting the traumatic rhabdomyolysis induced acute kidney injury; a systematic review and meta-analysis. BMC Nephrol. 2024, 25, 82. [Google Scholar] [CrossRef]
- Esmon, C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005, 131, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Tae, M.; Shin, S.; Choi, S.I. Predicting acute kidney injury in trauma using an extreme gradient boosting model. Clin. Kidney J. 2025, 18, sfaf002. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.L. Disseminated intravascular coagulation current concepts of etiology, pathophysiology, diagnosis, and treatment. Hematol. Oncol. Clin. N. Am. 2003, 17, 149–176. [Google Scholar] [CrossRef]
- Siracusa, J.; Koulmann, N.; Bourdon, S.; Goriot, M.E.; Banzet, S. Circulating miRNAs as Biomarkers of Acute Muscle Damage in Rats. Am. J. Pathol. 2016, 186, 1313–1327. [Google Scholar] [CrossRef]
- Minami, K.; Uehara, T.; Morikawa, Y.; Omura, K.; Kanki, M.; Horinouchi, A.; Ono, A.; Yamada, H.; Ohno, Y.; Urushidani, T. miRNA expression atlas in male rat. Sci. Data 2014, 1, 140005. [Google Scholar] [CrossRef]
- Chalchat, E.; Charlot, K.; Garcia-Vicencio, S.; Hertert, P.; Bauge, S.; Bourdon, S.; Bompard, J.; Farges, C.; Martin, V.; Bourrilhon, C.; et al. Circulating microRNAs after a 24-h ultramarathon run in relation to muscle damage markers in elite athletes. Scand. J. Med. Sci. Sports 2021, 31, 1782–1795. [Google Scholar] [CrossRef]
- Douvris, A.; Vinas, J.L.; Akbari, S.; Tailor, K.; Lalu, M.M.; Burger, D.; Burns, K.D. Systematic review of microRNAs in human acute kidney injury. Ren. Fail. 2024, 46, 2419960. [Google Scholar] [CrossRef] [PubMed]
- Tonomura, Y.; Matsushima, S.; Kashiwagi, E.; Fujisawa, K.; Takagi, S.; Nishimura, Y.; Fukushima, R.; Torii, M.; Matsubara, M. Biomarker panel of cardiac and skeletal muscle troponins, fatty acid binding protein 3 and myosin light chain 3 for the accurate diagnosis of cardiotoxicity and musculoskeletal toxicity in rats. Toxicology 2012, 302, 179–189. [Google Scholar] [CrossRef]
- Dihazi, H.; Koziolek, M.J.; Datta, R.R.; Wallbach, M.; Jung, K.; Heise, D.; Dihazi, G.H.; Markovic, I.; Asif, A.R.; Muller, G.A. FABP1 and FABP3 Have High Predictive Values for Renal Replacement Therapy in Patients with Acute Kidney Injury. Blood Purif. 2016, 42, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.A.; Vaidya, V.S.; Waikar, S.S.; Collings, F.B.; Sunderland, K.E.; Gioules, C.J.; Bonventre, J.V. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010, 77, 708–714. [Google Scholar] [CrossRef]
- Pommet, S.; Coisy, F.; Demattei, C.; Balaguer, L.; de Bauwere, D.P.; Grau-Mercier, L.; Markarian, T.; Bobbia, X.; Genre Grandpierre, R. Does serum neutrophil gelatinase-associated lipocalin level predict acute kidney injury in patients with acute rhabdomyolysis in the emergency department? A multicentre prospective study. BMJ Open 2024, 14, e088859. [Google Scholar] [CrossRef] [PubMed]
- Wolyniec, W.; Ratkowski, W.; Renke, J.; Renke, M. Changes in Novel AKI Biomarkers after Exercise. A Systematic Review. Int. J. Mol. Sci. 2020, 21, 5673. [Google Scholar] [CrossRef]
- Zengin, O.; Gore, B.; Yakut, M.; Yaylali, M.; Gov, M.; Donmez, S.; Celik, G.K.; Gunaydin, G.P.; Uzdogan, E.A.; Asfuroglu Kalkan, E.; et al. Development of the GDF-TRACK-AKI Score for Predicting Acute Kidney Injury in Patients with Rhabdomyolysis Due to Excessive Exercise or Trauma. Medicina 2025, 61, 1116. [Google Scholar] [CrossRef]
- Sawasawa, T.; Lin, J.D.; Wang, Y.H.; Chen, K.J.; Yang, Y.M.; Hu, S.W.; Cheng, C.W. Elevated serum GDF15 level as an early indicator of proximal tubular cell injury in acute kidney injury. Life Sci. 2024, 357, 123093. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, L.; Melchinger, I.; Thiessen-Philbrook, H.; Moledina, D.G.; Coca, S.G.; Hsu, C.Y.; Go, A.S.; Liu, K.D.; Siew, E.D.; et al. Longitudinal biomarkers and kidney disease progression after acute kidney injury. JCI Insight 2023, 8, e167731. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Packington, R.; Sewell, H.; Bartle, R.; McCole, E.; Kurth, M.J.; Richardson, C.; Shaw, S.; Akani, A.; Banks, R.E.; et al. Biomarkers During Recovery From AKI and Prediction of Long-term Reductions in Estimated GFR. Am. J. Kidney Dis. 2022, 79, 646–656.e1. [Google Scholar] [CrossRef]
- Loutradis, C.; Pickup, L.; Law, J.P.; Dasgupta, I.; Townend, J.N.; Cockwell, P.; Sharif, A.; Sarafidis, P.; Ferro, C.J. Acute kidney injury is more common in men than women after accounting for socioeconomic status, ethnicity, alcohol intake and smoking history. Biol. Sex. Differ. 2021, 12, 30. [Google Scholar] [CrossRef]
- Melli, G.; Chaudhry, V.; Cornblath, D.R. Rhabdomyolysis: An evaluation of 475 hospitalized patients. Medicine 2005, 84, 377–385. [Google Scholar] [CrossRef]
- Saverymuthu, A.; Teo, R.; Zain, J.M.; Cheah, S.K.; Yusof, A.M.; Rahman, R.A. Acute Kidney Injury following Rhabdomyolysis in Critically Ill Patients. J. Crit. Care Med. 2021, 7, 267–271. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, J.; Wang, X.; Wang, S.; Tang, Y.; Yang, L. Risk factors for severe acute kidney injury among patients with rhabdomyolysis. BMC Nephrol. 2020, 21, 498. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Ballew, S.H.; Gansevoort, R.T.; Kimm, H.; Kovesdy, C.P.; Naimark, D.; Oien, C.; Smith, D.H.; Coresh, J.; et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Age, Race, and Sex with Acute Kidney Injury. Am. J. Kidney Dis. 2015, 66, 591–601. [Google Scholar] [CrossRef]
- Tran, T.T.; Yun, G.; Kim, S. Artificial intelligence and predictive models for early detection of acute kidney injury: Transforming clinical practice. BMC Nephrol. 2024, 25, 353. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, T.; Koyner, J.L. Artificial Intelligence in Acute Kidney Injury Prediction. Adv. Chronic Kidney Dis. 2022, 29, 450–460. [Google Scholar] [CrossRef]
- Rehman, A.U.; Neyra, J.A.; Chen, J.; Ghazi, L. Machine learning models for acute kidney injury prediction and management: A scoping review of externally validated studies. Crit. Rev. Clin. Lab. Sci. 2025, 62, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.R.; Samuel, M.; Bonventre, J.V.; Celi, L.A.; Mattie, H. Machine Learning for Acute Kidney Injury Prediction in the Intensive Care Unit. Adv. Chronic Kidney Dis. 2022, 29, 431–438. [Google Scholar] [CrossRef]
- Vagliano, I.; Chesnaye, N.C.; Leopold, J.H.; Jager, K.J.; Abu-Hanna, A.; Schut, M.C. Machine learning models for predicting acute kidney injury: A systematic review and critical appraisal. Clin. Kidney J. 2022, 15, 2266–2280. [Google Scholar] [CrossRef]
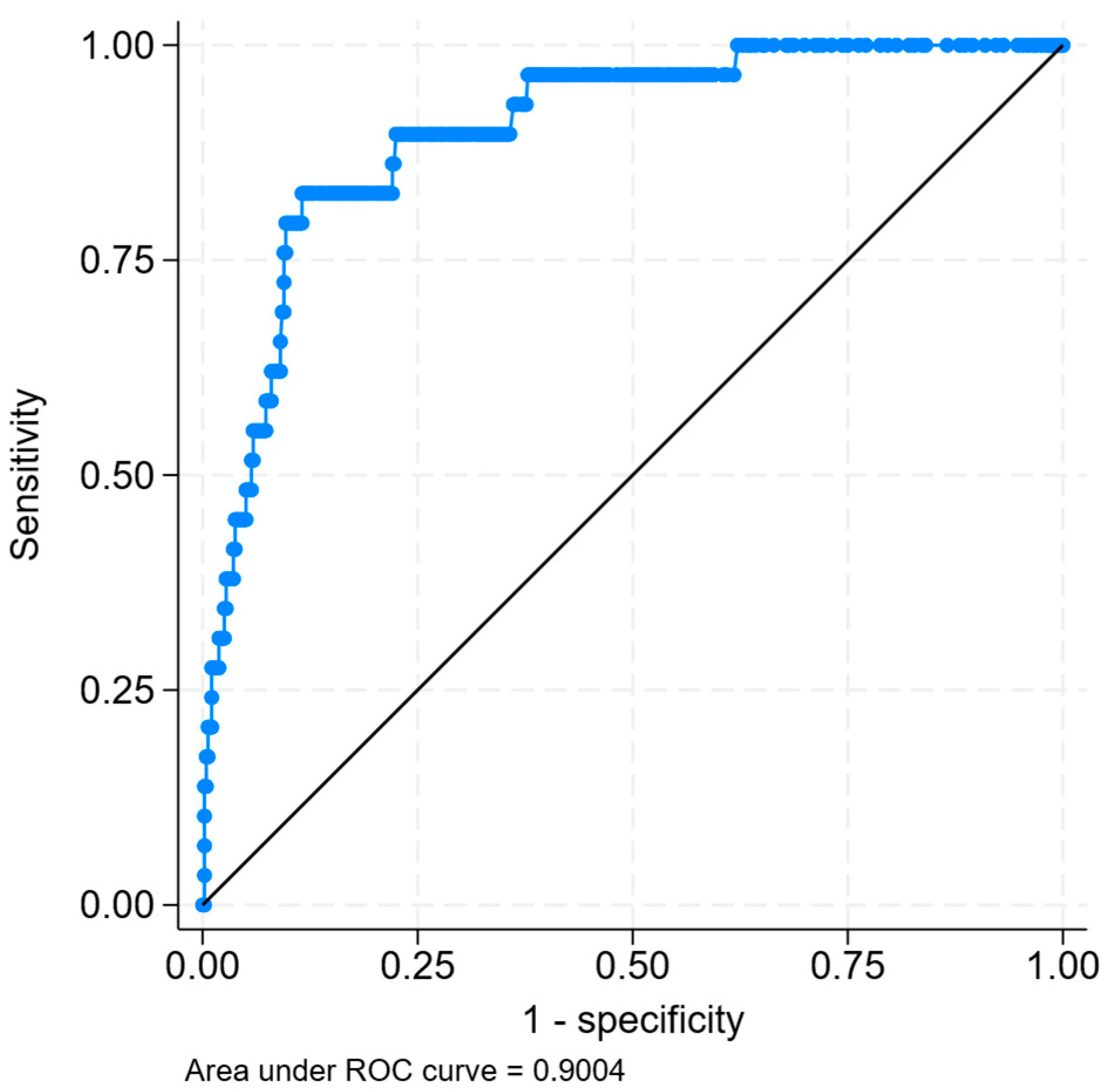
- Zhang, X.; Liang, X.; Fu, Z.; Zhou, Y.; Fang, Y.; Liu, X.; Yuan, Q.; Liu, R.; Hong, Q.; Liu, C. Interpretable machine learning model for early prediction of acute kidney injury in patients with rhabdomyolysis. Emerg. Crit. Care Med. 2024, 4, 155–162. [Google Scholar] [CrossRef]
- Poorsarvi Tehrani, P.; Malek, H. Early Detection of Rhabdomyolysis-Induced Acute Kidney Injury through Machine Learning Approaches. Arch. Acad. Emerg. Med. 2021, 9, e29. [Google Scholar] [CrossRef]
- Shi, T.; Lin, Y.; Zhao, H.; Kong, G. Artificial intelligence models for predicting acute kidney injury in the intensive care unit: A systematic review of modeling methods, data utilization, and clinical applicability. JAMIA Open 2025, 8, ooaf065. [Google Scholar] [CrossRef]
- Isshiki, R.; Asada, T.; Sumida, M.; Hamasaki, Y.; Nangaku, M.; Noiri, E.; Doi, K. Modest Impact of Serial Measurements of Acute Kidney Injury Biomarkers in an Adult Intensive Care Unit. Nephron 2018, 139, 243–253. [Google Scholar] [CrossRef]
- Horie, R.; Hayase, N.; Asada, T.; Yamamoto, M.; Matsubara, T.; Doi, K. Trajectory pattern of serially measured acute kidney injury biomarkers in critically ill patients: A prospective observational study. Ann. Intensive Care 2024, 14, 84. [Google Scholar] [CrossRef]
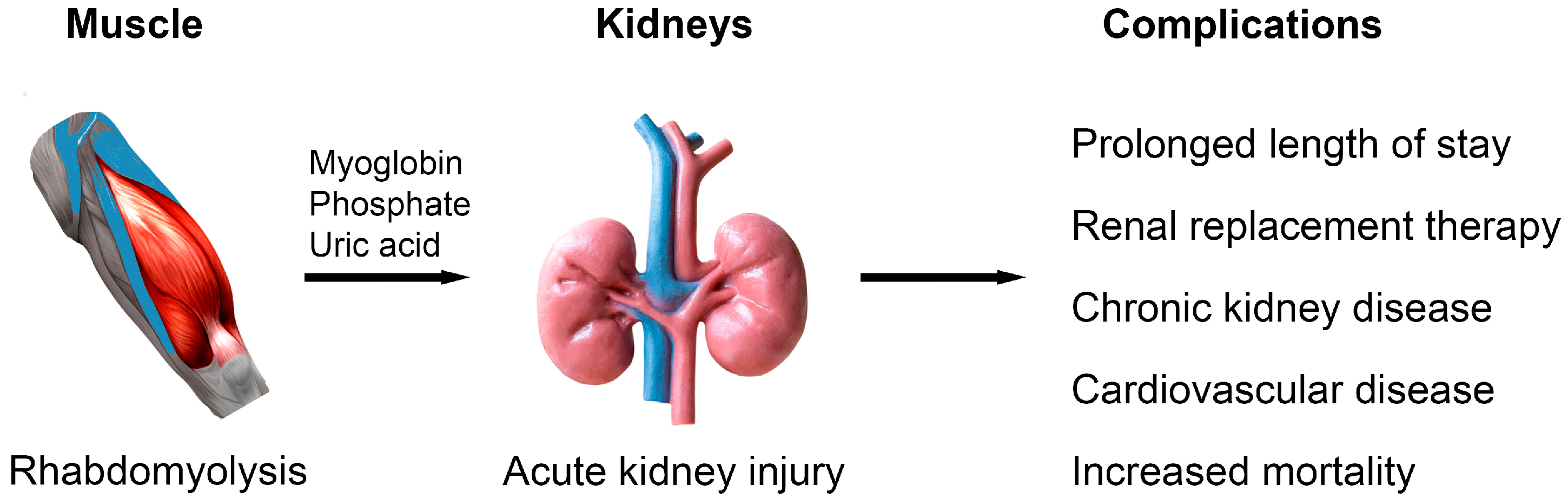
| Stage | Serum Creatinine Criteria 1 | Urine Output Criteria |
|---|---|---|
| 1 | 1.5–1.9 × baseline, or ≥0.3 mg/dL increase | <0.5 mL/kg/h for 6–12 h |
| 2 | 2.0–2.9 × baseline | <0.5 mL/kg/h for ≥12 h |
| 3 | 3.0 × baseline, or increase to ≥4.0 mg/dL, or RRT | <0.3 mL/kg/h for ≥24 h, or anuria ≥12 h |
| Raw CK | Log CK | |
|---|---|---|
| Correlation with peak creatinine, r | 0.161 (p < 0.001) | 0.250 (p < 0.001) |
| Correlation with change in creatinine, r | 0.118 (p = 0.003) | 0.161 (p < 0.001) |
| Logistic regression for RRT, pseudo-R2 | 0.052 | 0.100 |
| Logistic regression for RRT, Akaike IC | 233.6 | 221.9 |
| Logistic regression for RRT, Bayesian IC | 242.6 | 230.9 |
| Variable | Odds Ratio | 95% C.I. | p Value |
|---|---|---|---|
| ALT, per one log U/L increase | 2.26 | 1.48, 3.45 | <0.001 |
| Presence of sepsis syndrome | 4.67 | 1.88, 11.5 | 0.001 |
| Hyperkalemia, potassium > 6.0 mmol/L | 3.22 | 1.25, 8.27 | 0.016 |
| Hypocalcemia, calcium < 1.80 mmol/L | 4.35 | 1.73, 10.9 | 0.002 |
| CKD, eGFR < 30 mL/min/1.73 m2 | 7.55 | 1.33, 43.2 | 0.023 |
| McMahon et al. [16] | Liu et al. [29] | |
|---|---|---|
| Year of publication | 2013 | 2024 |
| Population studied | General hospitalized patients, combination of medical and surgical patients | Patients admitted to multiple intensive care units |
| CK inclusion threshold | 5000 U/L | 1000 U/L |
| Number of patients | Derivation, n = 1397 External validation, n = 974 | Derivation, n = 656 Internal validation, n = 282 External validation, n = 321 |
| Age of patients | Mean 52.4 (SD, 19.7) years | Mean 56.0 (SD, 12.1) years |
| Top 5 causes of rhabdomyolysis | Trauma (26.3%) Immobilization (18.1%) Sepsis (9.9%) Vascular surgery (8.1%) Cardiac surgery (5.9%) | Trauma (20.0%) Metabolic/electrolyte (15.9%) Infection (12.2%) Alcohol (9.3% Myopathy (8.5%) |
| Outcome | Composite RRT or death | RRT |
| AKI definition | KDIGO creatinine criteria | KDIGO criteria |
| Incidence of AKI | 47.7% | 71.3% |
| Incidence of RRT | 8.0% | 15.9% |
| Inpatient mortality | 14.1% | 10.7% |
| Model variables | Biochemical: Peak CK, initial creatinine, Ca2+, PO4− HCO3− Clinical: Age, female sex, cause of rhabdomyolysis | Biochemical: Peak CK, baseline creatinine, Ca2+, PO4−, aspartate aminotransferase, albumin Clinical: Age, atrial fibrillation |
| Model performance | Derivation, AUC 0.82 External validation, AUC 0.83 | Derivation, AUC 0.82 Internal validation, AUC 0.79 External validation, AUC 0.82 |
| Characteristic | Description |
|---|---|
| Discrimination | Correctly identifies patients who develop AKI. May be affected by poor quality or insufficient data, or unrecognized confounders. |
| Calibration | Good agreement between predicted and observed outcome. Poor calibration may be due to incorrect model specification or assumptions. |
| Validation | Performs accurately on new data in real-world scenarios. External validation ensures model performs consistently and fit-for-purpose. |
| Generalizable | Works across different populations and settings. Poor generalizability may be due to selection bias, AKI definitions, and model overfitting. |
| Pragmatic | Balances need for accuracy with real-world availability of predictor variables which are not routinely available or require special tests. |
| Parsimonious | Avoid unnecessary complexity and excessive predictors that contribute to model overfitting, poor generalizability or interpretability. |
| Clinical usefulness | Models can be easily implemented, easy to interpret, and helps with clinical decision making for interventions to prevent AKI. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.K.H. Predicting Acute Kidney Injury in Acute Rhabdomyolysis. J. Clin. Med. 2025, 14, 6892. https://doi.org/10.3390/jcm14196892
Lim AKH. Predicting Acute Kidney Injury in Acute Rhabdomyolysis. Journal of Clinical Medicine. 2025; 14(19):6892. https://doi.org/10.3390/jcm14196892
Chicago/Turabian StyleLim, Andy K. H. 2025. "Predicting Acute Kidney Injury in Acute Rhabdomyolysis" Journal of Clinical Medicine 14, no. 19: 6892. https://doi.org/10.3390/jcm14196892
APA StyleLim, A. K. H. (2025). Predicting Acute Kidney Injury in Acute Rhabdomyolysis. Journal of Clinical Medicine, 14(19), 6892. https://doi.org/10.3390/jcm14196892






