Targeted Therapies Modulating Mesenchymal–Epithelial Transition-Linked Oncogenic Signaling in the Tumor Microenvironment: Comparative Profiling of Capmatinib, Bemcentinib, and Galunisertib
Abstract
1. Introduction
2. Capmatinib–MET Inhibitor
3. Bemcentinib–AXL Inhibitor
4. Galunisertib–TGF-β Inhibitor
5. Future Perspectives and Clinical Implications in EMP Modulation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | Protein kinase B |
| ALK | Anaplastic lymphoma kinase |
| ALK5 | Activin receptor-like kinase 5 |
| ALT | Alanine aminotransferase |
| AML | Acute myeloid leukemia |
| AST | Aspartate aminotransferase |
| ASolT | Advanced Solid Tumors |
| ATP | Adenosine triphosphate |
| AXL | AXL receptor tyrosine kinase |
| bHLH | Basic helix–loop–helix |
| CDH1 | Gene encoding E-cadherin |
| CDK(s) | Cyclin-dependent kinase(s) |
| CIP1 | CDK-interacting protein 1 (p21CIP1) |
| CKD | Chronic kidney disease |
| CNS | Central nervous system |
| CRC | Colorectal cancer |
| CRC/RC | Colorectal and rectal cancers |
| ctDNA | Circulating tumor DNA |
| CTCs | Circulating tumor cells |
| CYP3A4 | Cytochrome P450 3A4 |
| DNA | Deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| ECM | Extracellular matrix |
| EGF(R) | Epidermal growth factor (receptor) |
| EMP | Epithelial–mesenchymal plasticity |
| EMT | Epithelial–mesenchymal transition |
| EMT TFs | Epithelial–mesenchymal transition transcription factors |
| ERK/ERKs | Extracellular signal-regulated kinase(s) |
| FAK | Focal adhesion kinase |
| FGFR1 | Fibroblast growth factor receptor 1 |
| GAB1 | GRB2-associated binder 1 |
| GAS6 | Growth arrest–specific 6 |
| GBM | Glioblastoma |
| GRB2 | Growth factor receptor–bound protein 2 |
| GSK3 | Glycogen synthase kinase 3 |
| GTPase | Guanosine triphosphatase |
| HCC | Hepatocellular carcinoma |
| HDAC | Histone deacetylase |
| HGF | Hepatocyte growth factor |
| HER3 | Human epidermal growth factor receptor 3 |
| IHC | Immunohistochemistry |
| ILD | Interstitial lung disease |
| INK4b | Inhibitor of CDK4 (p15INK4b) |
| JNK | c-Jun N-terminal kinase |
| Lgl2 | Lethal giant larvae homolog 2 |
| LVEF | Left ventricular ejection fraction |
| MAPK | Mitogen-activated protein kinase |
| MDS | Myelodysplastic syndromes |
| MEK | Mitogen-activated protein kinase kinase |
| MET | Mesenchymal–epithelial transition/MET receptor tyrosine kinase |
| miR/miRNA | MicroRNA |
| MM | Malignant Mesothelioma |
| MMP/MMPs | Matrix metalloproteinase(s) |
| MPNs | Myeloproliferative neoplasms |
| MRONJ | Medication-related osteonecrosis of the jaw |
| mRNA | Messenger RNA |
| mTOR | Mechanistic target of rapamycin |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NSCLC | Non-small cell lung cancer |
| ORR | Objective response rate |
| PAK1 | p21-activated kinase 1 |
| PATJ | PALS1-associated tight junction protein |
| PC | Pancreatic cancer |
| PCa | Prostate cancer |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDGFR | Platelet-derived growth factor receptor |
| PFS | Progression-free survival |
| PI3K | Phosphatidylinositol 3-kinase |
| PLCγ | Phospholipase C gamma |
| PTEN | Phosphatase and tensin homolog |
| QTc | the corrected QT interval |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 |
| RAF | Rapidly accelerated fibrosarcoma kinase |
| RAS | Rat sarcoma virus oncogene |
| RC | Rectal cancer |
| RHO | Ras homologous GTPase |
| RNA | Ribonucleic acid |
| RON | Recepteur d’origine nantais (RON receptor tyrosine kinase) |
| RTK(s) | Receptor tyrosine kinase(s) |
| SHC | SHC-transforming protein |
| SHP2 | Src homology region 2-containing protein tyrosine phosphatase 2 (PTPN11) |
| SLUG | Snail family transcriptional repressor 2 |
| SNAIL/SNAIL1/2 | Zinc finger transcription factors repressing E-cadherin |
| SMAD | Transcriptional mediators of TGF-β receptor signaling |
| SOS | Son of Sevenless (guanine nucleotide exchange factor) |
| SRC | Proto-oncogene tyrosine-protein kinase Src |
| STAT/STAT3 | Signal transducer and activator of transcription |
| STK11 | Serine/threonine kinase 11 |
| TAM | TYRO3, AXL, MER receptor tyrosine kinase family |
| TGF-β(R)/(RI) | Transforming growth factor-beta (receptor/receptor I) |
| TKI | Tyrosine kinase inhibitor |
| TNF-α | Tumor necrosis factor-alpha |
| TP53 | Tumor protein p53 |
| Tregs | Regulatory T cells |
| TWIST/TWIST1/2 | EMT-inducing transcription factors |
| VEGF(R) | Vascular endothelial growth factor (receptor) |
| ZEB1/2 | Zinc finger E-box-binding homeobox 1/2 |
| ZO-1 | Zonula occludens-1 |
References
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Jolly, M.K.; Ware, K.E.; Gilja, S.; Somarelli, J.A.; Levine, H. EMT and MET: Necessary or permissive for metastasis? Mol. Oncol. 2017, 11, 755–769. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The Molecular Mechanisms and Therapeutic Strategies of EMT in Tumor Progression and Metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Bangarh, R.; Saini, R.V.; Saini, A.K.; Singh, T.; Joshi, H.; Ramniwas, S.; Shahwan, M.; Tuli, H.S. Dynamics of epithelial-mesenchymal plasticity driving cancer drug resistance. Cancer Pathog. Ther. 2024, 3, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Hari, K.; Jolly, M.K. Design principles of regulatory networks underlying epithelial mesenchymal plasticity in cancer cells. Curr. Opin. Cell Biol. 2025, 92, 102445. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial–mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, O.H.; Córcoles, R.; Fabra, A.; Moreno-Bueno, G.; Acloque, H.; Vega, S.; Barrallo-Gimeno, A.; Cano, A.; Nieto, M.A. Metastatic colonization requires the repression of the epithelial–mesenchymal transition inducer Prrx1. Cancer Cell 2012, 22, 709–724. [Google Scholar] [CrossRef]
- Yang, J.; Du, X.; Wang, G.; Sun, Y.; Chen, K.; Zhu, X.; Lazar, A.J.; Hunt, K.K.; Pollock, R.E.; Zhang, W. Mesenchymal to epithelial transition in sarcomas. Eur. J. Cancer 2014, 50, 593–601. [Google Scholar] [CrossRef]
- Tsai, J.H.; Donaher, J.L.; Murphy, D.A.; Chau, S.; Yang, J. Spatiotemporal regulation of epithelial–mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 2012, 22, 725–736. [Google Scholar] [CrossRef]
- Lou, Y.; Diao, L.; Cuentas, E.R.; Denning, W.L.; Chen, L.; Fan, Y.H.; Byers, L.A.; Wang, J.; Papadimitrakopoulou, V.A.; Behrens, C.; et al. Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma. Clin. Cancer Res. 2016, 22, 3630–3642. [Google Scholar] [CrossRef]
- Ye, X.; Weinberg, R.A. Epithelial–mesenchymal plasticity: A central regulator of cancer progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Gaponova, A.V.; Rodin, S.; Mazina, A.A.; Volchkov, P.V. Epithelial-Mesenchymal Transition: Role in Cancer Progression and the Perspectives of Antitumor Treatment. Acta Naturae 2020, 12, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef]
- Heldin, C.H.; Moustakas, A. Role of Smads in TGFβ signaling. Cell Tissue Res. 2012, 347, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef]
- Meyer, A.S.; Miller, M.A.; Gertler, F.B.; Lauffenburger, D.A. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci. Signal. 2013, 6, ra66. [Google Scholar] [CrossRef]
- Goyette, M.A.; Côté, J.F. AXL Receptor Tyrosine Kinase as a Promising Therapeutic Target Directing Multiple Aspects of Cancer Progression and Metastasis. Cancers 2022, 14, 466. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; Benhadji, K.A.; et al. Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des. Devel. Ther. 2015, 9, 4479–4499. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.; Hida, T.; De Jonge, M.J.; Orlov, S.; et al. Capmatinib in MET exon 14–mutated or MET-amplified non–small-cell lung cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sharma, A.; Patne, K.; Tabasum, S.; Suryavanshi, J.; Rawat, L.; Machaalani, M.; Eid, M.; Singh, R.P.; Choueiri, T.K.; et al. AXL Signaling in Cancer: From Molecular Insights to Targeted Therapies. Signal Transduct. Target. Ther. 2025, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.; Chin, A.R.; et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Singh, A.; Settleman, J. EMT, cancer stem cells and drug resistance: An emerging axis of evil in the war on cancer. Oncogene 2010, 29, 4741–4751. [Google Scholar] [CrossRef]
- Du, B.; Shim, J.S. Targeting Epithelial-Mesenchymal Transition (EMT) to Overcome Drug Resistance in Cancer. Molecules 2016, 21, 965. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-β Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Sattler, M.; Salgia, R. The Expanding Role of the Receptor Tyrosine Kinase MET as a Therapeutic Target in Non-Small Cell Lung Cancer. Cell Rep. Med. 2025, 6, 101983. [Google Scholar] [CrossRef]
- Comoglio, P.M.; Giordano, S.; Trusolino, L. Drug development of MET inhibitors: Targeting oncogene addiction and expedience. Nat. Rev. Drug Discov. 2008, 7, 504–516. [Google Scholar] [CrossRef]
- Organ, S.L.; Tsao, M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011, 3 (Suppl. S1), S7–S19. [Google Scholar] [CrossRef]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef]
- Wood, G.E.; Hockings, H.; Hilton, D.M.; Kermorgant, S. The Role of MET in Chemotherapy Resistance. Oncogene 2021, 40, 1927–1941. [Google Scholar] [CrossRef]
- Rai, G.P.; Shanker, A. The Coevolutionary Landscape of Drug Resistance in Epidermal Growth Factor Receptor: A Cancer Perspective. Comput. Biol. Med. 2025, 189, 110001. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Hata, A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar] [CrossRef]
- Massagué, J. TGFbeta in cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Oettle, H.; Kozloff, M.; Cleverly, A.; et al. Galunisertib plus Gemcitabine vs. Gemcitabine for First-Line Treatment of Patients with Unresectable Pancreatic Cancer. Br. J. Cancer 2018, 119, 1208–1214. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Travis, M.A.; Sheppard, D. TGF-β activation and function in immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.G.; Burrows, J.; Salgia, R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 2005, 225, 1–26. [Google Scholar] [CrossRef]
- Mo, H.N.; Liu, P. Targeting MET in Cancer Therapy. Chronic Dis. Transl. Med. 2017, 3, 148–153. [Google Scholar] [CrossRef]
- Peng, W.T.; Sun, W.Y.; Li, X.R.; Sun, J.C.; Du, J.J.; Wei, W. Emerging Roles of G Protein-Coupled Receptors in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018, 19, 1366. [Google Scholar] [CrossRef]
- Engelsen, A.S.T.; Lotsberg, M.L.; Abou Khouzam, R.; Thiery, J.P.; Lorens, J.B.; Chouaib, S.; Terry, S. Dissecting the Role of AXL in Cancer Immune Escape and Resistance to Immune Checkpoint Inhibition. Front. Immunol. 2022, 13, 869676. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; He, J.; Liao, D.; Zu, X. Axl Inhibitors as Novel Cancer Therapeutic Agents. Life Sci. 2018, 198, 99–111. [Google Scholar] [CrossRef]
- Ou, X.; Gao, G.; Habaz, I.A.; Wang, Y. Mechanisms of Resistance to Tyrosine Kinase Inhibitor-Targeted Therapy and Overcoming Strategies. MedComm 2024, 5, e694. [Google Scholar] [CrossRef]
- Gjerdrum, C.; Tiron, C.; Høiby, T.; Stefansson, I.; Haugen, H.; Sandal, T.; Collett, K.; Li, S.; McCormack, E.; Gjertsen, B.T.; et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA 2010, 107, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wei, Y.; Wei, X. AXL Receptor Tyrosine Kinase as a Promising Anti-Cancer Approach: Functions, Molecular Mechanisms and Clinical Applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Z.; Gong, L.; Fan, Y. A Case of Resistance to Tyrosine Kinase Inhibitor Therapy: Small Cell Carcinoma Transformation Concomitant with Plasma-Genotyped T790M Positivity. Anticancer Drugs 2017, 28, 1056–1061. [Google Scholar] [CrossRef]
- Allgayer, H.; Mahapatra, S.; Mishra, B.; Swain, B.; Saha, S.; Khanra, S.; Kumari, K.; Panda, V.K.; Malhotra, D.; Patil, N.S.; et al. Epithelial-to-Mesenchymal Transition (EMT) and Cancer Metastasis: The Status Quo of Methods and Experimental Models 2025. Mol. Cancer 2025, 24, 167. [Google Scholar] [CrossRef]
- Guarino, M. Epithelial–mesenchymal transition and tumour invasion. Int. J. Biochem. Cell Biol. 2007, 39, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, J.; Vincent, T.; Garcia de Herreros, A. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle 2010, 9, 2363–2374. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Kalluri, R. EMT: When epithelial cells decide to become mesenchymal-like cells. J. Clin. Investig. 2009, 119, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, X.J.; Zhang, H.; Teng, Y.; Li, R.; Bai, F.; Elankumaran, S.; Xing, J. TGF-β–induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Sci. Signal. 2014, 7, ra91. [Google Scholar] [CrossRef]
- Moustakas, A.; Heldin, C.H. Mechanisms of TGFβ-induced epithelial–mesenchymal transition. J. Clin. Med. 2016, 5, 63. [Google Scholar] [CrossRef]
- Nieto, M.A. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu. Rev. Cell Dev. Biol. 2011, 27, 347–376. [Google Scholar] [CrossRef]
- Miyazawa, K.; Miyazono, K. Regulation of TGF-β Family Signaling by Inhibitory Smads. Cold Spring Harb. Perspect. Biol. 2017, 9, a022095. [Google Scholar] [CrossRef] [PubMed]
- Kowanetz, M.; Valcourt, U.; Bergström, R.; Heldin, C.H.; Moustakas, A. Id2 and Id3 Define the Potency of Cell Proliferation and Differentiation Responses to Transforming Growth Factor Beta and Bone Morphogenetic Protein. Mol. Cell. Biol. 2004, 24, 4241–4254. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef]
- Biddle, A.; Gammon, L.; Liang, X.; Costea, D.E.; Mackenzie, I.C. Phenotypic Plasticity Determines Cancer Stem Cell Therapeutic Resistance in Oral Squamous Cell Carcinoma. EBioMedicine 2016, 4, 138–145. [Google Scholar] [CrossRef]
- Huang, R.Y.; Wong, M.K.; Tan, T.Z.; Kuay, K.T.; Ng, A.H.; Chung, V.Y.; Chu, Y.S.; Matsumura, N.; Lai, H.C.; Lee, Y.F.; et al. An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis. 2013, 4, e915. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.J.; Ewald, A.J. A collective route to metastasis: Seeding by tumor cell clusters. Science 2016, 352, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Somarelli, J.A.; Sheth, M.; Biddle, A.; Tripathi, S.C.; Armstrong, A.J.; Hanash, S.M.; Bapat, S.A.; Rangarajan, A.; Levine, H. Hybrid epithelial/mesenchymal phenotypes promote metastasis and therapy resistance across carcinomas. Pharmacol. Ther. 2019, 194, 161–184. [Google Scholar] [CrossRef]
- Aquino, A.; Franzese, O. Reciprocal modulation of tumour and immune cell motility: Uncovering dynamic interplays and therapeutic approaches. Cancers 2025, 17, 1547. [Google Scholar] [CrossRef]
- Bahcall, M.; Sim, T.; Paweletz, C.; Patel, J.D.; Sholl, L.M.; Sacher, A.G.; Lydon, C.; Kirschmeier, P.; Lawrence, M.S.; Awad, M.M.; et al. Acquired METD1228V Mutation and Resistance to MET Inhibition in Lung Cancer. Cancer Discov. 2016, 6, 1334–1341. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Liang, Y.; Zhu, V.; Ou, S.H.I. The Race to Target MET Exon 14 Skipping Alterations in Non-Small Cell Lung Cancer: The Why, the How, the Who, the Unknown, and the Inevitable. Lung Cancer 2017, 103, 27–37. [Google Scholar] [CrossRef]
- Feng, H.; Xia, Y.; Wang, W.; Xu, C.; Wang, Q.; Song, Z.; Li, Z.; Yu, J.; Zhong, W.; Wang, Z.; et al. Expert consensus on the diagnosis and treatment of non-small cell lung cancer with MET alteration. Cancer Biol. Med. 2025, 22, 237–265. [Google Scholar] [CrossRef]
- Oksen, D.; Boutmy, E.; Wang, Y.; Stroh, C.; Johne, A.; Nisbett, A.R.; Ryder, A. Patients with advanced non-small cell lung cancer harboring MET alterations: A descriptive cohort study. Clin. Lung Cancer 2025, 26, e259–e269.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, J.; Xing, R.; Du, X.; Wei, C.; Wang, H. Exploring practical experience with different treatments in NSCLC patients with MET-deregulated: A retrospective analysis from the real world. BMC Pulm. Med. 2025, 25, 35. [Google Scholar] [CrossRef]
- Shek, R.C.M.; Li, P.S.N.; Leung, S.C.M.; Chu, H.T.; Hioe, F.; Tang, V.W.L.; Lui, Y.H.; Lam, L.R.S.; Ng, J.H.Y.; Wong, R.T.S.; et al. A novel digital PCR assay for accurate detection and differentiation of focal and non-focal subtypes of mesenchymal-epithelial transition (MET) gene amplification in lung cancer. Cancers 2025, 17, 811. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, G.; Chen, S.; Zhang, F.; Ma, S.; Bai, Y.; Zhang, Q.; Ding, Y. Natural product mediated mesenchymal-epithelial remodeling by covalently binding ENO1 to degrade m6A modified β-catenin mRNA. Acta Pharm. Sin. B 2025, 15, 467–483. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Zhang, J.; Wang, J.; Yang, S.; Zhao, H.; Wu, L.; Lei, J.; Zhou, Y.; Peng, J.; et al. RNASET2 deficiency induces hepatocellular carcinoma metastasis through cholesterol-triggered MET activation. Adv. Sci. 2025, 12, e2411888. [Google Scholar] [CrossRef]
- Miglio, U.; Berrino, E.; Avanzato, D.; Molineris, I.; Miano, V.; Milan, M.; Lanzetti, L.; Morelli, E.; Hughes, J.M.; De Bortoli, M.; et al. Inhibition of the LINE1-derived MET transcript induces apoptosis and oncoprotein knockdown in cancer cells. Mol. Ther. Nucleic Acids 2025, 36, 102529. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Huang, C.; Zhong, M.; Zhong, H.; Ruan, R.; Xiong, J.; Yao, Y.; Zhou, J.; Deng, J. Targeting HGF/c-MET signaling to regulate the tumor microenvironment: Implications for counteracting tumor immune evasion. Cell Commun. Signal. 2025, 23, 46. [Google Scholar] [CrossRef]
- Ma, P.C.; Tretiakova, M.S.; MacKinnon, A.C.; Ramnath, N.; Johnson, C.; Dietrich, S.; Maulik, G.; Christensen, J.G.; Salgia, R. Expression and Mutational Analysis of MET in Human Solid Cancers. Genes Chromosomes Cancer 2008, 47, 1025–1037. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Van De Kerkhove, C.; Wauters, E.; Van Mol, P. Capmatinib for the Treatment of Non-Small Cell Lung Cancer. Expert Rev. Anticancer Ther. 2019, 19, 659–671. [Google Scholar] [CrossRef]
- Kron, A.; Scheffler, M.; Wiesweg, M.; Hummel, H.D.; Kulhavy, J.; Gatteloehner, S.; Kollmeier, J.; Schubart, C.; Groß, T.; Demes, M.C.; et al. Indirect Comparison of Capmatinib Treatment from GEOMETRY Mono-1 Trial to SOC in German Patients with Locally Advanced or Metastatic NSCLC Harboring METex14 Skipping Mutations. Eur. J. Cancer 2024, 207, 114158. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Targeted and Cytotoxic Inhibitors Used in the Treatment of Lung Cancers. Pharmacol. Res. 2024, 209, 107465. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Stabile, L.P. Hepatocyte Growth Factor/c-Met Signaling in Head and Neck Cancer and Implications for Treatment. Cancers 2017, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Sepúlveda, R.V.; Tapia, I.; Estay, C.; Soto, V.; Blanco, A.; González, E.; Armisen, R. MET Exon 14 Skipping and Novel Actionable Variants: Diagnostic and Therapeutic Implications in Latin American Non-Small-Cell Lung Cancer Patients. Int. J. Mol. Sci. 2024, 25, 13715. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makimoto, G.; Sumii, R.; Omote, R.; Ando, Y.; Ninomiya, K.; Ichihara, E.; Ohashi, K.; Maeda, Y.; Tabata, M. Remarkable Efficacy of Capmatinib in a Patient with Cancer of Unknown Primary with MET Amplification: A Case Report. Intern. Med. 2025, (Online ahead of print). 1–5. [Google Scholar] [CrossRef] [PubMed]
- Akioka, T.; Kimura, S.; Katayama, Y.; Fujii, M.; Kiwaki, T.; Kawaguchi, M.; Fukushima, T.; Sato, Y.; Mukai, S.; Kamoto, T.; et al. Phosphorylation of MET Is Upregulated in Metastatic Sites of Renal Cell Carcinoma: Possible Role of MET and Hepatocyte Growth Factor Activation-Targeted Combined Therapy. Biomedicines 2025, 13, 811. [Google Scholar] [CrossRef]
- Bladt, F.; Friese-Hamim, M.; Ihling, C.; Wilm, C.; Blaukat, A. The c-Met Inhibitor MSC2156119J Effectively Inhibits Tumor Growth in Liver Cancer Models. Cancers 2014, 6, 1736–1752. [Google Scholar] [CrossRef]
- Mallareddy, J.R.; Yang, L.; Lin, W.H.; Feathers, R.; Ayers-Ringler, J.; Tolosa, E.; Kizhake, A.G.; Kizhake, S.; Kubica, S.P.; Boghean, L.; et al. Fluorescence Based Live Cell Imaging Identifies Exon 14 Skipped Hepatocyte Growth Factor Receptor (MET) Degraders. RSC Adv. 2025, 15, 10419–10425. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, W.; Shen, X.; Jiang, T.; Li, X.; Liu, H.; Zheng, Z. Molecular Mechanism of Type Ib MET Inhibitors and Their Potential for CNS Tumors. Sci. Rep. 2025, 15, 6926. [Google Scholar] [CrossRef]
- Wolf, J.; Hochmair, M.; Han, J.Y.; Reguart, N.; Souquet, P.J.; Smit, E.F.; Orlov, S.V.; Vansteenkiste, J.; Nishio, M.; de Jonge, M.; et al. Capmatinib in MET Exon 14-Mutated Non-Small-Cell Lung Cancer: Final Results from the Open-Label, Phase 2 GEOMETRY Mono-1 Trial. Lancet Oncol. 2024, 25, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.L.; Passiglia, F.; Cappuzzo, F.; Minuti, G.; Occhipinti, M.; Bulotta, A.; Delmonte, A.; Sini, C.; Galetta, D.; Roca, E.; et al. MET Exon 14 Skipping Mutations in Non-Small-Cell Lung Cancer: Real-World Data from the Italian Biomarker ATLAS Database. ESMO Open 2024, 9, 103680. [Google Scholar] [CrossRef]
- Novartis, A.G. Novartis Investigational Lung Cancer Therapy Capmatinib (INC280) Granted FDA Breakthrough Therapy Designation for Patients with MET-Mutated Advanced Non-Small Cell Lung Cancer. Available online: https://www.novartis.com/news/media-releases/novartis-investigational-lung-cancer-therapy-capmatinib-inc280-granted-fda-breakthrough-therapy-designation-patients-met-mutated-advanced-non-small-cell-lung-cancer (accessed on 9 June 2025).
- Schuler, M.; Berardi, R.; Lim, W.T.; de Jonge, M.; Bauer, T.M.; Azaro, A.; Gottfried, M.; Han, J.Y.; Lee, D.H.; Wollner, M.; et al. Molecular Correlates of Response to Capmatinib in Advanced Non-Small-Cell Lung Cancer: Clinical and Biomarker Results from a Phase I Trial. Ann. Oncol. 2020, 31, 789–797. [Google Scholar] [CrossRef]
- Drilon, A.; Cappuzzo, F.; Ou, S.H.I.; Camidge, D.R. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? J. Thorac. Oncol. 2017, 12, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, Y.; Miao, D.; Zhou, M.; Zhao, J.; Shao, Z.; Jin, R.; Le, X.; Li, W.; Xia, Y. Targeting MET in NSCLC: An Ever-Expanding Territory. JTO Clin. Res. Rep. 2024, 5, 100630. [Google Scholar] [CrossRef]
- Guo, X.J.; Cai, X.T.; Rong, Z.X.; Zhang, Y.P.; Wen, Y.X.; Bai, X.; Wang, J.; Fu, Q.J.; Guo, Z.Q.; Long, L.L.; et al. Interstitial Pneumonitis Associated with Combined Regimen of Immunotherapy and Conventional Therapies—Pharmacovigilance Database Analysis with Real-World Data Validation. BMC Med. 2023, 21, 6. [Google Scholar] [CrossRef]
- Weller, M.; Remon, J.; Rieken, S.; Vollmuth, P.; Ahn, M.J.; Minniti, G.; Le Rhun, E.; Westphal, M.; Brastianos, P.K.; Soo, R.A.; et al. Central Nervous System Metastases in Advanced Non-Small Cell Lung Cancer: A Review of the Therapeutic Landscape. Cancer Treat. Rev. 2024, 130, 102807. [Google Scholar] [CrossRef] [PubMed]
- Sisi, M.; Vitale, G.; Fusaroli, M.; Riefolo, M.; Giunchi, V.; D’Errico, A.; Ardizzoni, A.; Raschi, E.; Gelsomino, F. Capmatinib-Induced Liver Injury as Emerging Toxicity of MET Inhibitors in Patients with NSCLC Pretreated with Immune Checkpoint Inhibitors. JTO Clin. Res. Rep. 2023, 4, 100563. [Google Scholar] [CrossRef]
- Stanzione, B.; Del Conte, A.; Bertoli, E.; De Carlo, E.; Bortolot, M.; Torresan, S.; Spina, M.; Bearz, A. Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor (EGFR) Common Mutations: New Strategies. Cancers 2025, 17, 1515. [Google Scholar] [CrossRef]
- Asiedu, M.K.; Beauchamp-Perez, F.D.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. AXL Induces Epithelial-to-Mesenchymal Transition and Regulates the Function of Breast Cancer Stem Cells. Oncogene 2014, 33, 1316–1324. [Google Scholar] [CrossRef]
- Aiello, N.M.; Maddipati, R.; Norgard, R.J.; Balli, D.; Li, J.; Yuan, S.; Yamazoe, T.; Black, T.; Sahmoud, A.; Furth, E.E.; et al. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev. Cell 2018, 45, 681–695.e4. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Moonsamy, P.; Gainor, J.F.; Lennerz, J.K.; Piotrowska, Z.; Lin, J.J.; Lennes, I.T.; Sequist, L.V.; Shaw, A.T.; Goodwin, K.; et al. A Phase 2 Study of Capmatinib in Patients with MET-Altered Lung Cancer Previously Treated with a MET Inhibitor. J. Thorac. Oncol. 2021, 16, 850–859. [Google Scholar] [CrossRef]
- Cui, X.; Chen, X.; Pognan, N.; Sengupta, T.; Rahmanzadeh, G.; Kornberger, R.; Giovannini, M. Evaluation of the Pharmacokinetic Drug Interaction of Capmatinib with Itraconazole and Rifampicin and Potential Impact on Renal Transporters in Healthy Subjects. J. Clin. Pharmacol. 2023, 63, 228–238. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves Capmatinib for Metastatic Non-Small Cell Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capmatinib-metastatic-non-small-cell-lung-cancer (accessed on 9 June 2025).
- Capmatinib. Tabrecta. Eropean Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tabrecta (accessed on 5 June 2025).
- Wu, Y.L.; Smit, E.F.; Bauer, T.M. Capmatinib for Patients with Non-Small Cell Lung Cancer with MET Exon 14 Skipping Mutations: A Review of Preclinical and Clinical Studies. Cancer Treat. Rev. 2021, 95, 102173. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.; Muddasani, R.; Massarelli, E. Overcoming resistance to checkpoint inhibitors with combination strategies in the treatment of non-small cell lung cancer. Cancers 2024, 16, 2919. [Google Scholar] [CrossRef]
- Hu, D.; Hu, Y.; Lei, S.; Wu, D.; Wang, Y. MET Tyrosine Kinase Inhibitors in Combination with EGFR Tyrosine Kinase Inhibitors in NSCLC Patients with EGFR Mutations and Acquired MET Alterations: A Systematic Review and Meta-Analysis. BMC Cancer 2025, 25, 732. [Google Scholar] [CrossRef] [PubMed]
- Lara, M.S.; Riess, J.W.; Goldman, J.W.; Jiang, F.; Bivona, T.G.; Blakely, C.M. Current Trial Report: A Multicenter Phase I/Ib Study of Capmatinib Plus Trametinib in Patients with Metastatic Nonsmall Cell Lung Center Harboring MET Exon 14 Skipping Mutations and Other MET-Alterations. Clin. Lung Cancer 2024, 25, 732–737. [Google Scholar] [CrossRef]
- Cortot, A.; Le, X.; Smit, E.; Viteri, S.; Kato, T.; Sakai, H.; Park, K.; Camidge, D.R.; Berghoff, K.; Vlassak, S.; et al. Safety of MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14 Skipping Non-Small Cell Lung Cancer: A Clinical Review. Clin. Lung Cancer 2022, 23, 195–207. [Google Scholar] [CrossRef]
- DeAzevedo, R.; Steiner, M.; Turner, B.X.; Liu, A.; Newton, S.; Schmidt, J.; Fleming, R.; Tolentino, A.; Kaseb, A.O.; Curran, M.A. Type I MET Inhibitors Cooperate with PD-1 Blockade to Promote Rejection of Hepatocellular Carcinoma. J. Immunother. Cancer 2024, 12, e009690. [Google Scholar] [CrossRef]
- Carouge, E.; Burnichon, C.; Figeac, M.; Sebda, S.; Vanpouille, N.; Vinchent, A.; Truong, M.J.; Duterque-Coquillaud, M.; Tulasne, D.; Chotteau-Lelièvre, A. Functional Interaction between Receptor Tyrosine Kinase MET and ETS Transcription Factors Promotes Prostate Cancer Progression. Mol. Oncol. 2025, 19, 474–495. [Google Scholar] [CrossRef]
- Ueta, A.; Yamada, A.; Yoshioka, M.; Kanai, M.; Muto, M.; Okita, N. Remarkable Response to Capmatinib in a Patient with Intrahepatic Cholangiocarcinoma Harboring TFG-MET Fusion. Int. Cancer Conf. J. 2024, 13, 199–203. [Google Scholar] [CrossRef]
- Alves de Souza, G.; Dornellas, D.M.S.; Campregher, P.V.; Teixeira, C.H.A.; Schvartsman, G. Complete Response to Capmatinib in a Patient with Metastatic Lung Adenocarcinoma Harboring CD47-MET Fusion: A Case Report. Oncologist 2024, 29, 764–767. [Google Scholar] [CrossRef]
- Falchook, G.S.; Battiste, J.D.; Kalra, A.; Shastry, M.; Finney, L.; Hoekstra, S.J.; Shih, M.G.; Shih, K.C. A Phase Ib Study Evaluating the c-MET Inhibitor INC280 (Capmatinib) in Combination with Bevacizumab in Patients with High-Grade Glioma. Neurooncol. Adv. 2024, 7, vdae220. [Google Scholar] [CrossRef]
- Batra, U.; Singh, A.K.; Nathany, S.; Dewan, A.; Sharma, M.; Amrith, B.P.; Mehta, A.; Batra, V.; Noronha, V.; Prabhash, K. Real world experience with MET inhibitors in MET exon 14 skipping mutated non-small cell lung cancer: Largest Indian perspective. Discov. Oncol. 2025, 16, 286. [Google Scholar] [CrossRef]
- Solomon, B.J.; Mok, T.; Kim, D.W.; Wu, Y.L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef]
- Feng, Y.; Ma, P.C. MET targeted therapy for lung cancer: Clinical development and future directions. Lung Cancer 2012, 3, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Yu, J.H.; Lin, Y.C.; Chang, Y.M.; Liu, N.T.; Chen, S.F. Application of an Integrated Single-Cell and Three-Dimensional Spheroid Culture Platform for Investigating Drug Resistance Heterogeneity and Epithelial-Mesenchymal Transition (EMT) in Lung Cancer Subclones. Int. J. Mol. Sci. 2025, 26, 1766. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Zhao, J.; Yu, Y.; Tian, H.; Zhu, H.; Li, W.; Xia, Y.; Chen, L. Savolitinib Conferred Sensitivity in a Patient with D1228H Mutation-Induced Capmatinib-Resistant MET Exon 14 Skipping Mutated Lung Adenocarcinoma. J. Cancer Res. Clin. Oncol. 2024, 150, 395. [Google Scholar] [CrossRef] [PubMed]
- Jóri, B.; Bundschuh, O.; Falk, M.; Heukamp, L.C.; Kluge, A.; Tiemann, M.; Willborn, K.C.; Woitzik, J.; Griesinger, F. Intracranial Response to Capmatinib after Progression on Crizotinib in a Patient with MET Exon 14 Skipping Non-Small Cell Lung Cancer—A Case Report. Transl. Lung Cancer Res. 2024, 13, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET Amplification Leads to Gefitinib Resistance in Lung Cancer by Activating ERBB3 Signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Bardelli, A.; Corso, S.; Bertotti, A.; Hobor, S.; Valtorta, E.; Siravegna, G.; Sartore-Bianchi, A.; Scala, E.; Cassingena, A.; Zecchin, D.; et al. Amplification of the MET Receptor Drives Resistance to Anti-EGFR Therapies in Colorectal Cancer. Cancer Discov. 2013, 3, 658–673. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour Heterogeneity and Resistance to Cancer Therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Lai, Y.; Zhao, Z.; Zeng, T.; Liang, X.; Chen, D.; Duan, X.; Zeng, G.; Wu, W. Crosstalk between VEGFR and Other Receptor Tyrosine Kinases for TKI Therapy of Metastatic Renal Cell Carcinoma. Cancer Cell Int. 2018, 18, 31. [Google Scholar] [CrossRef]
- Tan, T.Z.; Miow, Q.H.; Miki, Y.; Noda, T.; Mori, S.; Huang, R.Y.; Thiery, J.P. Epithelial–Mesenchymal Transition Spectrum Quantification and Its Efficacy in Deciphering Survival and Drug Responses of Cancer Patients. EMBO Mol. Med. 2014, 6, 1279–1293. [Google Scholar] [CrossRef]
- Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S.; et al. Identification of the Tumour Transition States Occurring during EMT. Nature 2018, 556, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Boareto, M.; Debeb, B.G.; Aceto, N.; Farach-Carson, M.C.; Woodward, W.A.; Levine, H. Inflammatory Breast Cancer: A Model for Investigating Cluster-Based Dissemination. NPJ Breast Cancer 2017, 3, 21. [Google Scholar] [CrossRef]
- Cascetta, P.; Sforza, V.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Montanino, A.; Costanzo, R.; Sandomenico, C.; De Cecio, R.; et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Hendriks, L.E.L.; Mountzios, G.; García-Campelo, R.; Saw, S.P.L.; Uprety, D.; Recondo, G.; Villacampa, G.; Reck, M. MET alterations in NSCLC—Current perspectives and future challenges. J. Thorac. Oncol. 2023, 18, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Deng, Q.M.; Feng, W.; Chen, Z.H.; Su, J.W.; Chen, H.J.; Wang, W.X.; Zhang, S.; Wang, Q.; Chen, Z.; et al. Response and acquired resistance to MET inhibitors in de novo MET fusion-positive advanced non-small cell lung cancer. Lung Cancer 2023, 178, 66–74. [Google Scholar] [CrossRef]
- Jin, F.; Lin, Y.; Yuan, W.; Wu, S.; Yang, M.; Ding, S.; Liu, J.; Chen, Y. Recent advances in c-Met-based dual inhibitors in the treatment of cancers. Eur. J. Med. Chem. 2024, 272, 116477. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.Y.; Chow, K.C.; Lin, T.Y.; Chiang, I.P.; Fang, H.Y. Hepatocyte growth factor and HER2/neu downregulate expression of apoptosis-inducing factor in non-small cell lung cancer. Oncol. Rep. 2014, 31, 597–604. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef]
- Kazandjian, D.; Blumenthal, G.M.; Chen, H.Y.; He, K.; Patel, M.; Justice, R.; Keegan, P.; Pazdur, R. FDA approval summary: Crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist 2014, 19, e5–e11. [Google Scholar] [CrossRef]
- Nosaki, K.; Yoh, K.; Toyozawa, R.; Horinouchi, H.; Morise, M.; Ohashi, K.; Murakami, H.; Satouchi, M.; Sakakibara-Konishi, J.; Yano, S.; et al. Phase 2 trial of crizotinib in Japanese patients with advanced NSCLC harboring a MET gene alteration: A Co-MET study. Int. J. Clin. Oncol. 2024, 29, 1142–1151. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Owusu, B.Y.; Galemmo, R.; Janetka, J.; Klampfer, L. Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Microenvironment. Cancers 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Van Herpe, F.; Van Cutsem, E. The Role of cMET in Gastric Cancer-A Review of the Literature. Cancers 2023, 15, 1976. [Google Scholar] [CrossRef]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef]
- Linger, R.M.; Keating, A.K.; Earp, H.S.; Graham, D.K. TAM receptor tyrosine kinases: Biological functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 2008, 100, 35–83. [Google Scholar] [CrossRef]
- Mamun, Y.; Chadni, S.H.; Rayala, R.; Ferdous, S.; Pokhrel, R.; Nefzi, A.; Chapagain, P.; Tse-Dinh, Y.C. Identification of Novel Human Topoisomerase III Beta Inhibitors. bioRxiv 2025, preprint. [Google Scholar] [CrossRef]
- Pirson, S.; Gautier-Isola, M.; Baudin, L.; Rouaud, L.; Vanwynsberghe, A.; Deroye, J.; Bekisz, S.; Gucciardo, F.; Lebeau, A.; Buntinx, F.; et al. AXL Promotes Lymphangiogenesis by Amplifying VEGF-C-Mediated AKT Pathway. Cell Mol. Life Sci. 2025, 82, 95. [Google Scholar] [CrossRef]
- Majumder, A.; Hosseinian, S.; Stroud, M.; Adhikari, E.; Saller, J.J.; Smith, M.A.; Zhang, G.; Agarwal, S.; Creixell, M.; Meyer, B.S.; et al. Integrated Proteomics-Based Physical and Functional Mapping of AXL Kinase Signaling Pathways and Inhibitors Define Its Role in Cell Migration. Mol. Cancer Res. 2022, 20, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Holland, S.J.; Pan, A.; Franci, C.; Hu, Y.; Chang, B.; Li, W.; Duan, M.; Torneros, A.; Yu, J.; Heckrodt, T.; et al. R428, a selective small molecule inhibitor of AXL kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010, 70, 1544–1554. [Google Scholar] [CrossRef]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.M.; Balaji, K.; Byers, L.A. Giving AXL the axe: Targeting AXL in human malignancy. Br. J. Cancer 2017, 116, 415–423. [Google Scholar] [CrossRef]
- Wilson, C.; Ye, X.; Pham, T.; Lin, E.; Chan, S.; McNamara, E.; Neve, R.M.; Belmont, L.; Koeppen, H.; Yauch, R.L.; et al. AXL inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. Cancer Res. 2014, 74, 5878–5890. [Google Scholar] [CrossRef] [PubMed]
- Ben-Batalla, I.; Schultze, A.; Wroblewski, M.; Erdmann, R.; Heuser, M.; Waizenegger, J.S.; Riecken, K.; Binder, M.; Schewe, D.; Sawall, S.; et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood 2013, 122, 2443–2452. [Google Scholar] [CrossRef]
- Grøndal, S.M.; Blø, M.; Nilsson, L.I.H.; Rayford, A.J.; Jackson, A.; Gausdal, G.; Lorens, J.B. Targeting AXL Cellular Networks in Kidney Fibrosis. Front. Immunol. 2024, 15, 1446672. [Google Scholar] [CrossRef]
- Wu, S.; Liao, M.; Li, M.; Sun, M.; Xi, N.; Zeng, Y. Structure-based discovery of potent inhibitors of Axl: Design, synthesis, and biological evaluation. RSC Med. Chem. 2022, 13, 1246–1264. [Google Scholar] [CrossRef]
- Inoue, S.; Yamane, Y.; Tsukamoto, S.; Azuma, H.; Nagao, S.; Murai, N.; Nishibata, K.; Fukushima, S.; Ichikawa, K.; Nakagawa, T.; et al. Discovery of a potent and selective Axl inhibitor in preclinical model. Bioorg. Med. Chem. 2021, 39, 116137. [Google Scholar] [CrossRef] [PubMed]
- Kanlikilicer, P.; Ozpolat, B.; Aslan, B.; Bayraktar, R.; Gurbuz, N.; Rodriguez-Aguayo, C.; Bayraktar, E.; Denizli, M.; Gonzalez-Villasana, V.; Ivan, C.; et al. Therapeutic Targeting of AXL Receptor Tyrosine Kinase Inhibits Tumor Growth and Intraperitoneal Metastasis in Ovarian Cancer Models. Mol. Ther. Nucleic Acids 2017, 9, 251–262. [Google Scholar] [CrossRef]
- Byers, L.A.; Diao, L.; Wang, J.; Saintigny, P.; Girard, L.; Peyton, M.; Shen, L.; Fan, Y.; Giri, U.; Tumula, P.K.; et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies AXL as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 2013, 19, 279–290. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Dou, Y.; He, Y. AXL: Shapers of Tumor Progression and Immunosuppressive Microenvironments. Mol. Cancer 2025, 24, 11. [Google Scholar] [CrossRef]
- Lv, Y.; Zhu, J.; Ge, S.; Jiang, T.; Xu, Y.; Yao, W.; Jiang, C. The AXL-mediated modulation of myeloid-derived suppressor cells (MDSC) in nasopharyngeal carcinoma. Med. Oncol. 2024, 42, 17. [Google Scholar] [CrossRef]
- Woo, S.M.; Min, K.J.; Seo, S.U.; Kim, S.; Kubatka, P.; Park, J.W.; Kwon, T.K. Axl Inhibitor R428 Enhances TRAIL-Mediated Apoptosis Through Downregulation of c-FLIP and Survivin Expression in Renal Carcinoma. Int. J. Mol. Sci. 2019, 20, 3253. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Rychahou, P.G.; Qiu, S.; Evers, B.M.; Zhou, B.P. Stabilization of Snail by NF-κB is required for inflammation-induced cell migration and invasion. Cancer Cell 2009, 15, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.; Hope, C.; Huelse, J.M.; Graham, D.K. Prospects of Current AXL-Targeting Therapies in Early Phase Cancer Trials. Expert Opin. Investig. Drugs 2025, in press. [Google Scholar] [CrossRef]
- Bhalla, S.; Gerber, D.E. AXL Inhibitors: Status of Clinical Development. Curr. Oncol. Rep. 2023, 25, 521–529. [Google Scholar] [CrossRef]
- Taniguchi, H.; Yamada, T.; Wang, R.; Tanimura, K.; Adachi, Y.; Nishiyama, A.; Tanimoto, A.; Takeuchi, S.; Araujo, L.H.; Boroni, M.; et al. AXL Confers Intrinsic Resistance to Osimertinib and Advances the Emergence of Tolerant Cells. Nat. Commun. 2019, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.B.; Kim, J.H.; Kim, C.G.; Hong, M.H.; Kim, H.R.; Cho, B.C.; Lim, S.M. The Development of AXL Inhibitors in Lung Cancer: Recent Progress and Challenges. Front. Oncol. 2022, 12, 811247. [Google Scholar] [CrossRef]
- Veluswamy, R.; Bhalla, S.; Mehra, R.; Garassino, M.C.; Gligich, O.; Oliva, C.; Gorcea-Carson, C.; McCracken, N.W. Phase 1b/2a Safety and Tolerability Study of Bemcentinib (BEM) with Pembrolizumab/Carboplatin/Pemetrexed in First Line (1L) Advanced or Metastatic Non-Squamous Non-Small Cell Lung Cancer (NSCLC) Without/With a STK11 Mutation. J. Clin. Oncol. 2023, 41 (Suppl. S16), TPS9154. [Google Scholar] [CrossRef]
- Loges, S.; Heuser, M.; Chromik, J.; Sutamtewagul, G.; Kapp-Schwoerer, S.; Crugnola, M.; Di Renzo, N.; Lemoli, R.M.; Mattei, D.G.; Ben Batalla, I.; et al. Phase Ib/II Study (NCT02488408/BGBC003) of Bemcentinib Monotherapy or in Combination with Cytarabine or Decitabine in Acute Myeloid Leukemia (AML) or Myelodysplastic Syndrome (MDS): FINAL Results. Blood 2023, 142 (Suppl. S1), 4287. [Google Scholar] [CrossRef]
- Felip, E.; Brunsvig, P.; Vinolas, N.; Ponce Aix, S.; Carcereny Costa, E.; Dómine Gomez, M.; Trigo Perez, J.M.; Arriola, E.; Garcia Campelo, R.; Spicer, J.F.; et al. A phase II study of bemcentinib (BGB324), a first-in-class highly selective AXL inhibitor, with pembrolizumab in pts with advanced NSCLC: OS for stage I and preliminary stage II efficacy. J. Clin. Oncol. 2019, 37 (Suppl. S15), 9098. [Google Scholar] [CrossRef]
- Lorens, J.; Arce-Lara, C.E.; Arriola, E.; Brunsvig, P.; Carcereny Costa, E.; Domine, M.; Dragnev, K.H.; Felip, E.; Garcia Campelo, R.; Krebs, M.; et al. Phase II open-label, multi-centre study of bemcentinib (BGB324), a first-in-class selective AXL inhibitor, in combination with pembrolizumab in patients with advanced NSCLC. J. Clin. Oncol. 2018, 36 (Suppl. S15), 307. [Google Scholar] [CrossRef]
- Bhalla, S.; Fattah, F.J.; Williams, J.N.; Macchiaroli, A.; Padro, J.; Pogue, M.; Dowell, J.; Brekken, R.A.; Putnam, W.C.; McCracken, N.W.; et al. Phase 1 dose escalation and expansion study of bemcentinib (BGB324), a first-in-class, selective AXL inhibitor, with docetaxel in patients with previously treated advanced NSCLC. J. Clin. Oncol. 2022, 40 (Suppl. S16). [Google Scholar] [CrossRef]
- Felip, E.; Krebs, M.G.; Carcereny, E.; Smeland, K.B.; Arriola, E.; Llacer Perez, C.; Thompson, J.; Paz-Ares, L.; Domine Gomez, M.; Olivares, J.R.; et al. 1440P Final top-line results of the BGBC008 phase II, multicenter study of bemcentinib and pembrolizumab (bem+pembro) in second-line (2L) advanced non-squamous (NS) non-small cell lung cancer (NSCLC) (NCT03184571). Ann. Oncol. 2023, 34, S819. [Google Scholar] [CrossRef]
- Vandewalle, N.; De Beule, N.; De Becker, A.; De Bruyne, E.; Menu, E.; Vanderkerken, K.; Breckpot, K.; Devoogdt, N.; De Veirman, K. AXL as immune regulator and therapeutic target in acute myeloid leukemia: From current progress to novel strategies. Exp. Hematol. Oncol. 2024, 13, 99. [Google Scholar] [CrossRef]
- Kubasch, A.S.; Peterlin, P.; Cluzeau, T.; Götze, K.S.; Sockel, K.; Teipel, R.; Jentzsch, M.; Attalah, H.; Sebert, M.; Chermat, F.; et al. Efficacy and safety of bemcentinib in patients with advanced myelodysplastic neoplasms or acute myeloid leukemia failing hypomethylating agents—The EMSCO phase II BERGAMO trial. Leukemia 2023, 37, 2309–2313. [Google Scholar] [CrossRef]
- Dave, F.; Herrera, K.; Lockley, A.; van de Weijer, L.L.; Henderson, S.; Sofela, A.A.; Hook, L.; Adams, C.L.; Ercolano, E.; Hilton, D.A.; et al. Targeting MERTK on Tumour Cells and Macrophages: A Potential Intervention for Sporadic and NF2-Related Meningioma and Schwannoma Tumours. Oncogene 2024, 43, 3049–3061. [Google Scholar] [CrossRef]
- Li, K.M.; Deng, L.G.; Xue, L.J.; Tan, C.; Yao, S.K. AXL Inhibition Prevents RPA2/CHK1-Mediated Homologous Recombination to Increase PARP Inhibitor Sensitivity in Hepatocellular Carcinoma. Heliyon 2024, 10, e36283. [Google Scholar] [CrossRef]
- Arechederra, M.; Bazai, S.K.; Abdouni, A.; Sequera, C.; Mead, T.J.; Richelme, S.; Daian, F.; Audebert, S.; Dono, R.; Lozano, A.; et al. ADAMTSL5 Is an Epigenetically Activated Gene Underlying Tumorigenesis and Drug Resistance in Hepatocellular Carcinoma. J. Hepatol. 2021, 74, 893–906. [Google Scholar] [CrossRef]
- Grøndal, S.M.; Tutusaus, A.; Boix, L.; Reig, M.; Blø, M.; Hodneland, L.; Gausdal, G.; Jackson, A.; Garcia de Frutos, P.; Lorens, J.B.; et al. Dynamic changes in immune cell populations by AXL kinase targeting diminish liver inflammation and fibrosis in experimental MASH. Front. Immunol. 2024, 15, 1400553. [Google Scholar] [CrossRef] [PubMed]
- Danielli, S.G.; Wurth, J.; Morice, S.; Kisele, S.; Surdez, D.; Delattre, O.; Bode, P.K.; Wachtel, M.; Schäfer, B.W. Evaluation of the role of AXL in fusion-positive pediatric rhabdomyosarcoma identifies the small-molecule inhibitor bemcentinib (BGB324) as potent chemosensitizer. Mol. Cancer Ther. 2024, 23, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Gay, C.M.; Ramkumar, K.; Cargill, K.R.; Cardnell, R.J.; Nilsson, M.B.; Heeke, S.; Park, E.M.; Kundu, S.T.; Diao, L.; et al. Lung Cancer Models Reveal Severe Acute Respiratory Syndrome Coronavirus 2-Induced Epithelial-to-Mesenchymal Transition Contributes to Coronavirus Disease 2019 Pathophysiology. J. Thorac. Oncol. 2021, 16, 1821–1839. [Google Scholar] [CrossRef]
- Jordan, C.Z.; Tunbridge, M.; Husain, I.; Kitai, H.; Dilts, M.E.; Fay, O.K.; Abe, K.; Xiang, C.; Kwun, J.; Souma, T.; et al. AXL inhibition suppresses early allograft monocyte-to-macrophage differentiation and prolongs allograft survival. JCI Insight 2024, 9, e178502. [Google Scholar] [CrossRef]
- Gelebart, P.; Eriksen Gjerstad, M.; Benjaminsen, S.; Han, J.; Karlsen, I.; Safont, M.M.; Leitch, C.; Fandalyuk, Z.; Popa, M.; Helgeland, L.; et al. Inhibition of a New AXL Isoform, AXL3, Induces Apoptosis of Mantle Cell Lymphoma Cells. Blood 2023, 142, 1478–1493. [Google Scholar] [CrossRef]
- Lv, H.; Sun, H.; Wang, L.; Yao, S.; Liu, D.; Zhang, X.; Pei, Z.; Zhou, J.; Wang, H.; Dai, J.; et al. Targeting CD301⁺ Macrophages Inhibits Endometrial Fibrosis and Improves Pregnancy Outcome. EMBO Mol. Med. 2023, 15, e17601. [Google Scholar] [CrossRef] [PubMed]
- Batur, T.; Argundogan, A.; Keles, U.; Mutlu, Z.; Alotaibi, H.; Senturk, S.; Ozturk, M. AXL Knock-Out in SNU475 Hepatocellular Carcinoma Cells Provides Evidence for Lethal Effect Associated with G2 Arrest and Polyploidization. Int. J. Mol. Sci. 2021, 22, 13247. [Google Scholar] [CrossRef]
- Bian, Q.; Anderson, J.C.; Zhang, X.W.; Huang, Z.Q.; Ebefors, K.; Nyström, J.; Hall, S.; Novak, L.; Julian, B.A.; Willey, C.D.; et al. Mesangioproliferative Kidney Diseases and Platelet-Derived Growth Factor-Mediated AXL Phosphorylation. Kidney Med. 2021, 3, 1003–1013.e1. [Google Scholar] [CrossRef]
- Beitzen-Heineke, A.; Berenbrok, N.; Waizenegger, J.; Paesler, S.; Gensch, V.; Udonta, F.; Vargas Delgado, M.E.; Engelmann, J.; Hoffmann, F.; Schafhausen, P.; et al. AXL Inhibition Represents a Novel Therapeutic Approach in BCR-ABL Negative Myeloproliferative Neoplasms. Hemasphere 2021, 5, e630. [Google Scholar] [CrossRef] [PubMed]
- Hoel, A.; Osman, T.; Hoel, F.; Elsaid, H.; Chen, T.; Landolt, L.; Babickova, J.; Tronstad, K.J.; Lorens, J.B.; Gausdal, G.; et al. Axl-Inhibitor Bemcentinib Alleviates Mitochondrial Dysfunction in the Unilateral Ureter Obstruction Murine Model. J. Cell. Mol. Med. 2021, 25, 7407–7417. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.A.; Rodansky, E.S.; Johnson, L.A.; Berinstein, J.A.; Cushing, K.C.; Huang, S.; Spence, J.R.; Higgins, P.D.R. AXL Is a Potential Target for the Treatment of Intestinal Fibrosis. Inflamm. Bowel Dis. 2021, 27, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.A.; Ham, I.H.; Oh, H.J.; Lee, D.; Woo, J.; Son, S.Y.; Yoon, J.H.; Lorens, J.B.; Brekken, R.A.; Kim, T.M.; et al. Inhibiting the GAS6/AXL Axis Suppresses Tumor Progression by Blocking the Interaction between Cancer-Associated Fibroblasts and Cancer Cells in Gastric Carcinoma. Gastric Cancer 2020, 23, 824–836. [Google Scholar] [CrossRef]
- Flem-Karlsen, K.; Nyakas, M.; Farstad, I.N.; McFadden, E.; Wernhoff, P.; Jacobsen, K.D.; Flørenes, V.A.; Mælandsmo, G.M. Soluble AXL as a Marker of Disease Progression and Survival in Melanoma. PLoS ONE 2020, 15, e0227187. [Google Scholar] [CrossRef]
- Tutusaus, A.; de Gregorio, E.; Cucarull, B.; Cristóbal, H.; Aresté, C.; Graupera, I.; Coll, M.; Colell, A.; Gausdal, G.; Lorens, J.B.; et al. A Functional Role of GAS6/TAM in Nonalcoholic Steatohepatitis Progression Implicates AXL as Therapeutic Target. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 349–368. [Google Scholar] [CrossRef]
- Malvankar, C.; Kumar, D. AXL kinase inhibitors—A prospective model for medicinal chemistry strategies in anticancer drug discovery. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188786. [Google Scholar] [CrossRef]
- Loges, S.; Heuser, M.; Chromik, J.; Vigil, C.E.; Paschka, P.; Ben-Batalla, I.; Akyüz, N.; Micklem, D.; Holt, R.; Brown, A.; et al. Final analysis of the dose escalation, expansion and biomarker correlations in the Ph I/II trial BGBC003 with the selective oral AXL inhibitor bemcentinib (BGB324) in relapsed/refractory AML and MDS. Blood 2018, 132 (Suppl. S1), 2672. [Google Scholar] [CrossRef]
- Budha, N.R.; Ji, T.; Musib, L.; Eppler, S.; Dresser, M.; Chen, Y.; Jin, J.Y. Evaluation of cytochrome P450 3A4-mediated drug-drug interaction potential for cobimetinib using physiologically based pharmacokinetic modeling and simulation. Clin. Pharmacokinet. 2016, 55, 1435–1445. [Google Scholar] [CrossRef]
- Guengerich, F.P. Inhibition of cytochrome P450 enzymes by drugs—Molecular basis and practical applications. Biomol. Ther. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Wang, Q.; Liu, Z.; Dong, X.; Shi, S.; Xiao, H. Factors influencing the steady-state plasma concentration of imatinib mesylate in patients with gastrointestinal stromal tumors and chronic myeloid leukemia. Front. Pharmacol. 2020, 11, 569843. [Google Scholar] [CrossRef]
- Bemcentinib: A Promising New Cancer Treatment. Available online: https://clinicaltrials.eu/inn/bemcentinib/ (accessed on 9 June 2025).
- Wium, M.; Ajayi-Smith, A.F.; Paccez, J.D.; Zerbini, L.F. The role of the receptor tyrosine kinase Axl in carcinogenesis and development of therapeutic resistance: An overview of molecular mechanisms and future applications. Cancers 2021, 13, 1521. [Google Scholar] [CrossRef] [PubMed]
- Identification of Predictive and Pharmacodynamic Biomarkers Associated with the First-in-Class Selective AXL Inhibitor Bemcentinib Across Multiple Phase II Clinical Trials. Available online: https://www.asco.org/abstracts-presentations/ABSTRACT228259 (accessed on 9 June 2025).
- Bemcentinib (BGB324) in Combination with Pembrolizumab in Patients with Advanced NSCLC. Available online: https://clinicaltrials.gov/study/NCT03184571 (accessed on 9 June 2025).
- Spicer, J.; Helland, Å.; Carcereny, E.; Arriola, E.; Dómine Gomez, M.; Trigo Perez, J.M.; Thompson, J.; Strauss, J.; Ortega Granados, A.L.; Felip, E.; et al. A PhII study of bemcentinib, a first-in-class selective AXL kinase inhibitor, in combination with pembrolizumab in pts with previously-treated advanced NSCLC: Updated clinical & translational analysis. J. Immunother. Cancer 2020, 8 (Suppl. S2), A362. [Google Scholar] [CrossRef]
- Axl Inhibitors for Aggressive Disease. Available online: https://bgbwebpagefiles.fra1.cdn.digitaloceanspaces.com/wp-content/uploads/2020/01/BerGenBio-corp-Jan-2020.pdf (accessed on 9 June 2025).
- Phase 1b/2a Trial of Bemcentinib and SOC Doses First Patient with STK11m NSCLC. Available online: https://www.targetedonc.com/view/phase-1b-2a-trial-of-bemcentinib-and-soc-doses-first-patient-with-stk11m-nsclc (accessed on 9 June 2025).
- Malekinejad, Z.; Baghbanzadeh, A.; Nakhlband, A.; Baradaran, B.; Jafari, S.; Bagheri, Y.; Raei, F.; Montazersaheb, S.; Farahzadi, R. Recent clinical findings on the role of kinase inhibitors in COVID-19 management. Life Sci. 2022, 306, 120809. [Google Scholar] [CrossRef]
- Davis, F.M.; Stewart, T.A.; Thompson, E.W.; Monteith, G.R. Targeting EMT in cancer: Opportunities for pharmacological intervention. Trends Pharmacol. Sci. 2014, 35, 479–488. [Google Scholar] [CrossRef]
- Zhan, M.; Liu, D.; Yao, L.; Wang, W.; Zhang, R.; Xu, Y.; Wang, Z.; Yan, Q.; Fang, Q.; Du, J.; et al. Gas6/AXL Alleviates Hepatic Ischemia/Reperfusion Injury by Inhibiting Ferroptosis via the PI3K/AKT Pathway. Transplantation 2024, 108, e357–e369. [Google Scholar] [CrossRef]
- Zuo, R.C.; Apolo, A.B.; DiGiovanna, J.J.; Parnes, H.L.; Keen, C.M.; Nanda, S.; Dahut, W.L.; Cowen, E.W. Cutaneous Adverse Effects Associated with the Tyrosine-Kinase Inhibitor Cabozantinib. JAMA Dermatol. 2015, 151, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Loges, S.; Heuser, M.; Chromik, J.; Sutamtewagul, G.; Kapp-Schwoerer, S.; Crugnola, M.; Di Renzo, N.; Lemoli, R.; Mattei, D.; Fiedler, W.; et al. Bemcentinib as Monotherapy and in Combination with Low-Dose Cytarabine in Acute Myeloid Leukemia Patients Unfit for Intensive Chemotherapy: A Phase 1b/2a Trial. Nat. Commun. 2025, 16, 2846. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Shibata, S.; Koyama, A.; Li, L.; Sugimoto, E.; Taira, H.; Mizuno, Y.; Awaji, K.; Sato, S. Decreased Epidermal AXL Expression and Increased Infiltration of AXL-Expressing Dendritic Cells in Psoriasis. J. Cutan. Immunol. Allergy 2023, 6, 208–218. [Google Scholar] [CrossRef]
- Bumm, C.V.; Folwaczny, M.; Wölfle, U.C. Necrotizing Periodontitis or Medication-Related Osteonecrosis of the Jaw (MRONJ) in a Patient Receiving Bemcentinib—A Case Report. Oral Maxillofac. Surg. 2020, 24, 353–358. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Zhou, Y.L.; Guo, X.; Ge, J.; Fu, J. Immune-Related Adverse Events from Combination Immunotherapy in Cancer Patients: A Comprehensive Meta-Analysis of Randomized Controlled Trials. Int. Immunopharmacol. 2018, 63, 292–298. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, H.; He, Y.; Liu, L.; Huang, I.B.; Zhou, L.; Lin, C.Y.; Leung, R.W.; Loh, J.J.; Lee, T.K.; et al. Targeting AXL Induces Tumor-Intrinsic Immunogenic Response in Tyrosine Kinase Inhibitor-Resistant Liver Cancer. Cell Death Dis. 2024, 15, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, L.; Zhang, J.; Yang, J.; Xiao, X.; Shan, L.; Mao, W. Recent Discovery and Development of AXL Inhibitors as Antitumor Agents. Eur. J. Med. Chem. 2024, 272, 116475. [Google Scholar] [CrossRef] [PubMed]
- Tomuleasa, C.; Tigu, A.B.; Munteanu, R.; Moldovan, C.S.; Kegyes, D.; Onaciu, A.; Gulei, D.; Ghiaur, G.; Einsele, H.; Croce, C.M. Therapeutic Advances of Targeting Receptor Tyrosine Kinases in Cancer. Signal Transduct. Target Ther. 2024, 9, 201. [Google Scholar] [CrossRef]
- Shah, K.; Gopal, K.; Kumar, S.; Saha, S. Emerging AXL Inhibitors in Oncology: Chemical and Biological Advances in Targeted Cancer Therapy. Anticancer Agents Med. Chem. 2025, 25, 460–467. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, Y.; Feng, H.; Cai, J.; Liu, Y.; Li, Y.; Chen, S.; Zhou, Z.; Du, Y.; Zeng, X.; et al. PFKP Silencing Suppresses Tumor Growth via the AXL-MET Axis. Int. J. Biol. Sci. 2024, 20, 6056–6072. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Liu, L.; Zhang, H.; Han, C.; Girard, L.; Park, H.; Zhang, A.; Dong, C.; Ye, J.; et al. AXL Targeting Restores PD-1 Blockade Sensitivity of STK11/LKB1 Mutant NSCLC through Expansion of TCF1⁺ CD8 T Cells. Cell Rep. Med. 2022, 3, 100554. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, L.; Huang, Z.; Ruan, Z.; Yan, H.; Zou, C.; Xu, S.; Zhang, Y. Strategies Beyond 3rd EGFR-TKI Acquired Resistance: Opportunities and Challenges. Cancer Med. 2025, 14, e70921. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, X.; Koul, S.; Lee, C.Y.; Zhang, Z.; Halmos, B. AXL kinase as a novel target for cancer therapy. Oncotarget 2014, 5, 9546–9563. [Google Scholar] [CrossRef]
- Elkabets, M.; Vora, S.; Juric, D.; Morse, N.; Mino-Kenudson, M.; Muranen, T.; Tao, J.; Campos, A.B.; Rodon, J.; Ibrahim, Y.H.; et al. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci. Transl. Med. 2013, 5, 196ra99. [Google Scholar] [CrossRef]
- Zabludoff, S.D.; Deng, C.; Grondine, M.R.; Sheehy, A.M.; Ashwell, S.; Caleb, B.L.; Green, S.; Haye, H.R.; Horn, C.L.; Janetka, J.W.; et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol. Cancer Ther. 2008, 7, 2955–2966. [Google Scholar] [CrossRef]
- BerGenBio to Present Interim Clinical and Biomarker Data with Selective AXL Inhibitor Bemcentinib in AML and MDS at EHA. Available online: https://www.prnewswire.com/news-releases/bergenbio-to-present-interim-clinical-and-biomarker-data-with-selective-axl-inhibitor-bemcentinib-in-aml-and-mds-at-eha-300650898.html (accessed on 9 June 2025).
- Annexes to the Annual Report of the European Medicines Agency 2024. Available online: https://www.ema.europa.eu/en/documents/annual-report/annexes-2024-annual-report-european-medicines-agency_en.pdf (accessed on 9 June 2025).
- Yang, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: Molecular mechanisms and future perspective. Signal Transduct Target Ther. 2022, 7, 329. [Google Scholar] [CrossRef]
- Myers, S.H.; Brunton, V.G.; Unciti-Broceta, A. AXL Inhibitors in Cancer: A Medicinal Chemistry Perspective. J. Med. Chem. 2016, 59, 3593–3608. [Google Scholar] [CrossRef]
- Foley, C.N.; Qu, S.; Paladugu, S.R.; Lamani, M.; Grange, R.; Sharif, E.U.; Thomas, J.; Nareddy, P.; Zhao, G.; Chen, Y.; et al. Discovery and Characterization of Potent, Selective, and Orally Bioavailable 7-Azaindazole AXL Receptor Tyrosine Kinase Inhibitors. J. Med. Chem. 2025, 68, 10677–10703. [Google Scholar] [CrossRef] [PubMed]
- Study of Bemcentinib with Pembrolizumab, Carboplatin, and Pemetrexed for Patients with Advanced or Metastatic Non-Small Cell Lung Cancer with STK11 Mutation. Available online: https://clinicaltrials.eu/trial/study-of-bemcentinib-with-pembrolizumab-carboplatin-and-pemetrexed-for-patients-with-advanced-or-metastatic-non-small-cell-lung-cancer-with-stk11-mutation/ (accessed on 5 June 2025).
- Tang, Y.; Zang, H.; Wen, Q.; Fan, S. AXL in cancer: A modulator of drug resistance and therapeutic target. J. Exp. Clin. Cancer Res. 2023, 42, 148. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Denoyelle, C.; Ohashi, P.S.; De Bono, J.S.; Mottaghy, F.M. Molecularly targeted therapies in cancer: A guide for the nuclear medicine physician. Eur. J. Nucl. Med. Mol. Imaging 2017, 44 (Suppl. S1), 41–54. [Google Scholar] [CrossRef]
- Pfohl, U.; Pflaume, A.; Regenbrecht, M.; Finkler, S.; Graf Adelmann, Q.; Reinhard, C.; Regenbrecht, C.R.A.; Wedeken, L. Precision Oncology Beyond Genomics: The Future Is Here—It Is Just Not Evenly Distributed. Cells 2021, 10, 928. [Google Scholar] [CrossRef]
- AXL Inhibition Improves BRAF-Targeted Treatment in Melanoma. Cutaneous Metastatic Melanoma Searching for Biomarkers and New Treatment. Nyakas, M.S. Available online: https://www.duo.uio.no/bitstream/handle/10852/117853/PhD-Nyakas-2025.pdf%3Fsequence%3D1%26isAllowed%3Dy&ved=2ahUKEwjH3Nzs1IGOAxUdSvEDHYKrOL8QFnoECB4QAQ&usg=AOvVaw0as92GmBq8gp0KVvZzw7aW (accessed on 9 June 2025).
- Riillo, C.; Polerà, N.; Di Martino, M.T.; Juli, G.; Hokanson, C.A.; Odineca, T.; Signorelli, S.; Grillone, K.; Ascrizzi, S.; Mancuso, A.; et al. A Pronectin™ AXL-Targeted First-in-Class Bispecific T Cell Engager (pAXLxCD3ε) for Ovarian Cancer. J. Transl. Med. 2023, 21, 301. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.D.; Prieto-Dominguez, N.; Gowda, P.S.; Ubil, E. Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 2021, 12, 642285. [Google Scholar] [CrossRef]
- Onken, J.; Torka, R.; Korsing, S.; Radke, J.; Krementeskaia, I.; Nieminen, M.; Bai, X.; Ullrich, A.; Heppner, F.; Vajkoczy, P. Inhibiting Receptor Tyrosine Kinase AXL with Small Molecule Inhibitor BMS-777607 Reduces Glioblastoma Growth, Migration, and Invasion In Vitro and In Vivo. Oncotarget 2016, 7, 9876–9889. [Google Scholar] [CrossRef]
- Li, M.C.; Lai, Y.L.; Kuo, P.H.; Reddy, J.S.; Chen, C.M.; Manimala, J.; Wang, P.C.; Wu, M.S.; Chang, C.Y.; Yang, C.M.; et al. Discovery of Dual MER/AXL Kinase Inhibitors as Bifunctional Small Molecules for Inhibiting Tumor Growth and Enhancing Tumor Immune Microenvironment. J. Med. Chem. 2024, 67, 10906–10927. [Google Scholar] [CrossRef]
- Sakemura, R.L.; Hefazi, M.; Cox, M.J.; Siegler, E.L.; Sinha, S.; Hansen, M.J.; Stewart, C.M.; Feigin, J.M.; Manriquez Roman, C.; Schick, K.J.; et al. AXL Inhibition Improves the Antitumor Activity of Chimeric Antigen Receptor T Cells. Cancer Immunol. Res. 2023, 11, 1222–1236. [Google Scholar] [CrossRef]
- Datta, A.; Bahlmann, L.C.; Gong, D.N.; Tevonian, E.N.; Lorens, J.B.; Lauffenburger, D.A. Axl Inhibitor-Mediated Reprogramming of the Myeloid Compartment of the In Vitro Tumor Microenvironment Is Influenced by Prior Targeted Therapy Treatment. Front. Immunol. 2025, 16, 1601420. [Google Scholar] [CrossRef]
- Rayford, A.; Gärtner, F.; Ramnefjell, M.P.; Lorens, J.B.; Micklem, D.R.; Aanerud, M.; Engelsen, A.S.T. AXL Expression Reflects Tumor-Immune Cell Dynamics Impacting Outcome in Non-Small Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitor Monotherapy. Front. Immunol. 2024, 15, 1444007. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Yacoub, N.; Mishra, R.; White, A.; Long, Y.; Alanazi, S.; Garrett, J.T. Current Advances in the Treatment of BRAF-Mutant Melanoma. Cancers 2020, 12, 482. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Yang, Y.-X.; Liu, C.; Quan, X.-Y.; Zhao, Z.-H.; Jin, C.-H. Synthesis and Biological Evaluation of Sulfonamide Derivatives Containing Imidazole Moiety as ALK5 Inhibitors. Mol. Divers. 2025, 29, 2143–2156. [Google Scholar] [CrossRef]
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Yingling, J.M.; McMillen, W.T.; Yan, L.; Huang, H.; Sawyer, J.S.; Graff, J.; Clawson, D.K.; Britt, K.S.; Anderson, B.D.; Beight, D.W.; et al. Preclinical Assessment of Galunisertib (LY2157299 Monohydrate), a First-in-Class Transforming Growth Factor-β Receptor Type I Inhibitor. Oncotarget 2017, 9, 6659–6677. [Google Scholar] [CrossRef]
- Yen, Y.T.; Zhang, Z.; Chen, A.; Qiu, Y.; Liu, Q.; Wang, Q.; Li, C.; Wang, C.; Qian, X.; Shao, J.; et al. Enzymatically Responsive Nanocarriers Targeting PD-1 and TGF-β Pathways Reverse Immunotherapeutic Resistance and Elicit Robust Therapeutic Efficacy. J. Nanobiotechnol. 2025, 23, 124. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.J.; Bell, D.W. The Endometrial Cancer A230V-ALK5 (TGFBR1) Mutant Attenuates TGF-β Signaling and Exhibits Reduced In Vitro Sensitivity to ALK5 Inhibitors. PLoS ONE 2024, 19, e0312806. [Google Scholar] [CrossRef]
- Ikushima, H.; Miyazono, K. TGFβ signalling: A complex web in cancer progression. Nat. Rev. Cancer 2010, 10, 415–424. [Google Scholar] [CrossRef]
- David, C.J.; Massagué, J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef]
- Bataller, A.; Montalban-Bravo, G.; Soltysiak, K.A.; Garcia-Manero, G. The role of TGFβ in hematopoiesis and myeloid disorders. Leukemia 2019, 33, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Levy, L.; Hill, C.S. Smad4 dependency defines two classes of transforming growth factor β (TGF-β) target genes and distinguishes TGF-β-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol. Cell. Biol. 2005, 25, 8108–8125. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H. Autocrine TGF-β Signaling in the Pathogenesis of Systemic Sclerosis. J. Dermatol. Sci. 2008, 49, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, S.; Manning, C.; Hooper, S.; Jones, L.; Hill, C.S.; Sahai, E. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nat. Cell Biol. 2009, 11, 1287–1296. [Google Scholar] [CrossRef]
- Delle Cave, D.; Mangini, M.; Tramontano, C.; De Stefano, L.; Corona, M.; Rea, I.; De Luca, A.C.; Lonardo, E. Hybrid Biosilica Nanoparticles for In Vivo Targeted Inhibition of Colorectal Cancer Growth and Label-Free Imaging. Int. J. Nanomed. 2024, 19, 12079–12098. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-β signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Giannelli, G.; Villa, E.; Lahn, M. Transforming growth factor-β as a therapeutic target in hepatocellular carcinoma. Cancer Res. 2014, 74, 1890–1894. [Google Scholar] [CrossRef]
- Bloom, M.; Podder, S.; Dang, H.; Lin, D. Advances in Immunotherapy in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2025, 26, 1936. [Google Scholar] [CrossRef]
- Scialpi, R.; Espinosa-Sotelo, R.; Bertran, E.; Dituri, F.; Giannelli, G.; Fabregat, I. New Hepatocellular Carcinoma (HCC) Primary Cell Cultures as Models for Exploring Personalized Anti-TGF-β Therapies Based on Tumor Characteristics. Int. J. Mol. Sci. 2025, 26, 2430. [Google Scholar] [CrossRef] [PubMed]
- Panzarini, E.; Leporatti, S.; Tenuzzo, B.A.; Quarta, A.; Hanafy, N.A.N.; Giannelli, G.; Moliterni, C.; Vardanyan, D.; Sbarigia, C.; Fidaleo, M.; et al. Therapeutic Effect of Polymeric Nanomicelles Formulation of LY2157299-Galunisertib on CCl4-Induced Liver Fibrosis in Rats. J. Pers. Med. 2022, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Maas, R.J.A.; Hoogstad-van Evert, J.S.; Hagemans, I.M.; Brummelman, J.; van Ens, D.; de Jonge, P.K.J.D.; Hooijmaijers, L.; Mahajan, S.; van der Waart, A.B.; Hermans, C.K.J.C.; et al. Increased Peritoneal TGF-β1 Is Associated with Ascites-Induced NK-Cell Dysfunction and Reduced Survival in High-Grade Epithelial Ovarian Cancer. Front. Immunol. 2024, 15, 1448041. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Yang, Y.; Wang, P.; Xie, H.; Jin, S.; Zhao, L.; Wu, G.; Xing, H.; Chen, H.; Liu, B.; et al. CCDC113 Promotes Colorectal Cancer Tumorigenesis and Metastasis via TGF-β Signaling Pathway. Cell Death Dis. 2024, 15, 666. [Google Scholar] [CrossRef]
- Infante, A.; Alcorta-Sevillano, N.; Macías, I.; Cabodevilla, L.; Medhat, D.; Lafaver, B.; Crawford, T.K.; Phillips, C.L.; Bueno, A.M.; Sagastizabal, B.; et al. Galunisertib Downregulates Mutant Type I Collagen Expression and Promotes MSCs Osteogenesis in Pediatric Osteogenesis Imperfecta. Biomed. Pharmacother. 2024, 175, 116725. [Google Scholar] [CrossRef]
- Jank, B.J.; Lenz, T.; Haas, M.; Kadletz-Wanke, L.; Campion, N.J.; Schnoell, J.; Heiduschka, G.; Macfelda, K. Radiosensitizing Effect of Galunisertib, a TGF-β Receptor I Inhibitor, on Head and Neck Squamous Cell Carcinoma In Vitro. Investig. New Drugs 2022, 40, 478–486. [Google Scholar] [CrossRef]
- Gu, S.; Feng, X.H. TGF-β Signaling in Cancer. Acta Biochim. Biophys. Sin. 2018, 50, 941–949. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Nokihara, H.; Yamada, Y.; Yamamoto, N.; Sunami, K.; Utsumi, H.; Asou, H.; Takahashi, O.; Ogasawara, K.; Gueorguieva, I.; et al. Phase 1 Study of Galunisertib, a TGF-beta Receptor I Kinase Inhibitor, in Japanese Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2015, 76, 1143–1152. [Google Scholar] [CrossRef]
- Faivre, S.; Santoro, A.; Kelley, R.K.; Gane, E.; Costentin, C.E.; Gueorguieva, I.; Smith, C.; Cleverly, A.; Lahn, M.M.; Raymond, E.; et al. Novel Transforming Growth Factor Beta Receptor I Kinase Inhibitor Galunisertib (LY2157299) in Advanced Hepatocellular Carcinoma. Liver Int. 2019, 39, 1468–1477. [Google Scholar] [CrossRef]
- Feng, X.H.; Derynck, R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef]
- Heldin, C.H.; Moustakas, A. Signaling receptors for TGF-β family members. Cold Spring Harb. Perspect. Biol. 2016, 8, a022053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Hou, X.; Evans, B.J.; VanBlaricom, J.L.; Weroha, S.J.; Cliby, W.A. LY2157299 Monohydrate, a TGF-βR1 Inhibitor, Suppresses Tumor Growth and Ascites Development in Ovarian Cancer. Cancers 2018, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, Y.T.; Liu, J.; Gan, L.; Jiang, Y. Role of Transforming Growth Factor-β1 Pathway in Angiogenesis Induced by Chronic Stress in Colorectal Cancer. Cancer Biol. Ther. 2024, 25, 2366451. [Google Scholar] [CrossRef]
- Yin, L. Combination Therapy of Bevacizumab and Galunisertib Extends TVN Time Window. Mol. Ther. Oncol. 2024, 32, 200888. [Google Scholar] [CrossRef]
- Kuburich, N.A.; Sabapathy, T.; Demestichas, B.R.; Maddela, J.J.; den Hollander, P.; Mani, S.A. Proactive and reactive roles of TGF-β in cancer. Semin. Cancer Biol. 2023, 95, 120–139. [Google Scholar] [CrossRef]
- Yang, L.L.; Chen, X.; Huang, K.T.; Wang, J.L. Global Trends in Hepatocellular Carcinoma and TGF-β Research: A Bibliometric and Visualization Analysis from 2000 to 2024. Curr. Protein Pept. Sci. 2025, (Online ahead of print). 1–19. [Google Scholar] [CrossRef]
- Giannelli, G.; Santoro, A.; Kelley, R.K.; Gane, E.; Paradis, V.; Cleverly, A.; Smith, C.; Estrem, S.T.; Man, M.; Wang, S.; et al. Biomarkers and Overall Survival in Patients with Advanced Hepatocellular Carcinoma Treated with TGF-βRI Inhibitor Galunisertib. PLoS ONE 2020, 15, e0222259. [Google Scholar] [CrossRef]
- Gungor, M.Z.; Uysal, M.; Senturk, S. The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications. Cancers 2022, 14, 940. [Google Scholar] [CrossRef]
- Brandes, A.A.; Carpentier, A.F.; Kesari, S.; Sepulveda-Sanchez, J.M.; Wheeler, H.R.; Chinot, O.; Cher, L.; Steinbach, J.P.; Capper, D.; Specenier, P.; et al. A Phase II Randomized Study of Galunisertib Monotherapy or Galunisertib Plus Lomustine Compared with Lomustine Monotherapy in Patients with Recurrent Glioblastoma. Neuro Oncol. 2016, 18, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Hadizadeh, M.; AminJafari, A.; Parvizpour, S.; Ghasemi, S. Novel Targets to Overcome Antiangiogenesis Therapy Resistance in Glioblastoma Multiforme: Systems Biology Approach and Suggestion of Therapy by Galunisertib. Cell Biol. Int. 2022, 46, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Yang, H.H.; Liu, J.W.; Lee, J.H.; Harn, H.J.; Chiou, T.W. Pancreatic Adenocarcinoma Therapeutics Targeting RTK and TGF Beta Receptor. Int. J. Mol. Sci. 2021, 22, 8125. [Google Scholar] [CrossRef] [PubMed]
- Pietrobono, S.; Bertolini, M.; De Vita, V.; Sabbadini, F.; Fazzini, F.; Frusteri, C.; Scarlato, E.; Mangiameli, D.; Quinzii, A.; Casalino, S.; et al. CCL3 Predicts Exceptional Response to TGFβ Inhibition in Basal-Like Pancreatic Cancer Enriched in LIF-Producing Macrophages. NPJ Precis. Oncol. 2024, 8, 246. [Google Scholar] [CrossRef]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ Pathway for Cancer Therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massagué, J. TGF-β Inhibition and Immunotherapy: Checkmate. Immunity 2018, 48, 626–628. [Google Scholar] [CrossRef]
- Shi, L.; Sheng, J.; Wang, M.; Luo, H.; Zhu, J.; Zhang, B.; Liu, Z.; Yang, X. Combination Therapy of TGF-β Blockade and Commensal-Derived Probiotics Provides Enhanced Antitumor Immune Response and Tumor Suppression. Theranostics 2019, 9, 4115–4129. [Google Scholar] [CrossRef]
- Study of Galunisertib and Capecitabine for Patients with Advanced Chemotherapy-Resistant Colorectal Cancer with Peritoneal Metastases. Available online: https://clinicaltrials.eu/trial/study-of-galunisertib-and-capecitabine-for-patients-with-advanced-chemotherapy-resistant-colorectal-cancer-with-peritoneal-metastases/ (accessed on 9 June 2025).
- Tschernia, N.P.; Gulley, J.L. Tumor in the Crossfire: Inhibiting TGF-β to Enhance Cancer Immunotherapy. BioDrugs 2022, 36, 153–180. [Google Scholar] [CrossRef]
- Chiechi, A.; Waning, D.L.; Stayrook, K.R.; Buijs, J.T.; Guise, T.A.; Mohammad, K.S. Role of TGF-β in Breast Cancer Bone Metastases. Adv. Biosci. Biotechnol. 2013, 4, 15–30. [Google Scholar] [CrossRef]
- Peterson, J.M.; Jay, J.W.; Wang, Y.; Joglar, A.A.; Prasai, A.; Palackic, A.; Wolf, S.E.; El Ayadi, A. Galunisertib Exerts Antifibrotic Effects on TGF-β-Induced Fibroproliferative Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 6689. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad pathways in TGF-β signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Deng, P. Discovery of New Inhibitors of Transforming Growth Factor-Beta Type 1 Receptor by Utilizing Docking and Structure-Activity Relationship Analysis. Int. J. Mol. Sci. 2019, 20, 4090. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, W.; Wang, H.; Zhang, C.; Li, J.; Chen, W.; Xu, X.; Wang, L.; Ma, M.; Zhang, S.; et al. Helicobacter pylori Promotes Gastric Cancer Progression by Activating the TGF-β/Smad2/EMT Pathway through HKDC1. Cell. Mol. Life Sci. 2024, 81, 453. [Google Scholar] [CrossRef]
- Mao, Y.; Xu, W.; Chen, L.; Liao, H. Computational Drug Repurposing Screening Targeting Profibrotic Cytokine in Acute Respiratory Distress Syndrome. Cell Biochem. Biophys. 2025, 83, 3877–3888. [Google Scholar] [CrossRef]
- de Vasconcellos, J.F.; Westbrook, P.; Dingle, M.; Dimtchev, A.; Raiciulescu, S.; Schellhase, C.W.; Piscoya, A.; Putko, R.; Bedrin, M.; Cole, H.; et al. Preclinical validation of TGFβ inhibitors as a novel therapeutic strategy for post-traumatic heterotopic ossification. Sci. Rep. 2025, 15, 14277. [Google Scholar] [CrossRef]
- Heldin, C.H.; Vanlandewijck, M.; Moustakas, A. Regulation of EMT by TGFβ in Cancer. FEBS Lett. 2012, 586, 1959–1970. [Google Scholar] [CrossRef]
- Principe, D.R.; DeCant, B.; Mascariñas, E.; Wayne, E.A.; Diaz, A.M.; Akagi, N.; Hwang, R.; Pasche, B.; Dawson, D.W.; Fang, D.; et al. TGFβ Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res. 2016, 76, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, S.; Ferdous, U.T.; Nizela, A.; Hasan, A.; Shakoor, A.; Zia, A.W.; Uddin, S. Mechanistic Study of Cancer Drug Delivery: Current Techniques, Limitations, and Future Prospects. Eur. J. Med. Chem. 2025, 290, 117535. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Schlom, J.; Barcellos-Hoff, M.H.; Wang, X.J.; Seoane, J.; Audhuy, F.; Lan, Y.; Dussault, I.; Moustakas, A. Dual Inhibition of TGF-β and PD-L1: A Novel Approach to Cancer Treatment. Mol. Oncol. 2022, 16, 2117–2134. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, A.; Yang, Y.; Williams, K.; Gogineni, A.; Lane, R.S.; Wang, A.W.; Shyer, J.A.; Zhang, Z.; Mittman, S.; Gutierrez, A.; et al. Combined PD-L1/TGFβ Blockade Allows Expansion and Differentiation of Stem Cell-Like CD8 T Cells in Immune Excluded Tumors. Nat. Commun. 2023, 14, 4703. [Google Scholar] [CrossRef]
- Seoane, J.; Gomis, R.R. TGF-β Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef]
- Alsaffar, R.M.; Ali, S.; Rashid, S.; Rashid, S.M.; Majid, S.; Rehman, M.U. Immunomodulation: An Immune Regulatory Mechanism in Carcinoma Therapeutics. Int. Immunopharmacol. 2021, 99, 107984. [Google Scholar] [CrossRef]
- Wagner, L.I.; Cella, D. Fatigue and Cancer: Causes, Prevalence and Treatment Approaches. Br. J. Cancer 2004, 91, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Valcarcel, D.; Verma, A.; Platzbecker, U.; Santini, V.; Giagounidis, A.; Díez-Campelo, M.; Janssen, J.; Schlenk, R.F.; Gaidano, G.; Perez de Oteyza, J.; et al. Phase 2 Study of Monotherapy Galunisertib (LY2157299 Monohydrate) in Very Low-, Low-, and Intermediate-Risk Patients with Myelodysplastic Syndromes. Blood 2015, 126, 1669. [Google Scholar] [CrossRef]
- Niyongere, S.; Saltos, A.; Gray, J.E. Immunotherapy Combination Strategies (Non-Chemotherapy) in Non-Small Cell Lung Cancer. J. Thorac. Dis. 2018, 10 (Suppl. S3), S433–S450. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, R.J.; Maldonado, G.; Azaro, A.; Fernández, M.S.; Romero, F.L.; Sepulveda-Sánchez, J.M.; Corretti, M.; Carducci, M.; Dolan, M.; Gueorguieva, I.; et al. Cardiac Safety of TGF-β Receptor I Kinase Inhibitor LY2157299 Monohydrate in Cancer Patients in a First-in-Human Dose Study. Cardiovasc. Toxicol. 2015, 15, 309–323. [Google Scholar] [CrossRef]
- Chen, H.; Fan, L.; Peng, N.; Yin, Y.; Mu, D.; Wang, J.; Meng, R.; Xie, J. Galunisertib-Loaded Gelatin Methacryloyl Hydrogel Microneedle Patch for Cardiac Repair after Myocardial Infarction. ACS Appl. Mater. Interfaces 2022, 14, 40491–40500. [Google Scholar] [CrossRef] [PubMed]
- Yingling, J.M.; Blanchard, K.L.; Sawyer, J.S. Development of TGF-beta signalling inhibitors for cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 1011–1022. [Google Scholar] [CrossRef]
- Kelley, R.K.; Gane, E.; Assenat, E.; Siebler, J.; Galle, P.R.; Merle, P.; Hourmand, I.O.; Cleverly, A.; Zhao, Y.; Gueorguieva, I.; et al. A Phase 2 Study of Galunisertib (TGF-β1 Receptor Type I Inhibitor) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2019, 10, e00056. [Google Scholar] [CrossRef]
- Melisi, D.; Garcia-Carbonero, R.; Macarulla, T.; Pezet, D.; Deplanque, G.; Fuchs, M.; Trojan, J.; Kozloff, M.; Simionato, F.; Cleverly, A.; et al. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother. Pharmacol. 2019, 83, 975–991. [Google Scholar] [CrossRef]
- Gonzalez-Sanchez, E.; Vaquero, J.; Férnandez-Barrena, M.G.; Lasarte, J.J.; Avila, M.A.; Sarobe, P.; Reig, M.; Calvo, M.; Fabregat, I. The TGF-β Pathway: A Pharmacological Target in Hepatocellular Carcinoma? Cancers 2021, 13, 3248. [Google Scholar] [CrossRef]
- Strauss, J.; Heery, C.R.; Schlom, J.; Madan, R.A.; Cao, L.; Kang, Z.; Lamping, E.; Marté, J.L.; Donahue, R.N.; Grenga, I.; et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 1287–1295. [Google Scholar] [CrossRef]
- Jung, B.; Staudacher, J.J.; Beauchamp, D. Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology 2017, 152, 36–52. [Google Scholar] [CrossRef]
- Kim, B.G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef]
- Malek, E.; Rana, P.S.; Swamydas, M.; Daunov, M.; Miyagi, M.; Murphy, E.; Ignatz-Hoover, J.J.; Metheny, L.; Kim, S.J.; Driscoll, J.J. The TGFβ Type I Receptor Kinase Inhibitor Vactosertib in Combination with Pomalidomide in Relapsed/Refractory Multiple Myeloma: A Phase 1b Trial. Nat. Commun. 2024, 15, 7388. [Google Scholar] [CrossRef]
- Yap, T.A.; Vieito, M.; Baldini, C.; Sepúlveda-Sánchez, J.M.; Kondo, S.; Simonelli, M.; Cosman, R.; van der Westhuizen, A.; Atkinson, V.; Carpentier, A.F.; et al. First-In-Human Phase I Study of a Next-Generation, Oral, TGFβ Receptor 1 Inhibitor, LY3200882, in Patients with Advanced Cancer. Clin. Cancer Res. 2021, 27, 6666–6676. [Google Scholar] [CrossRef]
- Stein, E.M.; Tallman, M.S. Emerging therapeutic drugs for AML. Blood 2016, 127, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.N.; Chan, A.S.; Lai, F.P.; Leung, S.Y. Organoid cultures for cancer modeling. Cell Stem Cell 2023, 30, 917–937. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Library of Medicine. Capmatinib. Available online: https://www.ncbi.nlm.nih.gov/books/NBK595111/ (accessed on 26 May 2025).
- BerGenBio ASA. Bemcentinib Pipeline—NSCLC and Other Indications. Available online: https://www.bergenbio.com/pipeline/bemcentinib-cornerstone-therapy-nsclc (accessed on 26 May 2025).
- Krebs, M.G.; Branson, A.; Barber, S.; Poile, C.; King, A.; Greystoke, A.; Moody, S.; Nolan, L.; Scotland, M.; Darlison, L.; et al. Bemcentinib and Pembrolizumab in Patients with Relapsed Mesothelioma: MIST3, a Phase IIa Trial with Cellular and Molecular Correlates of Efficacy. J. Clin. Oncol. 2023, 41 (Suppl. S16), 8511. [Google Scholar] [CrossRef]
- Harding, J.J.; Cloughesy, T.F.; Mellinghoff, I.K.; Wen, P.Y.; Abrey, L.E.; Ellingson, B.M.; Aldape, K.D.; Penas-Prado, M.; Kuhn, J.; Mack, F.; et al. Phase 1b Study of Galunisertib and Ramucirumab in Patients with Advanced Hepatocellular Carcinoma. Cancer Med. 2021, 10, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Nadal, E.; Saleh, M.; Aix, S.P.; Ochoa-de-Olza, M.; Patel, S.P.; Antonia, S.; Zhao, Y.; Gueorguieva, I.; Man, M.; Estrem, S.T.; et al. A Phase Ib/II Study of Galunisertib in Combination with Nivolumab in Solid Tumors and Non-Small Cell Lung Cancer. BMC Cancer 2023, 23, 708. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Oh, D.Y.; Hollebecque, A.; Calvo, E.; Varghese, A.; Borazanci, E.; Macarulla, T.; Merz, V.; Zecchetto, C.; Zhao, Y.; et al. Safety and Activity of the TGFβ Receptor I Kinase Inhibitor Galunisertib Plus the Anti-PD-L1 Antibody Durvalumab in Metastatic Pancreatic Cancer. J. Immunother. Cancer 2021, 9, e002068. [Google Scholar] [CrossRef]
- Brazel, D.; Zhang, S.; Nagasaka, M. Spotlight on Tepotinib and Capmatinib for Non-Small Cell Lung Cancer with MET Exon 14 Skipping Mutation. Lung Cancer 2022, 13, 33–45. [Google Scholar] [CrossRef]
- Wick, A.; Desjardins, A.; Suarez, C.; Forsyth, P.; Gueorguieva, I.; Burkholder, T.; Cleverly, A.L.; Estrem, S.T.; Wang, S.; Lahn, M.M.; et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Investig. New Drugs 2020, 38, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
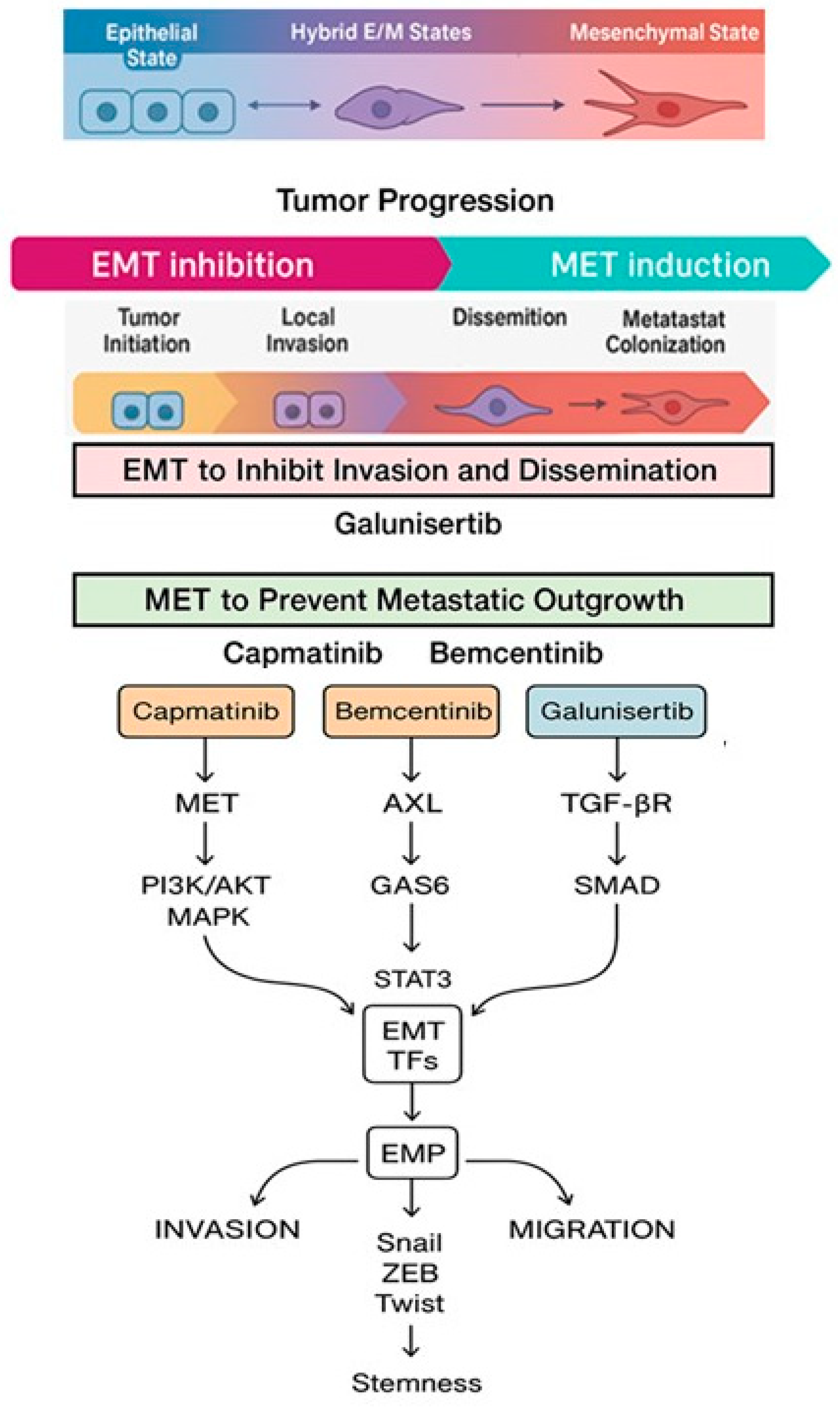
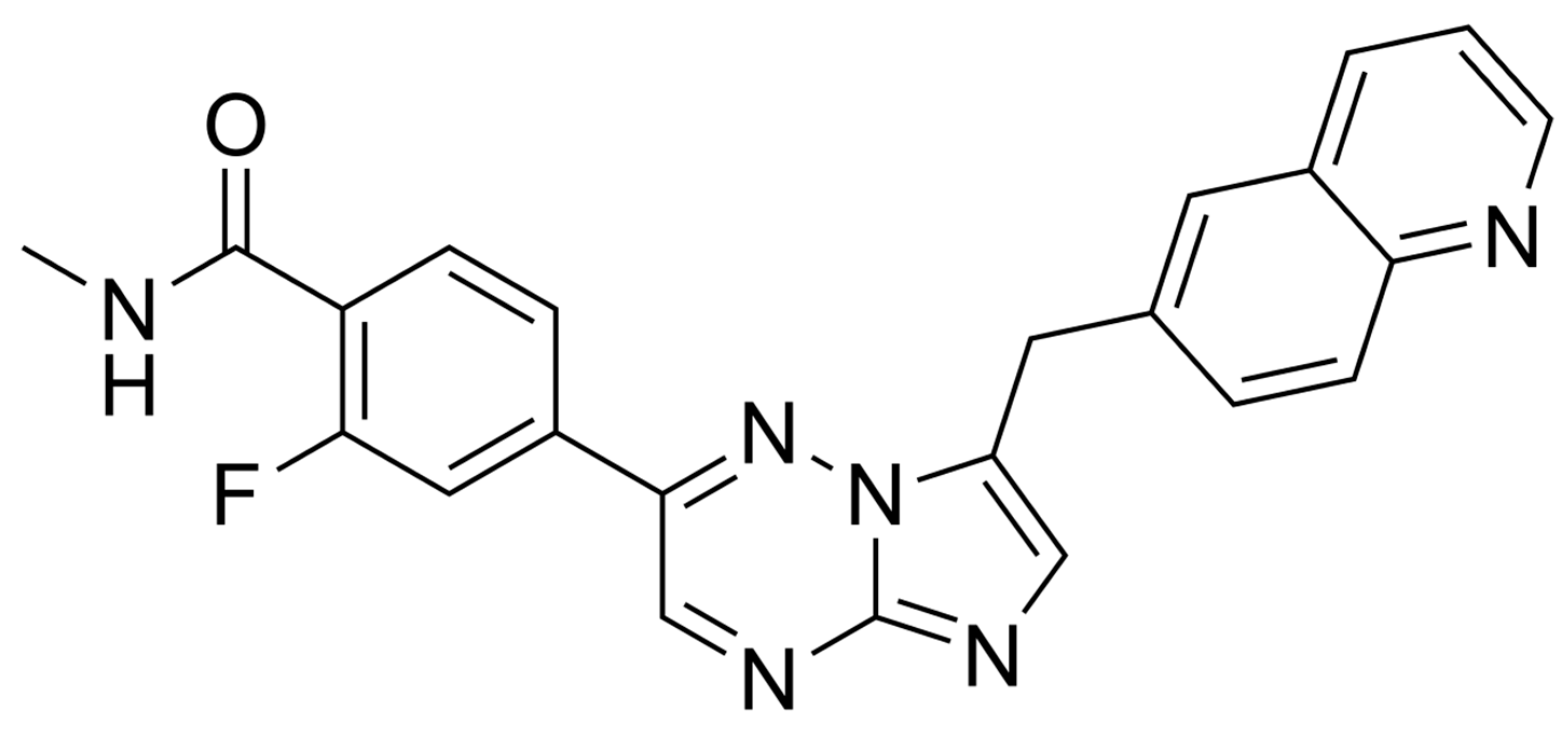

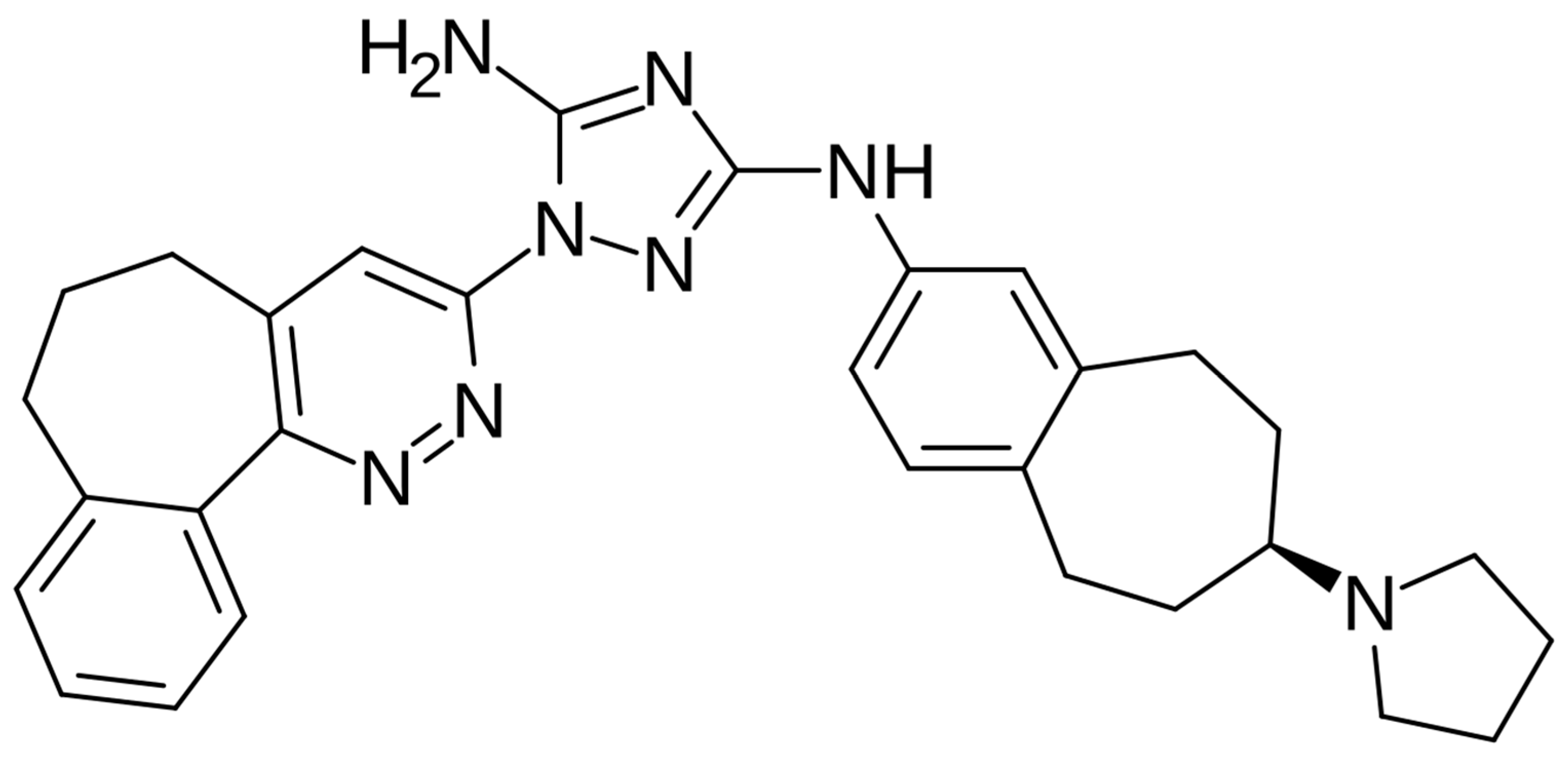
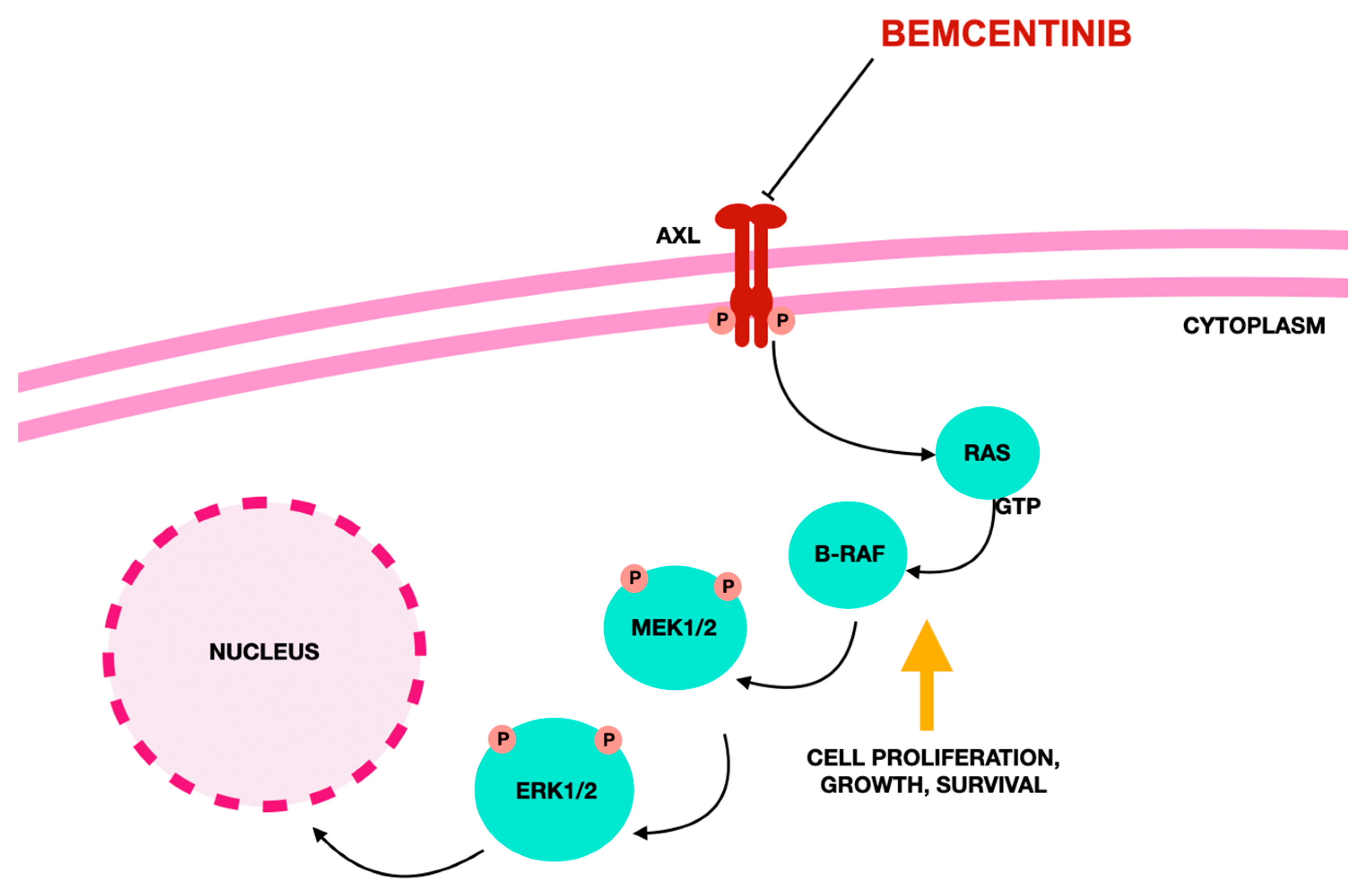
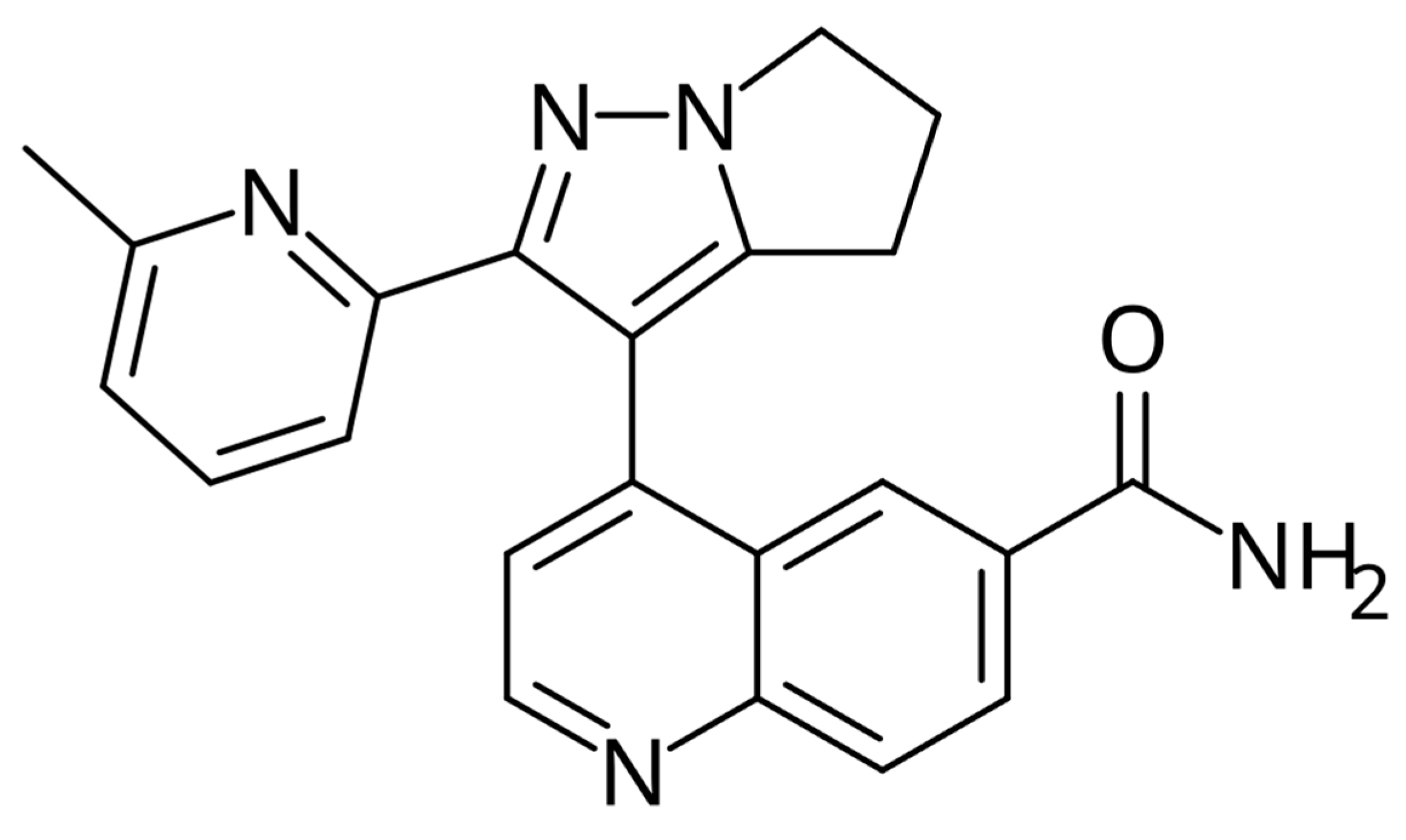
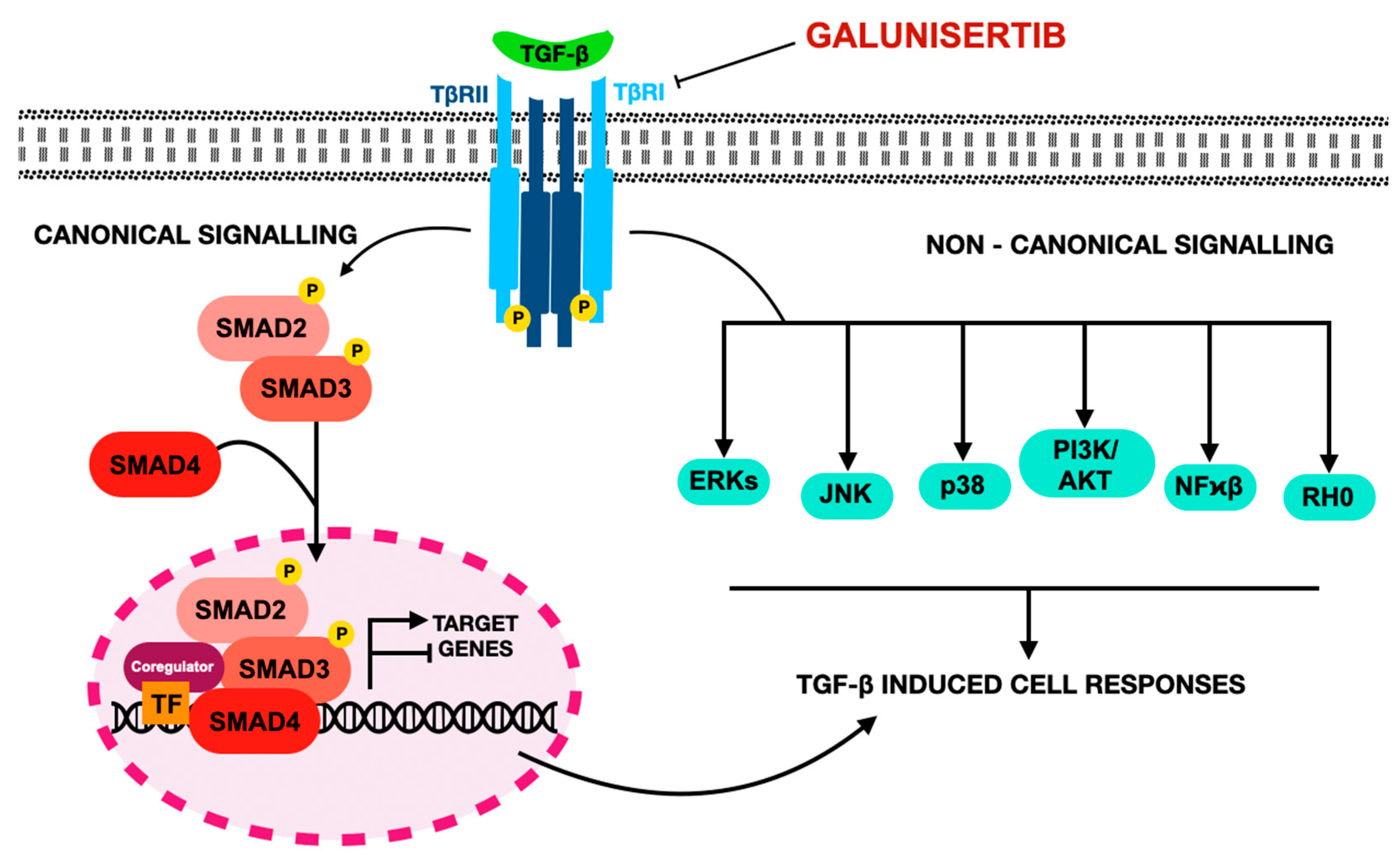
| EMT-TF | Family | Key Functions | Regulation | Target Genes & Pathways | Impact on Cancer Biology |
|---|---|---|---|---|---|
| SNAIL1/2 | Zinc finger (SNAIL family) | Repress epithelial genes (e.g., E-cadherin); Suppress tight junction proteins (claudins, occludins, ZO-1, connexins); Inhibit CRUMBS3 (affects polarity) | Activated by TGF-β, EGF, IGF-1, HGF, Wnt/β-catenin, NOTCH; Regulated by GSK3 (degradation); PAK1 phosphorylation (promotes nuclear localization) | E-cadherin, MMPs (context-dependent); CRUMBS3; Alters glucose metabolism (glycolysis shift) | Promotes stemness, invasion, therapy resistance; Associated with poor prognosis and recurrence |
| TWIST1/2 | bHLH | Downregulates E-cadherin; Upregulates fibronectin, N-cadherin, vimentin; Supports stemness and invasion | Regulated by TGF-β2, AKT2, PDGFR; Enhanced by MAPK/AKT phosphorylation | E-cadherin, fibronectin, N-cadherin, vimentin; Controls TGF-β2, AKT2, PDGFR; | Drives EMT and metastasis; Enhances cancer motility and stem-like traits |
| ZEB1/2 | Zinc finger (E-box-binding homeobox) | Represses epithelial genes (e.g., E-cadherin); Activates mesenchymal genes; Represses polarity genes (CDH1, Lgl2, PATJ, Crumbs3) | Induced by estrogen, TGF-β, Wnt/β-catenin-Modulated by SNAIL1 and TWIST1 | E-cadherin, MMPs, polarity genes | Promotes EMT and metastasis; Associated with therapy resistance and poor prognosis |
| Mechanism/ Inhibitor | Target/ Pathway Activity | Effect on cMET Signaling | Clinical Relevance/ Notes |
|---|---|---|---|
| Selective cMET TKI; Direct cMET inhibition (Capmatinib) | Inhibits cMET, GAB1, SRC, PI3K; blocks AKT, MAPK | Prevents activation of downstream signaling | Approved for NSCLC with MET exon 14 skipping mutations |
| Selective cMET inhibitor; Direct inhibition (Savolitinib) | Inhibits cMET phosphorylation; blocks survival and proliferation pathways | Inhibits cMET downstream signaling | Investigated in NSCLC and gastric cancer |
| Selective cMET inhibitor; Direct inhibition (Tepotinib) | Prevents cMET dimerization and phosphorylation; inhibits STAT3, PI3K/AKT, MAPK | Prevents cMET signaling and downstream cascade activation | Approved for NSCLC with MET exon 14 skipping mutations |
| Monoclonal antibody; HGF binding inhibitor (Onartuzumab) | Blocks HGF binding; prevents receptor dimerization and downstream PI3K, RAS/MAPK signaling | Prevents receptor activation at extracellular level | Explored in clinical trials; limited efficacy alone, combined therapies being explored |
| Multi-kinase inhibitor (cMET, VEGFR, AXL) (Cabozantinib) | Inhibits cMET and angiogenesis pathways (VEGFR) | Inhibits tumor growth via cMET and VEGFR pathways | Approved for renal cell carcinoma and thyroid cancer |
| Multi-targeted TKI (cMET, VEGFR, RON) (Foretinib) | Inhibits migration/invasion pathways (FAK, RAC1/JNK) | Broad inhibition including cMET, VEGFR pathways | Evaluated in trials for gastric and other cancers |
| Non-ATP competitive cMET inhibitor (Tivantinib) | Disrupts cMET signaling; inhibits survival/proliferation; mechanism partially unclear | Blocks downstream effects despite unclear kinase binding | Clinical trials in lung and liver cancers |
| TKI; Direct cMET kinase inhibition (Crizotinib) | Inhibits cMET autophosphorylation; blocks AKT/mTOR, RAS/RAF/MAPK, STAT3 | Prevents downstream signaling cascades | Approved for NSCLC with cMET alterations; also inhibits ALK |
| Feature | Targeting Aberrantly Activated AXL |
|---|---|
| Key Inhibitors | Bemcentinib (AXL inhibitor), Selumetinib (MEK inhibitor combo) |
| Primary Cell Type Targeted | Mesenchymal cells (AXL), epithelial cells (MEK inhibitor) |
| Mechanism of Action | Blocks AXL-driven EMT signaling activated by TGF-β and hypoxia |
| Effect on EMT | Attenuates TGF-β and hypoxia-induced EMT |
| Impact on Drug Resistance | Restores TKI sensitivity, reduces growth of EMT and drug-resistant tumors |
| Combination Strategies | Combined with MEK inhibitor selumetinib for dual epithelial and mesenchymal targeting |
| Apoptosis Induction | Indirect via EMT attenuation |
| Clinical Implications | Promising for overcoming resistance in tumors with EMT and drug resistance |
| Challenges | Requires targeting multiple pathways due to EMT complexity |
| Stage/Component | Details |
|---|---|
| Ligand Interaction | The function of TGFβ ligands is influenced by the variety and concentration of ligands and receptors present, alongside extracellular inhibitors and accessory molecules. Proteins like Gremlin 1 (GREM1) can suppress related signaling pathways (such as BMP), affecting cellular characteristics. Co-receptors like β-glycan and CRIPTO facilitate or hinder receptor activation based on context. |
| Receptor Complex Formation | TGFβ receptors assemble as heterotetramers comprising type I and type II subunits, each with multiple variants. Binding of ligands prompts type II receptors to phosphorylate type I receptors, initiating downstream signaling. The specific pairing of receptor subtypes with ligands dictates the cellular response. Regulatory factors like FKBP12 and BAMBI inhibit receptor activation to fine-tune signaling output. |
| Canonical Intracellular Signaling | Activated type I receptors phosphorylate receptor-specific SMAD proteins (R-SMADs), which combine with SMAD4 to form complexes that translocate to the nucleus. Different R-SMADs respond to TGFβ-like or BMP-like signals, while inhibitory SMADs (SMAD6/7) suppress the pathway. Scaffold proteins such as SARA regulate SMAD phosphorylation and activation. |
| Non-Canonical Intracellular Pathways | In addition to SMAD-dependent signaling, TGFβ receptors engage alternative pathways including MAPK cascades (JNK, p38, ERK), PI3K-AKT signaling, and Rho GTPase activation. These non-canonical routes interact with SMAD signaling to shape cellular outcomes, though their roles depend on the specific physiological or pathological setting. |
| Nuclear Regulation | SMAD complexes are imported into the nucleus via specialized transport proteins, where they partner with cell-specific transcription factors to control gene expression. They also recruit chromatin remodeling enzymes that either promote or repress transcription. Alternative SMAD interactions, such as with TRIM33, facilitate opening of chromatin regions critical for differentiation and development. |
| Feature/Drug | Capmatinib (Tabrecta®) | Bemcentinib (BGB324) | Galunisertib (LY2157299) |
|---|---|---|---|
| Mechanism of Action | Selective MET tyrosine kinase inhibitor; targets MET exon 14 skipping mutations | Selective AXL receptor tyrosine kinase inhibitor; blocks EMT, metastasis, immune evasion | Oral inhibitor of TGF-β receptor I kinase (ALK5); suppresses TGF-β-driven EMT and immune evasion |
| Clinical Applications | Approved for metastatic NSCLC with MET exon 14 skipping mutations | NSCLC (especially STK11-mutant), AML, MM | CRC/RC, HCC, GBM, MDS, NSCLC, PC |
| Clinical Trials | GBM (NCT02386826), NSCLC (NCT04427072, NCT01911507, NCT05435846) | AML (NCT02488408, NCT03824080), MM (MiST3 NCT03654833), NSCLC (NCT05469178, NCT03184571, NCT02424617) | ASolT (NCT01682187), CRC (NCT05700656), HCC (NCT02240433), GBM (NCT01582269), PC (NCT02734160), PCa (NCT02452008), RC (NCT02688712) |
| Common Adverse Effects | Peripheral edema, nausea, fatigue, vomiting, dyspnea, elevated liver enzymes, pancreatitis, interstitial lung disease (ILD) | Elevated liver enzymes, fatigue, diarrhea, anemia, thrombocytopenia, QTc prolongation | Fatigue, diarrhea, nausea, vomiting, constipation, transaminase elevation, cytopenias, thrombosis, neutropenia, dyspnea, hypophosphatemia, hand-foot syndrome |
| Regulatory Status | FDA-approved for METex14 NSCLC | Fast Track designation by FDA for elderly relapsed AML | Investigational; in various phase trials for multiple cancers |
| Efficacy | High in METex14-mutated NSCLC; ORR ~68% in treatment-naïve patients | Modest; ORR ~46% in NSCLC with pembrolizumab | Variable; improved OS in pancreatic cancer with gemcitabine |
| Toxicity | Generally mild; nausea, fatigue, peripheral edema | Mild to moderate; rash, diarrhea, fatigue, elevated liver enzymes | Mild; no dose-limiting toxicities reported |
| Resistance | Low; rare mutations reported | Moderate; common in ~20% of cases | High; frequent relapse observed |
| Key Trial Outcomes | Improved OS and PFS in METex14-mutated NSCLC | No significant improvement in efficacy with combination therapies | Prolonged OS with gemcitabine in pancreatic cancer; minimal added toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawczak, P.; Feszak, I.J.; Bączek, T. Targeted Therapies Modulating Mesenchymal–Epithelial Transition-Linked Oncogenic Signaling in the Tumor Microenvironment: Comparative Profiling of Capmatinib, Bemcentinib, and Galunisertib. J. Clin. Med. 2025, 14, 6853. https://doi.org/10.3390/jcm14196853
Kawczak P, Feszak IJ, Bączek T. Targeted Therapies Modulating Mesenchymal–Epithelial Transition-Linked Oncogenic Signaling in the Tumor Microenvironment: Comparative Profiling of Capmatinib, Bemcentinib, and Galunisertib. Journal of Clinical Medicine. 2025; 14(19):6853. https://doi.org/10.3390/jcm14196853
Chicago/Turabian StyleKawczak, Piotr, Igor Jarosław Feszak, and Tomasz Bączek. 2025. "Targeted Therapies Modulating Mesenchymal–Epithelial Transition-Linked Oncogenic Signaling in the Tumor Microenvironment: Comparative Profiling of Capmatinib, Bemcentinib, and Galunisertib" Journal of Clinical Medicine 14, no. 19: 6853. https://doi.org/10.3390/jcm14196853
APA StyleKawczak, P., Feszak, I. J., & Bączek, T. (2025). Targeted Therapies Modulating Mesenchymal–Epithelial Transition-Linked Oncogenic Signaling in the Tumor Microenvironment: Comparative Profiling of Capmatinib, Bemcentinib, and Galunisertib. Journal of Clinical Medicine, 14(19), 6853. https://doi.org/10.3390/jcm14196853






