Abstract
Background/Objectives: Periradicular disease is largely microbial in origin. Even gutta-percha (GP) cones manufactured under aseptic conditions can acquire contaminants during handling or storage, undermining otherwise adequate canal preparation. To assess residual antimicrobial activity on GP cones after brief exposure to five endodontic disinfectants: sodium hypochlorite (NaOCl) 1%, 2.5%, 5.25%; chlorhexidine (CHX) 2%; and glutaraldehyde 2% against Enterococcus faecalis and Candida albicans. Methods: Standardized GP cones were dipped for 5–120 s, blotted on neutralizing gauze, and placed on agar inoculated with either organism. Using an agar diffusion approach, inhibition-zone diameters were recorded at 0, 24, and 48 h. Data were summarized using descriptive statistics (means, standard deviations, and 95% confidence intervals) for each disinfectant–dip-time combination. Results: By 24 h, inhibition zones were observed for most disinfectants; for C. albicans, glutaraldehyde 2% showed no measurable effect. At later time points, performance depended on both disinfectant and contact time. For E. faecalis, NaOCl 2.5% and 5.25% yielded the largest zones at 48 h (20–21 mm at 120 s), whereas NaOCl 1% was smaller (10 mm) and glutaraldehyde 2% modest (9 mm). For C. albicans, NaOCl 2.5% and CHX 2% were most effective at 48 h (17–19 mm at 120 s); NaOCl 5.25% was intermediate, NaOCl 1% weak, and glutaraldehyde 2% showed no measurable antifungal effect. Longer immersions (≥45 s) consistently increased inhibition zone diameters. Conclusions: Residual antimicrobial activity on GP cones depends on both the agent and the immersion time. For E. faecalis, higher concentration NaOCl produced the largest zones at short contact time, whereas for C. albicans, CHX 2% and NaOCl 2.5% provided the most reliable carryover. Selecting an appropriate concentration and allowing sufficient dip time may reduce reinfection risk at obturation.
1. Introduction
The primary objective of endodontic treatment is to eliminate microbial pathogens and prevent reinfection of the canal [1]. For effective infection control in endodontics, every instrument and material that enters the root canal should be free of microorganisms. In daily practice, gutta-percha (GP) remains the main choice for obturation because it is easy to handle, dimensionally stable, and generally well-tolerated by tissues [2,3]. Even though the cones come in sealed packages, they are not completely sterile, and once the package is opened, they can easily be contaminated. Some studies have detected microbial growth even in cones taken from newly opened boxes [2]. This highlights why a disinfection step is necessary before these cones are used on patients. Conventional sterilization methods, such as moist or dry heat, are not suitable for GP due to concerns about structural integrity [3]. In a busy clinical setting, even brief exposure to gloves, instruments, or surrounding surfaces may introduce microorganisms that compromise the cleanliness of the root canal right before obturation. This poses a risk of introducing bacteria or fungi into the canal during obturation, which could compromise treatment outcomes [4].
Persistent endodontic infections are frequently associated with resilient microorganisms such as Enterococcus faecalis and Candida albicans [5,6]. Both E. faecalis and C. albicans have been linked to recurrent infections and endodontic failure, largely due to their resistance to disinfectants, ability to form biofilms, and capacity to invade dentin [7,8], characteristics that can contribute to less favorable clinical outcomes [9,10]. To reduce the risk of contaminating the root canal system during obturation, various chemical solutions have been tested for disinfecting GP cones chairside. Among them, sodium hypochlorite (NaOCl) remains one of the most commonly used agents. Its antimicrobial effectiveness is well established, particularly at higher concentrations (2.5% to 5.25%), and it also has the added advantage of dissolving organic tissue [11]. Nevertheless, its potential to alter the physical integrity of GP raises concerns [12]. Chlorhexidine gluconate (CHX), by contrast, is valued for its substantivity and relatively lower impact on GP properties [13]. Glutaraldehyde is not commonly used in endodontics, but its effectiveness as a high-level disinfectant has been well established in broader medical practice, and in recent years it has also attracted attention for possible dental applications [14].
Although many studies have evaluated individual disinfectants, only a limited number have compared their performance against both E. faecalis and C. albicans at different exposure times. There is still no clear agreement on how long GP cones should remain in disinfectant solutions to achieve effective microbial control without compromising their physical properties [15,16].
The main aim of this study was to evaluate and compare the short-term antimicrobial effectiveness of five commonly used disinfectants, NaOCl (at concentrations of 1%, 2.5%, and 5.25%), CHX 2%, and glutaraldehyde 2%, against E. faecalis and C. albicans in an agar diffusion model. The null hypothesis was that all tested disinfectants would exhibit equal antimicrobial effectiveness against E. faecalis and C. albicans, regardless of concentration or exposure time.
2. Materials and Methods
2.1. GP Sample Preparation
A total of 1050 standardized GP cones (#25 and #30, ISO color coding; VDW GmbH, Munich, Germany), from a single manufacturer, were used to ensure uniform product characteristics and packaging. Cones were removed directly from sealed manufacturer packs using sterile tweezers, previously autoclaved, under aseptic laboratory conditions to prevent contamination. The cones were randomly divided into seven groups (n = 150) according to the disinfectant to be tested.
2.2. Disinfectants
Five disinfectant solutions used in endodontics were evaluated: NaOCl at 1%, 2.5%, and 5.25%; CHX 2%; and glutaraldehyde 2%. Two controls were included as described in Section 2.5. All solutions were used at room temperature (approximately 22–24 °C) as supplied by the manufacturers; pH was not measured. The disinfectants, their concentrations, and manufacturers are summarized in Table 1.

Table 1.
Disinfectants evaluated in the study, including concentrations and manufacturers.
2.3. Experimental Design and Exposure Protocol
Each disinfectant group (n = 150) was divided into two subgroups (n = 75 each) for E. faecalis (ATCC 29212, Liofilchem, Italy) or C. albicans (ATCC 10231, Liofilchem, Italy). Each subgroup was divided into five exposure times (n = 15: 5, 20, 45, 60, and 120 s. Sterile cones were immersed in the assigned disinfectant for the specified time, removed aseptically, lightly blotted, and placed on inoculated agar plates. For E. faecalis, blood agar (nutrient agar with 5% defibrinated sheep blood) was used; for C. albicans, Mueller–Hinton agar. Inoculum density was adjusted to approximately 0.5 McFarland, and a uniform lawn was prepared by swabbing in three directions. Plates were allowed to dry for 10–15 min before cone placement. Cones were positioned radially, spaced ≥ 20 mm apart and ≥15 mm from the plate edge. Plates were incubated aerobically at 37 °C and read at 0, 24, and 48 h for growth inhibition zones, according to the agar diffusion assay (Chart 1).
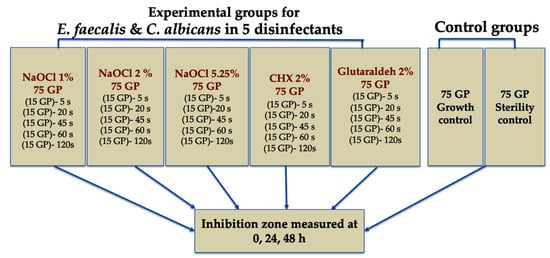
Chart 1.
Allocation of the GP cones into disinfectant/control groups, microorganism subgroups, and immersion time sets.
2.4. Evaluation of Antimicrobial Activity (Agar Diffusion-Based Assay)
The standard agar diffusion test [17] was performed with minor modifications to assess residual antimicrobial activity retained by the disinfectant-treated cones. Briefly, cones exposed to the various disinfectants for different times were placed onto freshly inoculated agar plates (either E. faecalis or C. albicans). Before transfer, cones were briefly blotted on sterile gauze moistened with a neutralizing solution (for example, 0.5% sodium thiosulfate for NaOCl and sterile saline for CHX and glutaraldehyde) to minimize carry-over artefacts from residual disinfectant. Growth inhibition zones (clear areas around each cone) were measured in millimetres at 0, 24, and 48 h using a calibrated digital calliper. For non-circular halos, the mean of two perpendicular diameters was recorded. Measurements were performed under blinded conditions to reduce bias. Placing disinfectant-treated cones onto inoculated agar was chosen solely to evaluate residual antimicrobial activity retained on the cone surface after brief chemical exposure, not to measure pre-existing contamination.
2.5. Control Validation
A sterility control consisted of sterile GP cones placed on uninoculated agar to confirm the absence of contamination. A growth control consisted of cones exposed only to sterile saline before placement on inoculated agar.
2.6. Statistical Analysis
Fifteen GP cones were measured for each disinfectant–dip-time combination (n = 15 per group). Zone diameters are summarized descriptively as mean, standard deviation, minimum, maximum, and 95% confidence intervals for each combination. No formal inferential hypothesis test or between-group significance testing was performed. Calculations were performed in SPSS Statistics, version 21 (IBM Corp., Armonk, NY, USA). Line plots were created to illustrate trends across dip times and disinfectants.
2.7. Ethical Consideration
This in vitro study used standard laboratory materials only; therefore, ethical approval was not applicable.
3. Results
3.1. Antimicrobial Activity Against E. faecalis
The diffusion assay was performed as described. Sterility and growth controls behaved as expected, confirming cone sterility and the absence of intrinsic antimicrobial activity of GP. Where zones were present at 24 h, they remained visible at 48 h, as detailed in Table 2. Zone diameters varied by disinfectant and immersion time; very short dips (5–20 s) produced little or no effect, except for small halos for 5.25% NaOCl. Once exposure reached 45–60 s, clear zones appeared, and the largest diameters were recorded at 120 s. At 48 h after a 120 s dip (n = 15), the mean ± SD (min–max) were 20.9 ± 0.4 mm (20–21) for 5.25% NaOCl, 19.9 ± 0.4 mm (19–20) for 2.5% NaOCl, 12.7 ± 0.5 mm (12–13) for 2% CHX, 9.1 ± 0.3 mm (9–10) for 2% glutaraldehyde, and 9.6 ± 0.6 mm (8–10) for 1% NaOCl; values at 48 h were slightly lower than at 24 h, but the rank order was unchanged.

Table 2.
Inhibition-zone diameters (mm) at 24 h and 48 h for E. faecalis by disinfectant and immersion time. GP: gutta-percha; SD: standard deviation.
3.2. Antimicrobial Activity Against C. albicans
In the agar-diffusion assay with C. albicans (Table 3), short immersions of 5–20 s produced no measurable zones for any agent. Once exposure reached 45–60 s, inhibition became evident and peaked at 120 s. At 48 h after a 120 s dip (n = 15), the mean diameters ± SD (min–max) were 18.8 ± 0.4 mm (18–19) for 2.5% NaOCl and 16.7 ± 0.5 mm (16–17) for 2% CHX; 5.25% NaOCl showed an intermediate effect at 13.0 ± 0.0 mm (13–13), 1% NaOCl was weak at 5.7 ± 0.5 mm (5–6), and 2% glutaraldehyde showed no zones at any time point. This pattern indicates that, beyond the 45–60 s threshold, extended contact yields clear antifungal carryover, with 2.5% NaOCl and 2% CHX providing the strongest effects, 5.25% NaOCl a moderate effect, and 1% NaOCl a limited one, while glutaraldehyde remains inactive.

Table 3.
Inhibition-zone diameters (mm) at 24 h and 48 h for C. albicans by disinfectant and immersion time. GP: gutta-percha; SD: standard deviation.
3.3. Relevance of the Dip Times
Figure 1, Figure 2, Figure 3 and Figure 4 show how cone exposure time affected residual antifungal and antibacterial activity.

Figure 1.
Inhibition zones at 24 h—C. albicans. Line plots show inhibition-zone diameter (mm) versus dip time (s) for CHX 2%, Glutaraldehyde 2%, NaOCl 1%, NaOCl 2.5%, and NaOCl 5.25%. Data are descriptive (no hypothesis testing).
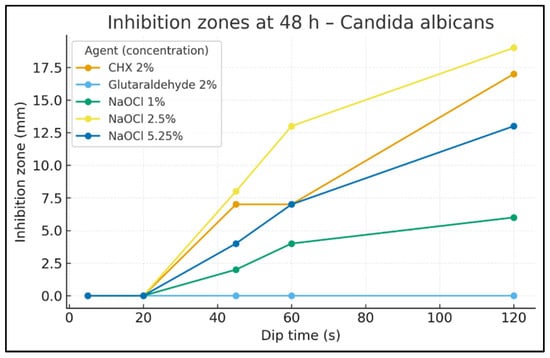
Figure 2.
Inhibition zones at 48 h—C. albicans. Inhibition-zone diameter (mm) by dip time (s) for CHX 2%, Glutaraldehyde 2%, NaOCl 1%, NaOCl 2.5%, and NaOCl 5.25%. Data are descriptive (no hypothesis testing).
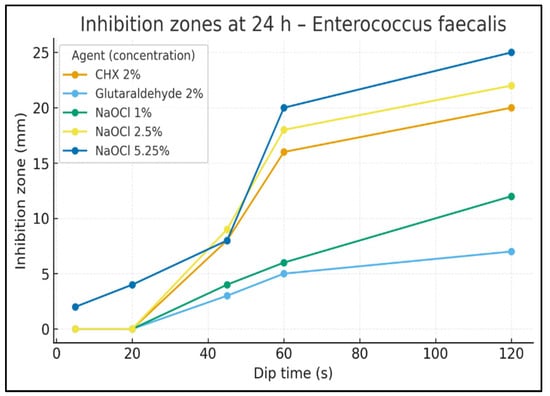
Figure 3.
Inhibition zones at 24 h—E. faecalis. Inhibition-zone diameter (mm) vs. dip time (s) for CHX 2%, Glutaraldehyde 2%, NaOCl 1%, NaOCl 2.5%, and NaOCl 5.25%. Data are descriptive (no hypothesis testing).
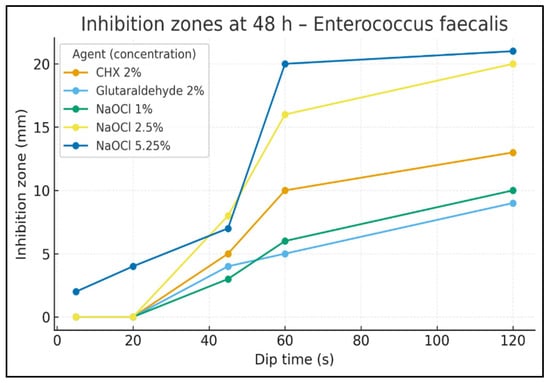
Figure 4.
Inhibition zones at 48 h—E. faecalis. Inhibition-zone diameter (mm) vs. dip time (s) for CHX 2%, Glutaraldehyde 2%, NaOCl 1%, NaOCl 2.5%, and NaOCl 5.25%. Data are descriptive (no hypothesis testing).
At 24 h (Figure 1), dip times ≤ 20 s produced no halos for any agent. At 45 s, activity was evident for NaOCl 2.5% (8 mm) and CHX 2% (7 mm), with smaller zones for NaOCl 5.25% (6 mm) and NaOCl 1% (3 mm); glutaraldehyde 2% stayed inactive (0 mm). At 60 s, the pattern intensified: NaOCl 2.5% reached 15 mm, CHX 11 mm, NaOCl 5.25% 9 mm, NaOCl 1% 5 mm, and glutaraldehyde 0 mm. By 120 s, the largest halos were 20 mm for both NaOCl 2.5% and CHX 2%, followed by NaOCl 5.25% (15 mm) and NaOCl 1% (6 mm); glutaraldehyde remained 0 mm.
At 48 h (Figure 2), the time–response persisted but with slightly different magnitudes. Halos were absent at 20 s. At 45 s, zones measured 8 mm for NaOCl 2.5%, 7 mm for CHX 2%, 4 mm for NaOCl 5.25%, 2 mm for NaOCl 1%, and 0 mm for glutaraldehyde. At 60 s, NaOCl 2.5% was 13 mm, NaOCl 5.25% 7 mm, CHX 2% stayed 7 mm, NaOCl 1% 4 mm, and glutaraldehyde 0 mm. By 120 s, NaOCl 2.5% was highest at 19 mm, CHX 2% 17 mm, NaOCl 5.25% 13 mm, NaOCl 1% 6 mm, and glutaraldehyde 0 mm.
At 24 h (Figure 3), a small early effect was already visible for NaOCl 5.25% at 20 s (4 mm), while other agents were inactive. At 45 s, halos measured 9 mm for NaOCl 2.5%, 8 mm for CHX 2% and NaOCl 5.25%, 4 mm for NaOCl 1%, and 3 mm for glutaraldehyde. At 60 s, NaOCl 5.25% rose to 20 mm, NaOCl 2.5% 18 mm, CHX 16 mm, NaOCl 1% 6 mm, and glutaraldehyde 5 mm. At 120 s, NaOCl 5.25% was highest at 25 mm, followed by NaOCl 2.5% (22 mm), CHX (20 mm), NaOCl 1% (12 mm), and glutaraldehyde (7 mm).
At 48 h (Figure 4), NaOCl 5.25% again showed measurable inhibition as early as 20 s (4 mm), whereas other agents remained at 0 mm. At 45 s, zones were 8 mm for NaOCl 2.5%, 7 mm for NaOCl 5.25%, 5 mm for CHX 2%, 4 mm for glutaraldehyde, and 3 mm for NaOCl 1%. At 60 s, NaOCl 5.25% reached 20 mm, NaOCl 2.5% 16 mm, CHX 10 mm, NaOCl 1% 6 mm, and glutaraldehyde 5 mm. At 120 s, the final diameters were 21 mm for NaOCl 5.25%, 20 mm for NaOCl 2.5%, 13 mm for CHX 2%, 10 mm for NaOCl 1%, and 9 mm for glutaraldehyde.
Dip times below 20 s were ineffective except for a small early effect of NaOCl 5.25% against E. faecalis. The steepest increases occurred between 45 s and 60 s, with near-maximal halos by 120 s. NaOCl 2.5% and CHX 2% were the most reliable against C. albicans, while NaOCl 5.25% consistently produced the largest zones against E. faecalis.
4. Discussion
This study evaluated the short-term residual antimicrobial activity of five endodontic disinfectants that had been used to treat GP cones at different concentrations and for different times. The disinfectant-treated GP cones were then analyzed by an agar diffusion-based assay. Differences in residual antimicrobial effects emerged at 24 and 48 h across agents and immersion times. Patterns were consistent with the descriptive data: for E. faecalis, NaOCl 2.5% and 5.25% produced the largest inhibition zones at 48 h, CHX 2% was intermediate, whereas NaOCl 1% and glutaraldehyde 2% were little or not effective. For C. albicans, CHX 2% and NaOCl 2.5% were the most effective, NaOCl 5.25% was intermediate, NaOCl 1% was weak, and glutaraldehyde 2% showed no measurable effect.
NaOCl’s strong early antimicrobial action but uncertain lasting effect has been noted by other authors [17,18,19,20], and our findings fit that pattern. In our plates, the halos from higher-strength NaOCl tended to shrink by 24–48 h, which could be explained by biofilm-related tolerance or adaptive responses described by Arias-Moliz et al. (2015) and Fiegler-Rudol et al. (2025) [15,21]. Interestingly, NaOCl at 1% behaved more steadily over time, a result that may indicate a better balance between microbial suppression and preservation of gutta-percha integrity, in line with Mishra et al. (2024) [22]. Although NaOCl is highly effective, its caustic nature and the risk of tissue damage if extruded beyond the apex, particularly at higher concentrations, mean that careful handling is essential in clinical practice [23].
CHX at 2% performed reliably against E. faecalis but showed inconsistent antifungal effects against C. albicans. The variability in inhibition-zone diameters at 24 and 48 h may reflect CHX’s limited penetration into fungal biofilms and its reduced activity in the presence of organic material, as reported by Dudás et al. (2025), Antonelli et al. (2019), and Rossi-Fedele et al. (2011) [24,25,26]. Despite these limitations, CHX produced intermediate but comparatively stable antibacterial halos, reinforcing its usefulness as a steady antibacterial agent rather than a primary antifungal disinfectant [12,13]. In contrast, glutaraldehyde 2% produced only modest inhibition against E. faecalis and no measurable effect against C. albicans in this agar-diffusion model. This outcome contrasts with its well-established role as a high-level disinfectant in broader medical practice [14,15] and with prior suggestions of its potential chairside use in endodontics [27,28]; further work in dentin or biofilm models is needed to clarify any residual benefits. Across all groups, E. faecalis was more susceptible than C. albicans, underscoring the latter’s clinical relevance in persistent endodontic infections [5,6]. Disinfectant selection and immersion time should therefore be tailored to the clinical scenario. In our agar-diffusion model, NaOCl 2.5–5.25% produced the largest residual inhibition zones for E. faecalis at 48 h, whereas for C. albicans, CHX 2% and NaOCl 2.5% performed best; NaOCl 5.25% was intermediate, NaOCl 1% weak, and glutaraldehyde 2% showed no measurable antifungal effect. CHX can serve as a useful adjunct for bacterial contamination but may require extended exposure or combination strategies for antifungal coverage [24,25,26]. Claims of prolonged suppression with glutaraldehyde 2% or 1% NaOCl are not supported by our data and, if mentioned based on external reports, should be presented as literature context rather than conclusions from this study [22,27,28].
This study has limitations that must be acknowledged. The in vitro design cannot fully replicate the complexity of clinical conditions, including anatomical variations, the buffering effects of dentin, and host immune responses. The agar diffusion method, while standardized and reproducible, does not adequately simulate biofilm resistance or agent penetration into dentin. Incubation was controlled at 37 °C. Furthermore, during the in vitro disinfection step, solution temperature and pH were not controlled, and no mechanical agitation was applied, although all these factors can enhance disinfectant penetration in clinical practice [29]. Possible carryover effects from residual disinfectant cannot be entirely excluded, even after blotting/neutralization. The biochemical properties of each disinfectant and the choice of the culture media may also have influenced halo sizes. Beyond antimicrobial carryover, in vitro studies have shown that disinfecting solutions can alter GP surface properties; because we did not assess surface integrity (e.g., via SEM/FTIR), our clinical recommendations should be interpreted with this limitation in mind [8,22,26].
In future work, researchers could employ molecular tools such as quantitative PCR (qPCR) or confocal microscopy to examine microbial survival and agent penetration into dentin more directly. Other methods, including scanning electron microscopy or infrared spectroscopy, could reveal how these chemicals affect the surface or composition of GP cones [3]. It would also be worthwhile to explore combination approaches, using NaOCl followed by CHX or newer tools like nanoparticle-based disinfectants and photodynamic therapy. Such studies could help strike a better balance between eliminating microbes and preserving cone integrity, making chairside disinfection both safer and more dependable.
5. Conclusions
Within the limits of this in vitro model, most disinfectants produced measurable halo zones by 24 h, with size depending on the agent and the contact time. For E. faecalis, NaOCl 2.5% and 5.25% led at 48 h; for C. albicans, CHX 2% and NaOCl 2.5% performed best, while glutaraldehyde 2% showed no antifungal effect. These findings open up dentin/biofilm models as a further step towards clinical practice changes.
Author Contributions
Conceptualization, T.K. and D.B.S.; methodology, T.K.; software, A.M.; validation, H.O., D.B.S. and A.M.; formal analysis, H.O.; investigation, T.K.; resources, D.B.S.; data curation, D.B.S.; writing—original draft preparation, T.K.; writing—review and editing, D.B.S. and A.M.; visualization, A.M.; supervision, H.O.; project administration, T.K.; funding acquisition, D.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as it was an in vitro investigation conducted exclusively with standard laboratory materials; hence, ethical approval was not applicable.
Informed Consent Statement
Informed consent is not applicable, as the study did not involve human participants, but rather microbial species.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We are grateful to Prof. Elisabetta Blasi (Department of CHIMOMO, University of Modena and Reggio Emilia, Modena, Italy) for her invaluable microbiological advice, and to Ph.D. Stefania Paduano (Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, Modena, Italy) for assistance with the statistical analyses.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kandaswamy, D.; Venkateshbabu, N. Root canal filling materials: A review. J. Conserv. Dent. 2010, 13, 195–199. [Google Scholar] [CrossRef]
- Chandrappa, M.M.; Mundathodu, N.; Srinivasan, R.; Nasreen, F.; Kavitha, P.; Shetty, A. Disinfection of gutta-percha cones using three reagents and their residual effects. J. Conserv. Dent. 2014, 17, 571–574. [Google Scholar] [CrossRef]
- Kowalski, J.; Rygas, J.; Homa, K.; Dobrzyński, W.; Wiglusz, R.J.; Matys, J.; Dobrzyński, M. Antibacterial Activity of Endodontic Gutta-Percha—A Systematic Review. Appl. Sci. 2024, 14, 388. [Google Scholar] [CrossRef]
- Bracciale, F.; Marino, N.; Noronha, A.; Manso, M.D.; Gavinha, S.; Cardoso, I.L.; Pina, C.; Teles, A.M. Bacterial Contamination of Gutta-Percha Points From Different Brands and the Efficacy of a Chairside Disinfection Protocol. Eur. Endod. J. 2020, 5, 282–287. [Google Scholar] [CrossRef]
- Brundin, M.; Figdor, D.; Sundqvist, G.; Sjögren, U. DNA binding to hydroxyapatite: A potential mechanism for preservation of microbial DNA. J. Endod. 2013, 39, 211–216. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, B.; Gupta, K.; Gupta, N.; Singh, R.; Singh, S. Comparative Evaluation of Antifungal Efficacy of Five Root Canal Sealers against Clinical Isolates of Candida albicans: A Microbiological Study. Int. J. Clin. Pediatr. Dent. 2020, 13, 119–123. [Google Scholar] [CrossRef]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef]
- Basrani, B.; Haapasalo, M. Update on endodontic irrigating solutions. Endod. Top. 2013, 27, 74–102. [Google Scholar] [CrossRef]
- Naladkar, K.; Chandak, M.; Sarangi, S.; Agrawal, P.; Jidewar, N.; Suryawanshi, T.; Hirani, P. Breakthrough in the Development of Endodontic Irrigants. Cureus 2024, 16, e66981. [Google Scholar] [CrossRef]
- Abraham, S.B.; Al Marzooq, F.; Himratul-Aznita, W.H.; Ahmed, H.M.A.; Samaranayake, L.P. Prevalence, virulence and antifungal activity of C. albicans isolated from infected root canals. BMC Oral Health 2020, 20, 347. [Google Scholar] [CrossRef]
- Zehnder, M.; Belibasakis, G.N. On the dynamics of root canal infections-what we understand and what we don’t. Virulence 2015, 6, 216–222. [Google Scholar] [CrossRef]
- Agrawal, M.; Kotalwar, G.; Gelda, A.; Kadtane, S.; Badade, A.; Hegde, V. Effectiveness of different agents for disinfection of gutta-percha cones: An in vitro study. J. Res. Med. Dent. Sci. 2020, 8, 169–172. [Google Scholar]
- Weissheimer, T.; Pinto, K.P.; da Silva, E.J.N.L.; Hashizume, L.N.; da Rosa, R.A.; Só, M.V.R. Disinfectant effectiveness of chlorhexidine gel compared to sodium hypochlorite: A systematic review with meta-analysis. Restor. Dent. Endod. 2023, 48, e37. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection and Sterilization in Health Care Facilities: An Overview and Current Issues. Infect. Dis. Clin. N. Am. 2016, 30, 609–637. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moliz, M.T.; Ordinola-Zapata, R.; Baca, P.; Ruiz-Linares, M.; García García, E.; Hungaro Duarte, M.A.; Monteiro Bramante, C.; Ferrer-Luque, C.M. Antimicrobial activity of Chlorhexidine, Peracetic acid and Sodium hypochlorite/etidronate irrigant solutions against Enterococcus faecalis biofilms. Int. Endod. J. 2015, 48, 1188–1193. [Google Scholar] [CrossRef]
- Shabani, D.B.; Teneqja, A.D. In Vitro Comparisons of Minimal Inhibitory Concentrations between NaOCL, CHX, MTAD and EDTA against Candida Albicans. Int. J. Biomed. 2023, 13, 345–349. [Google Scholar] [CrossRef]
- Devaraj, S.; Venkatakrishnan, S.; Ramkumar, S.; Bharadwaj, S.K.; Balaji, D.; Govindaraju, L. Effectiveness of Microwave Disinfection on Gutta Percha Cones Against Staphylococcus aureus and Enterococcus faecalis—An In vitro Study. J. Pharm. Bioallied Sci. 2025, 17, S1823–S1826. [Google Scholar] [CrossRef]
- Nacif, M.; Marceliano-Alves, M.F.V.; Alves, F.R.F. Contamination of gutta-percha cones in clinical use by endodontic specialists and clinicians. Rev. Fac. Odontol. Univ. Antioq. 2017, 28, 327–340. [Google Scholar] [CrossRef]
- Teles, A.M.; Pina, C.; Cardoso, I.L.; Tramontana, A.; Cardoso, M.; Duarte, A.S.; Bartolomeu, M.; Noites, R. Degree of Contamination of Gutta-Percha Points by Staphylococcus aureus (MRSA/MSSA) Strains. Int. J. Mol. Sci. 2024, 25, 8566. [Google Scholar] [CrossRef]
- García, J.M.; Barba, M.B.; García, M.G.; Ruíz, V.G.; García, A.G. Comparison of disinfection of different brands of gutta-percha tip with sodium hypochlorite. Rev. ADM 2020, 77, 185–190. [Google Scholar] [CrossRef]
- Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Tkaczyk, M.; Grzech-Leśniak, K.; Zawilska, A.; Wiench, R. Enhancing Root Canal Disinfection with Er:YAG Laser: A Systematic Review. Dent. J. 2025, 13, 101. [Google Scholar] [CrossRef]
- Mishra, L.; Dash, G.; Singh, N.R.; Kumar, M.; Panda, S.; Diemer, F.; Lukomska-Szymanska, M.; Lapinska, B.; Khan, A.S. Influence of disinfecting solutions on the surface topography of gutta-percha cones: A systematic review of in vitro studies. Restor. Dent. Endod. 2024, 49, e42. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Meto, A.; Mehta, V.; Lo Giudice, G.; Cervino, G. Sodium Hypochlorite Accidents in Endodontic Practice: Clinical Evidence and State of the Art. J. Craniofac. Surg. 2024, 35, e636–e645. [Google Scholar] [CrossRef] [PubMed]
- Dudás, C.; Bardocz-Veres, Z.; Gyulai, A.I.; Pop, S.I.; Székely, M.; Kerekes-Máthé, B.; Kovács, M. Comparative in Vitro Study on the Antimicrobial Efficacy of Endodontic Sealers Against Common Oral Pathogens. Dent. J. 2025, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Giovannini, L.; Baccani, I.; Giuliani, V.; Pace, R.; Rossolini, G.M. In Vitro Antimicrobial Activity of the Decontaminant HybenX® Compared to Chlorhexidine and Sodium Hypochlorite against Common Bacterial and Yeast Pathogens. Antibiotics 2019, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Fedele, G.; Guastalli, A.R.; Doğramacı, E.J.; Steier, L.; De Figueiredo, J.A. Influence of pH changes on chlorine-containing endodontic irrigating solutions. Int. Endod. J. 2011, 44, 792–799. [Google Scholar] [CrossRef]
- Thienngern, P.; Panichuttra, A.; Ratisoontorn, C.; Aumnate, C.; Matangkasombut, O. Efficacy of chitosan paste as intracanal medication against Enterococcus faecalis and Candida albicans biofilm compared with calcium hydroxide in an in vitro root canal infection model. BMC Oral Health 2022, 22, 354. [Google Scholar] [CrossRef]
- Pauletto, G.; Guerim, P.H.F.; Barbosa, A.B.; Lopes, L.Q.S.; Bier, C.A.S.; Marquezan, P.K. Efficacy of calcium hypochlorite in disinfection of gutta-percha cones contaminated with Candida albicans. Braz. J. Microbiol. 2024, 55, 403–410. [Google Scholar] [CrossRef]
- Karata, Ş.E.; Ayaz, N.; Uluk, Ö.E.; Baltaci, M.Ö.; Adig, Ü.A. Effect of final irrigation with sodium hypochlorite at different temperatures on postoperative pain level and antibacterial activity: A randomized controlled clinical study. J. Appl. Oral Sci. 2021, 29, e20200502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).