New Approaches to Treatment of Tricuspid Regurgitation
Abstract
1. Introduction
2. Case Reports
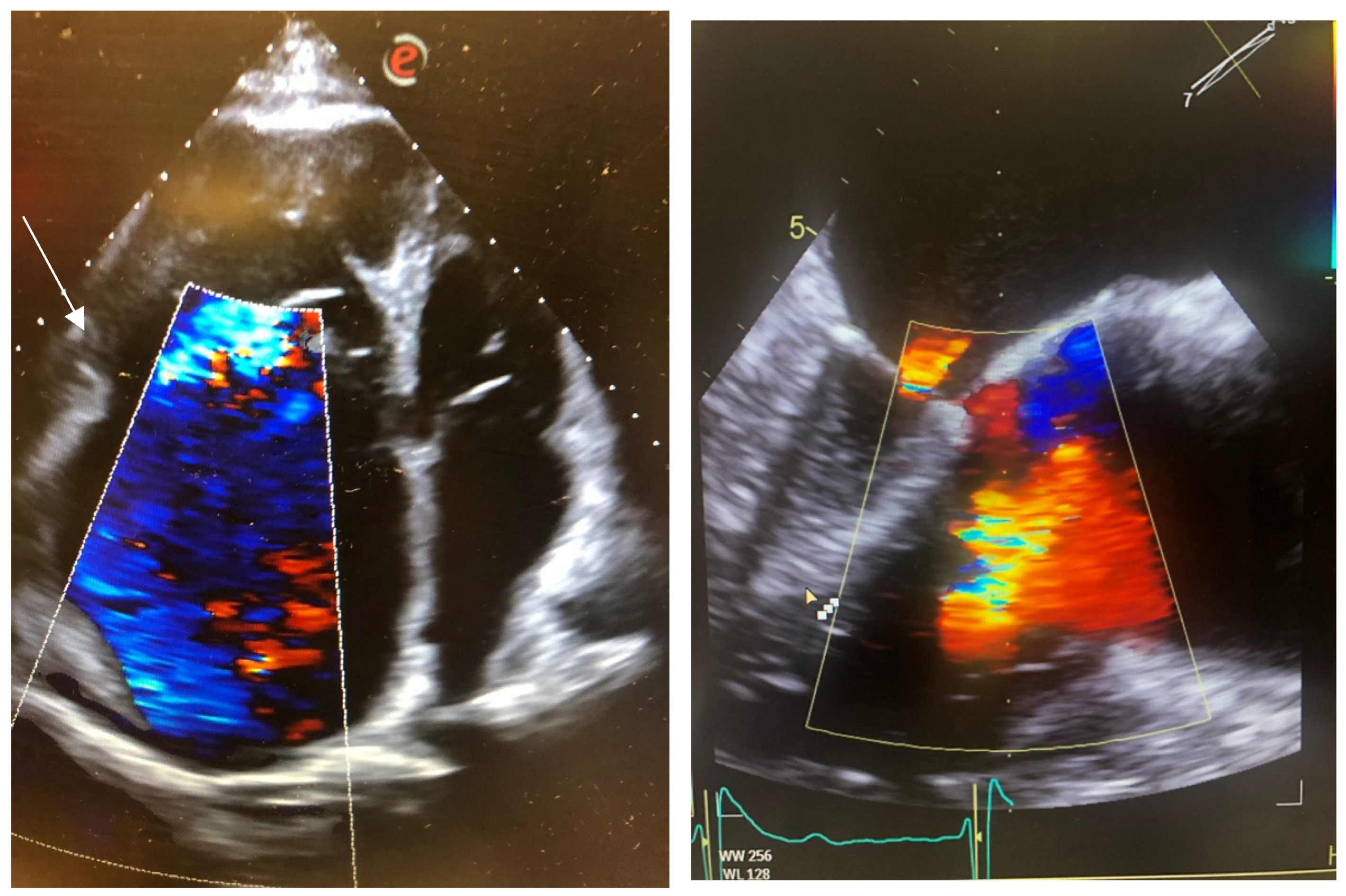
2.1. Case 1
2.2. Case 2
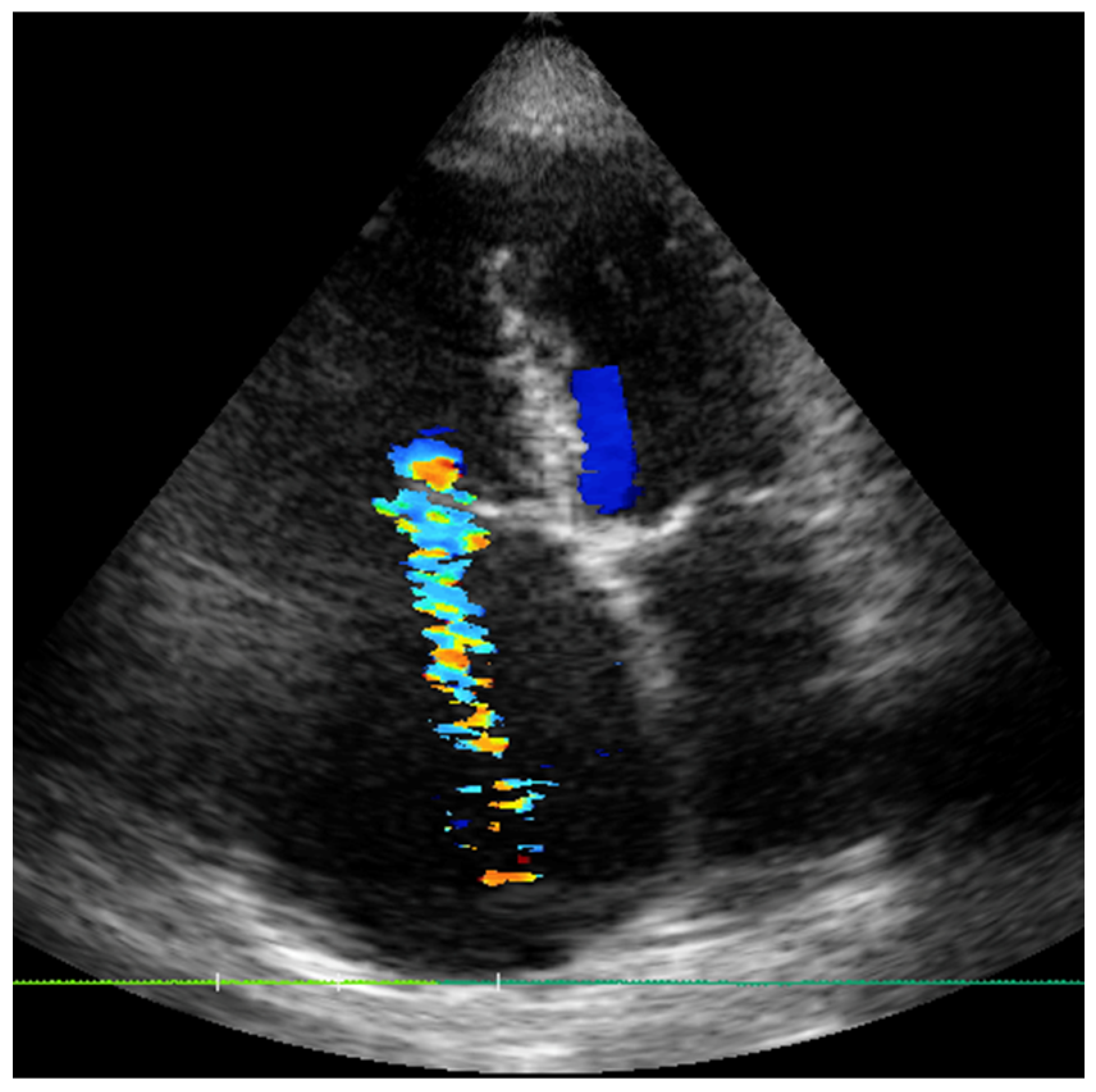
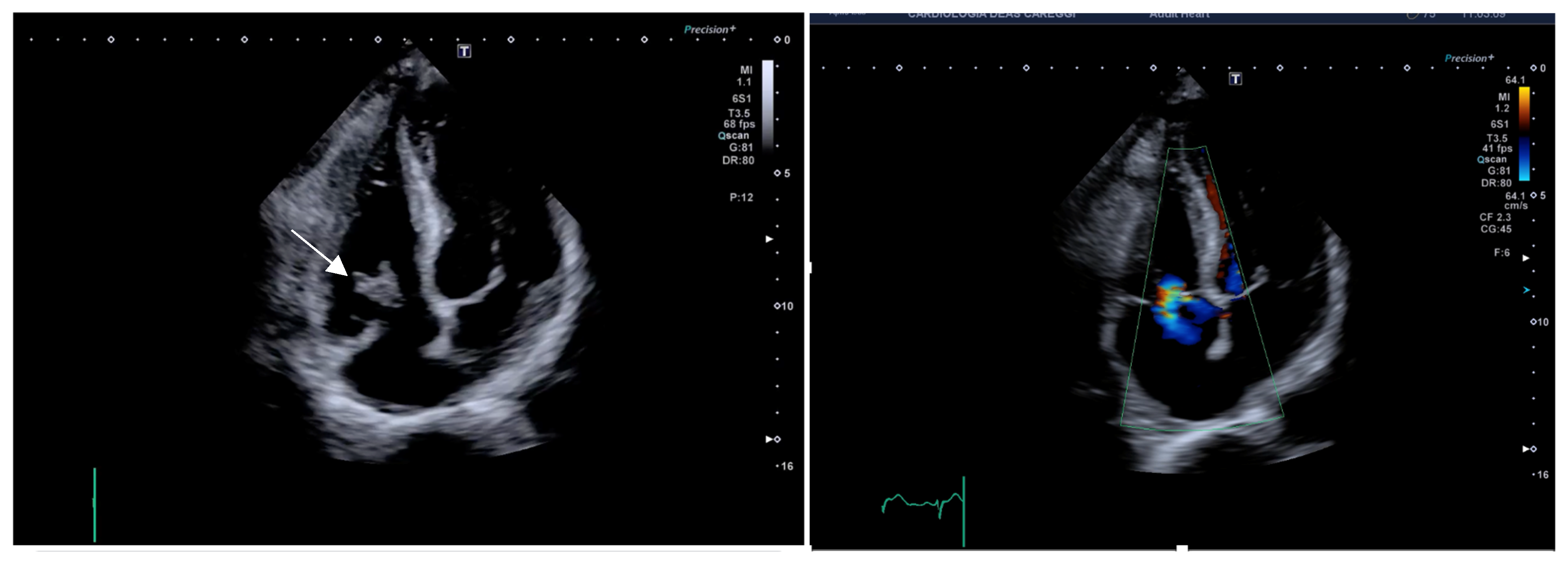
2.3. Case 3
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TR | Tricuspid regurgitation |
| OMT | Optimal medical therapy |
References
- Coffey, S.; d’Arcy, J.L.; Loudon, M.A.; Mant, D.; Farmer, A.J.; Prendergast, B.D.; OxVALVEPCS Group. The OxVALVE population cohort study (OxVALVE-PCS)-population screening for undiagnosed valvular heart disease in the elderly: Study design and objectives. Open Heart 2014, 1, e000043. [Google Scholar] [CrossRef]
- Taramasso, M.; Vanermen, H.; Maisano, F.; Guidotti, A.; La Canna, G.; Alfieri, O. The growing clinical importance of secondary tricuspid regurgitation. J. Am. Coll. Cardiol. 2012, 59, 703–710. [Google Scholar] [CrossRef]
- Ansari, Y.; Raja, A.; Raja, S.; Ali, S.M.E.; Ali, F.; Noor, I.; Siddique, A.; Shakil, S.; Abdullah Keen, M.A.; Zafar, B.; et al. Investigating mortality trends and disparities in tricuspid valve disorder: A U.S. nationwide study from 1999 to 2023. BMC Cardiovasc. Disord. 2025, 25, 208. [Google Scholar] [CrossRef]
- Lebehn, M.A.; Hahn, R.T. Valvular heart failure due to tricuspid regurgitation: Surgical and transcatheter management options. Heart Fail. Clin. 2023, 19, 329–343. [Google Scholar] [CrossRef]
- Agarwal, V.; Hahn, R. Tricuspid regurgitation and right heart failure: The role of imaging in defining pathophysiology, presentation, and novel management strategies. Heart Fail. Clin. 2023, 19, 505–523. [Google Scholar] [CrossRef]
- Ortiz-Leon, X.A.; Posada-Martinez, E.L.; Trejo-Paredes, M.C.; Ivey-Miranda, J.B.; Pereira, J.; Crandall, I.; DaSilva, P.; Bouman, E.; Brooks, A.; Gerardi, C.; et al. Understanding tricuspid valve remodelling in atrial fibrillatiusing three-dimensional echocardiography. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 747–755. [Google Scholar] [CrossRef]
- Kim, J.B.; Spevack, D.M.; Tunick, P.A.; Bullinga, J.R.; Kronzon, I.; Chinitz, L.A.; Reynolds, H.R. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: An observational study. J. Am. Soc. Echocardiogr. 2008, 21, 284–287. [Google Scholar] [CrossRef]
- Hoke, U.; Auger, D.; Thijssen, J.; Wolterbeek, R.; van der Velde, E.T.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Significant lead-induced tricuspid regurgitation is associated with poor prognosis at long-term follow-up. Heart 2014, 100, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.J.; Kim, Y.J.; Kim, M.K.; Kim, H.K.; Park, J.S.; Kim, K.H.; Kim, K.B.; Ahn, H.; Sohn, D.W.; Oh, B.H.; et al. Development of tricuspid regurgitation late after left-sided valve surgery: A single-center experience with long-term echocardiographic examinations. Am. Heart J. 2008, 155, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Topilsky, Y.; Khanna, A.; Le Tourneau, T.; Park, S.; Michelena, H.; Suri, R.; Mahoney, D.W.; Enriquez-Sarano, M. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ. Cardiovasc. Imaging 2012, 5, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Prihadi, E.A.; van der Bijl, P.; Gursoy, E.; Abou, R.; Mara Vollema, E.; Hahn, R.T.; Stone, G.W.; Leon, M.B.; Ajmone Marsan, N.; Delgado, V.; et al. Development of significant tricuspid regurgitation over time and prognostic implications: New insights into natural history. Eur. Heart J. 2018, 39, 3574–3581. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Frazzetto, M.; Bonanni, M.; Matteucci, A.; Leo, L.A.; Umar, R.; Imperatore, G.; de Coelho Castro, P.; Attizzani, G.; Sangiorgi, G.M.; et al. Transcatheter treatment of tricuspid regurgitation: A state of art review. Cardiovasc. Revasc. Med. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Zack, C.J.; Fender, E.A.; Chandrashekar, P.; Reddy, Y.N.V.; Bennett, C.E.; Stulak, J.M.; Miller, V.M.; Nishimura, R.A. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J. Am. Coll. Cardiol. 2017, 70, 2953–2960. [Google Scholar] [CrossRef]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Ajmone Marsan, N.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2025; ehaf194. [Google Scholar] [CrossRef]
- Nickenig, G.; Lurz, P.; Sorajja, P.; von Bardeleben, R.S.; Sitges, M.; Tang, G.H.L.; Hausleiter, J.; Trochu, J.N.; Näbauer, M.; Heitkemper, M.; et al. Percutaneous Edge-to-Edge Repair for Tricuspid Regurgitation: 3-Year Outcomes From the TRILUMINATE Study. JACC Cardiovasc. Interv. 2024, 17, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Donal, E.; Dreyfus, J.; Leurent, G.; Coisne, A.; Leroux, P.Y.; Ganivet, A.; Sportouch, C.; Lavie-Badie, Y.; Guerin, P.; Rouleau, F.; et al. Transcatheter Edge-to-Edge Repair for Severe IsolatedTricuspid Regurgitation The Tri.Fr Randomized Clinical Trial. JAMA 2025, 333, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Schlotter, F.; Stolz, L.; Kresoja, K.P.; von Stein, J.; Fortmeier, V.; Koell, B.; Rottbauer, W.; Kassar, M.; Schöber, A.; Goebel, B.; et al. Tricuspid Regurgitation Disease Stages and Treatment Outcomes After Transcatheter Tricuspid Valve Repair. JACC Cardiovasc. Interv. 2025, 18, 339–348. [Google Scholar] [CrossRef]
- Suc, G.; Mesnier, J.; Cailliau, A.; Habib, M.; Delhomme, C.; Arangalage, D.; Himbert, D.; Ducrocq, G.; Brochet, E.; Vahanian, A.; et al. Survival outcomes in isolated severe tricuspid regurgitation according to therapeutic modalities: A systematic review and meta-analysis. Open Heart 2025, 12, e002986. [Google Scholar] [CrossRef]
- von Bardeleben, R.S.; Lurz, P.; Sorajja, P.; Ruf, T.; Hausleiter, J.; Sitges, M.; Da Rocha E Silva, J.; Näbauer, M.; Weber, M.; Tang, G.H.L.; et al. Two-Year Outcomes for Tricuspid Repair With a Transcatheter Edge-to-Edge Valve Repair From the Transatlantic TRILUMINATE Trial. Circ. Cardiovasc. Interv. 2023, 16, e012888. [Google Scholar] [CrossRef]
- Stolz, L.; Weckbach, L.T.; Hahn, R.T.; Chatfield, A.G.; Fam, N.P.; von Bardeleben, R.S.; Davidson, C.J.; Grayburn, P.A.; Zahr, F.; Hausleiter, J. 2-Year Outcomes Following Transcatheter Tricuspid Valve Replacement Using the EVOQU System. J. Am. Coll. Cardiol. 2023, 81, 2374–2376. [Google Scholar] [CrossRef]
- Gerçek, M.; Narang, A.; Körber, M.I.; Friedrichs, K.P.; Puthumana, J.J.; Ivannikova, M.; Al-Kazaz, M.; Cremer, P.; Baldridge, A.S.; Meng, Z.; et al. GLIDE Score: Scoring System for Prediction of Procedural Success in Tricuspid Valve Transcatheter Edge-to-Edge Repair. JACC Cardiovasc. Imaging 2024, 17, 729–742. [Google Scholar] [CrossRef]
- Pizzino, F.; Trimarchi, G.; D’Agostino, A.; Bonanni, M.; Benedetti, G.; Paradossi, U.; Manzo, R.; Capasso, R.; Di Bella, G.; Zito, C.; et al. Impact of Leaflet-to-Annulus Index on Residual Regurgitation Following Transcatheter Edge-to-Edge Repair of the Tricuspid Valve. J. Clin. Med. 2024, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Weber, M.; Schueler, R.; Hausleiter, J.; Näbauer, M.; von Bardeleben, R.S.; Sotiriou, E.; Schäfer, U.; Deuschl, F.; Kuck, K.H.; et al. 6-Month Outcomes of Tricuspid Valve Reconstruction for Patients With Severe Tricuspid Regurgitation. J. Am. Coll. Cardiol. 2019, 73, 1905–1915. [Google Scholar] [CrossRef]
- Nickenig, G.; Weber, M.; Schüler, R.; Hausleiter, J.; Nabauer, M.; von Bardeleben, R.S.; Sotiriou, E.; Schäfer, U.; Deuschl, F.; Alessandrini, H.; et al. Tri-cuspid valve repair with the Cardioband system: Two-year outcomes of the multicentre, prospective TRI-REPAIR study. EuroIntervention 2021, 16, e1264–e1271. [Google Scholar] [CrossRef]
- Nickenig, G.; Friedrichs, K.P.; Baldus, S.; Arnold, M.; Seidler, T.; Hakmi, S.; Linke, A.; Schäfer, U.; Dreger, H.; Reinthaler, M.; et al. Thirty-day outcomes of the Cardioband tricuspid system for patients with symptomatic functional tricuspid regurgitation: The TriBAND study. EuroIntervention 2021, 17, 809–817. [Google Scholar] [CrossRef]
- Bingöl, G.; Güven, B.; Yıldız, A.; Ökçün, B. TricValve Pop-Out: Management of Transcatheter Caval Valve Migration. Anatol. J. Cardiol. 2022, 26, 414–418. [Google Scholar] [CrossRef]
- Blasco-Turrión, S.; Briedis, K.; Estévez-Loureiro, R.; Sánchez-Recalde, A.; Cruz-González, I.; Pascual, I.; Mascherbauer, J.; Abdul-Jawad Altisent, O.; Nombela-Franco, L.; Pan, M.; et al. Bicaval TricValve Implantation in Patients With Severe Symptomatic Tricuspid Regurgitation: 1-Year Follow-Up Outcomes. JACC Cardiovasc. Interv. 2024, 17, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Dormio, S.; Morosato, M.; Belletti, A.; Silvestri, D.; Montorfano, M.; Monaco, F. Shaping the Anesthetic Approach to TricValve Implantation: Insights From a Case Series. J. Cardiothorac. Vasc. Anesth. 2024, 38, 911–917. [Google Scholar] [CrossRef]
- Poliwoda, S.D.; Durbach, J.R.; Castro, A.; Herman, J.; Caltagirone, C.; Kurup, A.; Rosen, G.; Tuda, C.; La Pietra, A. AngioVac system for infective endocarditis: A new treatment for an old disease. Ann. Card. Anaesth. 2023, 26, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Worku, B.; Salemi, A.; D’Ayala, M.D.; Tranbaugh, R.F.; Girardi, L.N.; Gulkarov, I.M. The angiovac device: Understanding the failures on the road to success. Innovations 2016, 11, 430–433. [Google Scholar] [CrossRef] [PubMed]
- George, B.J.; Voelkel, A.; Kotter, J.; Leventhal, A.; Gurley, J. A novel approach to percutaneous removal of large tricuspid valve vegetations using suction filtration and veno-venous bypass: A single center experience. Catheter. Cardiovasc. Interv. 2017, 90, 1009–1015. [Google Scholar] [CrossRef]
- George, B.J.; Santos, P.; Donaldson, K.; Musa, T.; Kotter, J.; Smyth, S.S.; Leventhal, A.R.; Gurley, J.C. A retrospective comparison of tricuspid valve surgery to tricuspid valve vegetation debulking with angiovac for isolated tricuspid valve endocarditis. J. Am. Coll. Cardiol. 2019, 73 (Suppl. 1), 1973. [Google Scholar] [CrossRef]
- Starck, C.T.; Dreizler, T.; Falk, V. The AngioVac system as a bail-out option in infective valve endocarditis. Ann. Cardiothorac. Surg. 2019, 8, 675–677. [Google Scholar] [CrossRef] [PubMed]
| Score | 1 Point | 2 Points | 3 Points |
|---|---|---|---|
| LVEF (%) | ≥50 | 40–49 | <40 |
| TAPSE (mm) | >17 | 13–17 | <13 |
| eGFR (mL/min/1.73 m2) | >60 | 30–60 | <30 |
| NT pro BNP or BNP (pg/mL) | <125 or 35 | 125–1249 or 35–349 | >1250 or >350 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostagno, C.; Cerillo, A.; Manca, A.R.; Tozzetti, C.; Stefàno, P.L. New Approaches to Treatment of Tricuspid Regurgitation. J. Clin. Med. 2025, 14, 6878. https://doi.org/10.3390/jcm14196878
Rostagno C, Cerillo A, Manca AR, Tozzetti C, Stefàno PL. New Approaches to Treatment of Tricuspid Regurgitation. Journal of Clinical Medicine. 2025; 14(19):6878. https://doi.org/10.3390/jcm14196878
Chicago/Turabian StyleRostagno, Carlo, Alfredo Cerillo, Anna Rita Manca, Camilla Tozzetti, and Pier Luigi Stefàno. 2025. "New Approaches to Treatment of Tricuspid Regurgitation" Journal of Clinical Medicine 14, no. 19: 6878. https://doi.org/10.3390/jcm14196878
APA StyleRostagno, C., Cerillo, A., Manca, A. R., Tozzetti, C., & Stefàno, P. L. (2025). New Approaches to Treatment of Tricuspid Regurgitation. Journal of Clinical Medicine, 14(19), 6878. https://doi.org/10.3390/jcm14196878







