A Closer Look at Potential Underlying Factors Related to Possible Disparity Between Sexes in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage
Abstract
1. Introduction
Objectives
2. Methods
2.1. Setting and Participants
2.2. Study Design and Outcome Variables
2.3. Treatment Algorithm
2.4. Statistics
3. Results
3.1. Demographics
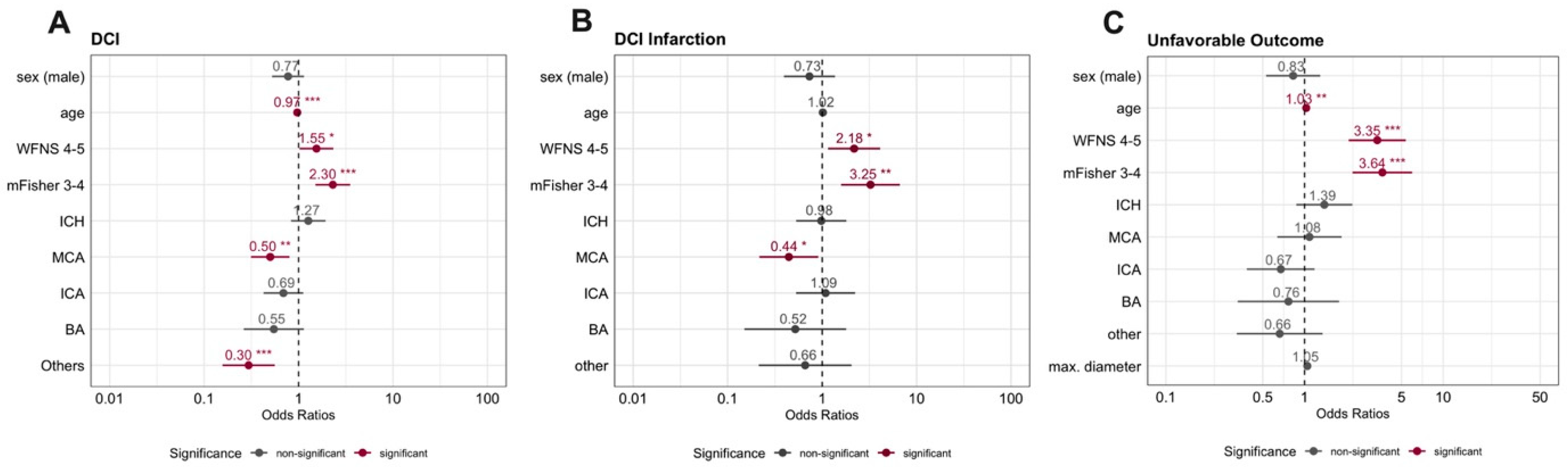
3.2. Occurrence and Timing of DCI
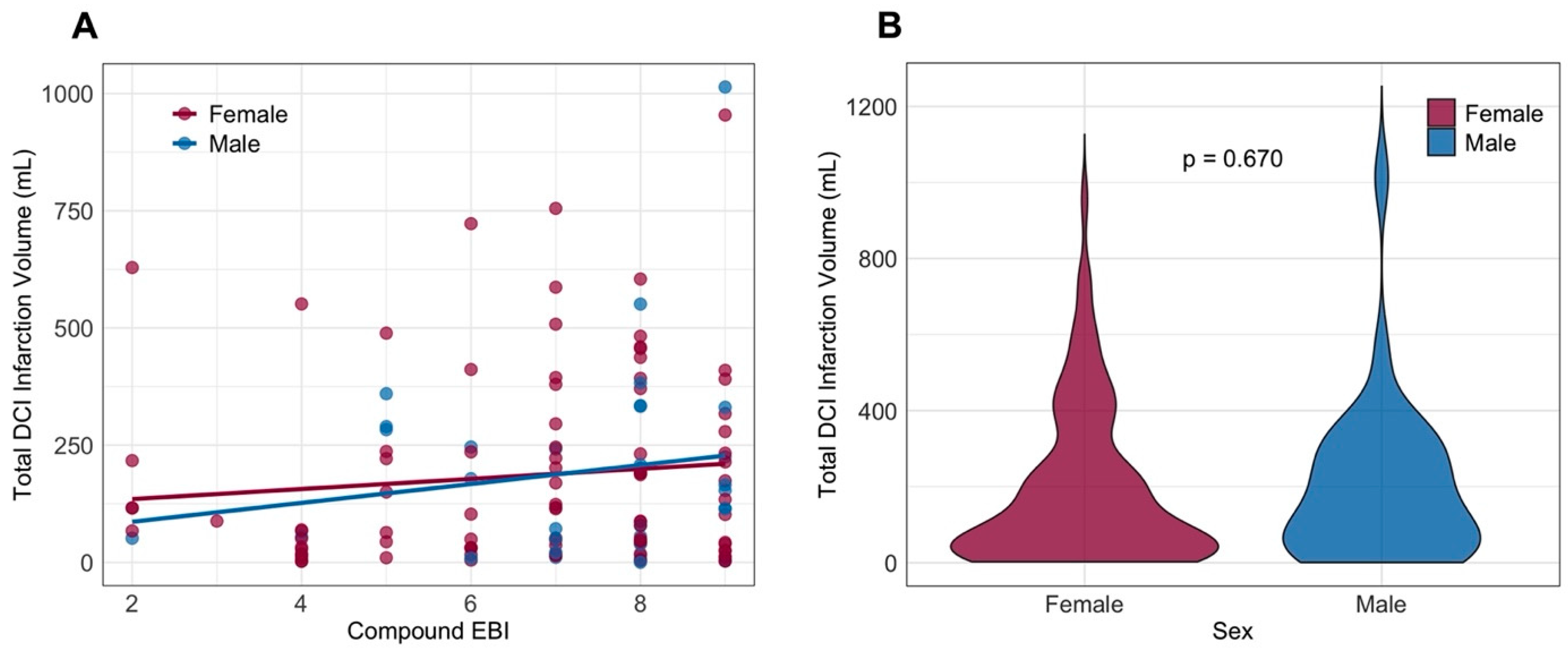
3.3. Occurrence and Timing of DCI-Related Infarction
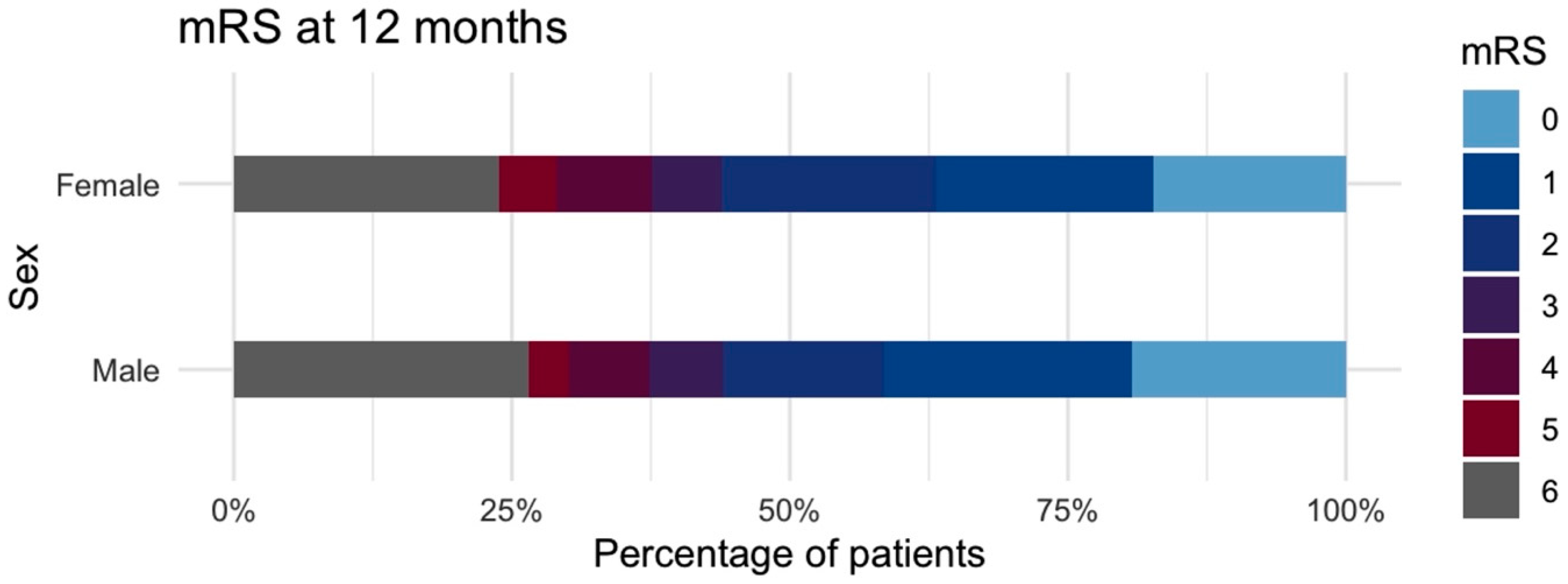
3.4. Recovery After SAH
3.5. Comparison of Post-Menopausal Outcomes
3.6. Post Hoc Power Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Acomm | Anterior communication artery |
| CT | Computed tomography |
| CTP | Computed tomography perfusion scanning |
| DCI | Delayed cerebral ischemia |
| EBI | Early brain injury |
| EVD | External ventricular drain |
| ERT | Endovascular rescue treatment |
| GLMM | Generalized linear mixed-effects model |
| mFisher | Modified Fisher scale |
| mL | Milliliter |
| mRS | Modified Rankin scale |
| PSM | Propensity score matching |
| Q1 | First quartile |
| Q3 | Third quartile |
| SAH | Aneurysmal subarachnoid hemorrhage |
| TCD | Transcranial Doppler |
| WFNS | World Federation of Neurosurgical Societies |
References
- Claassen, J.; Park, S. Spontaneous subarachnoid haemorrhage. Lancet 2022, 400, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Zacharia, B.E.; Hickman, Z.L.; Grobelny, B.T.; DeRosa, P.; Kotchetkov, I.; Ducruet, A.F.; Connolly, E.S., Jr. Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg. Clin. N. Am. 2010, 21, 221–233. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Zuurbier, C.C.M.; Molenberg, R.; Mensing, L.A.; Wermer, M.J.H.; Juvela, S.; Lindgren, A.E.; Jääskeläinen, J.E.; Koivisto, T.; Yamazaki, T.; Uyttenboogaart, M.; et al. Sex Difference and Rupture Rate of Intracranial Aneurysms: An Individual Patient Data Meta-Analysis. Stroke 2022, 53, 362–369. [Google Scholar] [CrossRef]
- Shen, D.; Cai, M.; Luo, Y.; Li, Z.; Zhang, P.; Wang, Y.; Fan, W.; Wu, H.; Yu, Y.; Gong, X.; et al. Sex disparities in the risk of intracranial aneurysm rupture: A case-control study. Front. Neurol. 2024, 15, 1483679. [Google Scholar] [CrossRef]
- Germans, M.R.; Jaja, B.N.R.; de Oliviera Manoel, A.L.; Cohen, A.H.; Macdonald, R.L. Sex differences in delayed cerebral ischemia after subarachnoid hemorrhage. J. Neurosurg. 2018, 129, 458–464. [Google Scholar] [CrossRef]
- Swiatek, V.M.; Amini, A.; Marinescu, M.; Dumitru, C.A.; Spitz, L.; Stein, K.-P.; Saalfeld, S.; Rashidi, A.; Sandalcioglu, I.E.; Neyazi, B. Sex Differences in Intracranial Aneurysms: A Matched Cohort Study. J. Pers. Med. 2024, 14, 1038. [Google Scholar] [CrossRef]
- Darkwah Oppong, M.; Iannaccone, A.; Gembruch, O.; Pierscianek, D.; Chihi, M.; Dammann, P.; Köninger, A.; Müller, O.; Forsting, M.; Sure, U.; et al. Vasospasm-related complications after subarachnoid hemorrhage: The role of patients’ age and sex. Acta Neurochir. 2018, 160, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Ghods, A.J.; Lopes, D.; Chen, M. Gender differences in cerebral aneurysm location. Front. Neurol. 2012, 3, 78. [Google Scholar] [CrossRef] [PubMed]
- Ballester, B.R.; Maier, M.; Duff, A.; Cameirão, M.; Bermúdez, S.; Duarte, E.; Cuxart, A.; Rodríguez, S.; San Segundo Mozo, R.M.; Verschure, P. A critical time window for recovery extends beyond one-year post-stroke. J. Neurophysiol. 2019, 122, 350–357. [Google Scholar] [CrossRef]
- Schmidt, T.P.; Weiss, M.; Hoellig, A.; Nikoubashman, O.; Schulze-Steinen, H.; Albanna, W.; Clusmann, H.; Schubert, G.A.; Veldeman, M. Revisiting the Timeline of Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage: Toward a Temporal Risk Profile. Neurocrit. Care 2022, 37, 735–743. [Google Scholar] [CrossRef]
- Goertz, L.; Pflaeging, M.; Hamisch, C.; Kabbasch, C.; Pennig, L.; von Spreckelsen, N.; Laukamp, K.; Timmer, M.; Goldbrunner, R.; Brinker, G.; et al. Delayed hospital admission of patients with aneurysmal subarachnoid hemorrhage: Clinical presentation, treatment strategies, and outcome. J. Neurosurg. 2021, 134, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Charles, G.D. Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J. Neurosurg. 1988, 68, 985–986. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S., Jr.; MacDonald, R.L.; Mayer, S.A. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery 2006, 59, 21–27. [Google Scholar] [CrossRef]
- Vergouwen, M.D.; Vermeulen, M.; van Gijn, J.; Rinkel, G.J.; Wijdicks, E.F.; Muizelaar, J.P.; Mendelow, A.D.; Juvela, S.; Yonas, H.; Terbrugge, K.G.; et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: Proposal of a multidisciplinary research group. Stroke 2010, 41, 2391–2395. [Google Scholar] [CrossRef]
- Wilson, J.T.L.; Hareendran, A.; Grant, M.; Baird, T.; Schulz, U.G.R.; Muir, K.W.; Bone, I. Improving the Assessment of Outcomes in Stroke. Stroke 2002, 33, 2243–2246. [Google Scholar] [CrossRef]
- Stute, P.; Eversheim, H.; Ortius-Lechner, D.; May, M.; Feig, C. Care reality of menopausal women in Germany: Healthcare research using quantitative (SHI claims data) and qualitative (survey) data collection. Arch. Gynecol. Obstet. 2022, 306, 513–521. [Google Scholar] [CrossRef]
- Veldeman, M.; Albanna, W.; Weiss, M.; Conzen, C.; Schmidt, T.P.; Schulze-Steinen, H.; Wiesmann, M.; Clusmann, H.; Schubert, G.A. Invasive neuromonitoring with an extended definition of delayed cerebral ischemia is associated with improved outcome after poor-grade subarachnoid hemorrhage. J. Neurosurg. 2020, 134, 1527–1534. [Google Scholar] [CrossRef]
- Vossen, L.V.; Weiss, M.; Albanna, W.; Conzen-Dilger, C.; Schulze-Steinen, H.; Rossmann, T.; Schmidt, T.P.; Höllig, A.; Wiesmann, M.; Clusmann, H.; et al. Intra-arterial nimodipine for the treatment of refractory delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. J. Neurointerv. Surg. 2025, 17, e31–e40. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Chen, W.; Ruan, L.; Chen, Y.; Zhong, M.; Zhuge, Q.; Fan, L.H.; Zhao, B.; Yang, Y. Sex differences in aneurysm morphologies and clinical outcomes in ruptured anterior communicating artery aneurysms: A retrospective study. BMJ Open 2016, 6, e009920. [Google Scholar] [CrossRef]
- Schupper, A.J.; Hardigan, T.A.; Mehta, A.; Yim, B.; Yaeger, K.A.; De Leacy, R.; Fifi, J.T.; Mocco, J.; Majidi, S. Sex and Racial Disparity in Outcome of Aneurysmal Subarachnoid Hemorrhage in the United States: A 20-Year Analysis. Stroke 2023, 54, 1347–1356. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Z.; Jia, C.; Zhao, J.; Chai, S.; Li, Z.; Xu, C.; Zhang, T.; Ma, Y.; Ma, C.; et al. Comparison of Sex Differences in Outcomes of Patients With Aneurysmal Subarachnoid Hemorrhage: A Single-Center Retrospective Study. Front. Neurol. 2022, 13, 853513. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, B.; Qi, X.; Yuan, G.; Li, X.; Hao, G.; Liang, G. Comparison of sex differences on outcomes after aneurysmal subarachnoid hemorrhage: A propensity score-matched analysis. BMC Neurol. 2024, 24, 153. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhao, Z.; Yang, B.; Wang, K.; Zhu, G.; Miao, H. Sex Differences in Outcome of Aneurysmal Subarachnoid Hemorrhage and Its Relation to Postoperative Cerebral Ischemia. Neurocrit. Care 2024, 41, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Berli, S.; Barbagallo, M.; Keller, E.; Esposito, G.; Pagnamenta, A.; Brandi, G. Sex-Related Differences in Mortality, Delayed Cerebral Ischemia, and Functional Outcomes in Patients with Aneurysmal Subarachnoid Hemorrhage: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 2781. [Google Scholar] [CrossRef]

| All | Female (%) | Male (%) | Univariate p-Value | |
|---|---|---|---|---|
| n = 650 | n = 455 (70) | n = 195 (30) | ||
| Demographics and comorbidity | ||||
| age—yrs.—mean ± SD (range) | 55.1 ± 13.2 (19–90) | 54.7 ± 13.1 (19–87) | 56.0 ± 13.4 (19–90) | 0.234 |
| BMI—median (Q1 to Q3) | 24.9 (22.5 to 27.7) | 24.8 (22.3 to 27.7) | 25.5 (23.2 to 27.7) | 0.353 |
| hypertension—no. (%) | 280 (43.1) | 199 (43.7) | 81 (41.5) | 0.708 |
| smoking—no. (%) | 195 (30.0) | 133 (29.2) | 62 (31.8) | 0.531 |
| type 2 diabetes—no. (%) | 22 (3.4) | 16 (3.5) | 6 (3.1) | 0.969 |
| coronary artery disease—no. (%) | 49 (7.5) | 27 (5.9) | 22 (11.3) | 0.026 |
| Aneurysm location—no. (%) | ||||
| Acomm | 204 (31.4) | 127 (27.9) | 77 (39.5) | 0.005 |
| ICA | 137 (21.1) | 104 (22.9) | 33 (16.9) | 0.111 |
| MCA | 170 (26.2) | 130 (28.6) | 40 (20.5) | 0.041 |
| BA | 49 (7.5) | 33 (7.3) | 16 (8.2) | 0.795 |
| others | 90 (13.8) | 61 (13.4) | 29 (14.9) | 0.710 |
| posterior circulation | 140 (21.5) | 99 (21.8) | 41 (21.0) | 0.994 |
| max. diameter (mm)—median (Q1 to Q3) | 6.0 (4.0 to 8.2) | 6.0 (4.0 to 8.0) | 7.0 (5.0 to 9.0) | 0.038 |
| multiplicity | 163 (25.1) | 125 (27.5) | 35 (17.9) | 0.048 |
| giant aneurysms—no. (%) | 2 (0.3) | 0 | 2 (1.0) | 0.086 |
| Occlusion modality—no. (%) | ||||
| clipping/endovascular | 298 (45.8)/352 (54.2) | 212 (46.6)/26 (53.4) | 86 (44.1)/109 (55.9) | 0.720 |
| Hemorrhage severity | ||||
| WFNS grade—no. (%) | 0.055 | |||
| grade 1 | 148 (22.8) | 93 (20.4) | 55 (28.2) | |
| grade 2 | 118 (18.2) | 91 (20.0) | 27 (13.8) | |
| grade 3 | 115 (17.7) | 78 (17.1) | 37 (19.0) | |
| grade 4 | 122(18.8) | 93 (20.4) | 29 (14.9) | |
| grade 5 | 147 (22.6) | 100 (22.0) | 47 (24.1) | |
| poor-grade SAH (WFNS 3–5) | 269 (41.4) | 193 (42.4) | 76 (39.0) | 0.466 |
| modified Fisher scale—no. (%) | 0.009 | |||
| grade 1 | 153 (23.5) | 101 (22.2) | 52 (26.7) | |
| grade 2 | 93 (14.3) | 68 (14.9) | 25 (12.8) | |
| grade 3 | 193 (29.7) | 151 (33.2) | 42 (21.5) | |
| grade 4 | 211 (32.5) | 135 (29.7) | 76 (39.0) | |
| intracerebral hemorrhage | 217 (33.4) | 152 (33.4) | 65 (33.3) | 0.989 |
| acute hydrocephalus | 467 (71.8) | 333(73.2) | 134 (68.7) | 0.322 |
| DCI diagnostics—no. (%) | ||||
| INM available | 328 (50.5) | 217 (47.7) | 111 (56.9) | 0.656 |
| PtiO2 | 136 (20.9) | 93 (20.4) | 43 (22.1) | 0.548 |
| CMD | 105 (16.2) | 69 (15.2) | 36 (18.5) | 0.988 |
| Dual monitoring | 67 (10.3) | 67 (14.7) | 35 (17.9) | 0.376 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldeman, M.; Schmidt, T.P.; Seyfried, K.; Weyland, C.; Hakvoort, K.; Rossmann, T.; Vossen, L.V.; Hoellig, A.; Conzen-Dilger, C. A Closer Look at Potential Underlying Factors Related to Possible Disparity Between Sexes in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. J. Clin. Med. 2025, 14, 6856. https://doi.org/10.3390/jcm14196856
Veldeman M, Schmidt TP, Seyfried K, Weyland C, Hakvoort K, Rossmann T, Vossen LV, Hoellig A, Conzen-Dilger C. A Closer Look at Potential Underlying Factors Related to Possible Disparity Between Sexes in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Journal of Clinical Medicine. 2025; 14(19):6856. https://doi.org/10.3390/jcm14196856
Chicago/Turabian StyleVeldeman, Michael, Tobias Philip Schmidt, Katharina Seyfried, Charlotte Weyland, Karlijn Hakvoort, Tobias Rossmann, Laura Victoria Vossen, Anke Hoellig, and Catharina Conzen-Dilger. 2025. "A Closer Look at Potential Underlying Factors Related to Possible Disparity Between Sexes in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage" Journal of Clinical Medicine 14, no. 19: 6856. https://doi.org/10.3390/jcm14196856
APA StyleVeldeman, M., Schmidt, T. P., Seyfried, K., Weyland, C., Hakvoort, K., Rossmann, T., Vossen, L. V., Hoellig, A., & Conzen-Dilger, C. (2025). A Closer Look at Potential Underlying Factors Related to Possible Disparity Between Sexes in Delayed Cerebral Ischemia After Aneurysmal Subarachnoid Hemorrhage. Journal of Clinical Medicine, 14(19), 6856. https://doi.org/10.3390/jcm14196856






