Neurocognitive Interventions Informed by Cognitive–Behavioral Therapy (CBT) Principles and Physical Exercise for Complex Regional Pain Syndrome: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
- Population: adult patients (≥18 years) diagnosed with complex regional pain syndrome.
- Intervention: physical exercise and cognitive–behavioral interventions.
- Comparison: Conventional therapy or usual care, including hydrotherapy, early stimulation (e.g., TENS), physical modalities, occupational therapy, and facilitation techniques, without neurocognitive elements.
- Outcomes: Pain: Measured using the Numerical Rating Scale (NRS), Visual Analog Scale (VAS), or McGill Pain Questionnaire.; Physical function: Assessed through passive range of motion (via goniometry) and strength (using a hand dynamometer); and Functional Outcomes or Disability Related to Daily Activities: Evaluated using instruments such as the Patient-Rated Wrist/Hand Evaluation (PRWHE), QuickDASH, the 5-item version of the EQ-5D (EQ-5D-5L), the Oswestry Low Back Pain Disability Questionnaire, and the Roland-Morris Disability Questionnaire.
2.2. Source of Information and Strategy Search
2.3. Selection of Articles
2.4. Process for Collecting Information
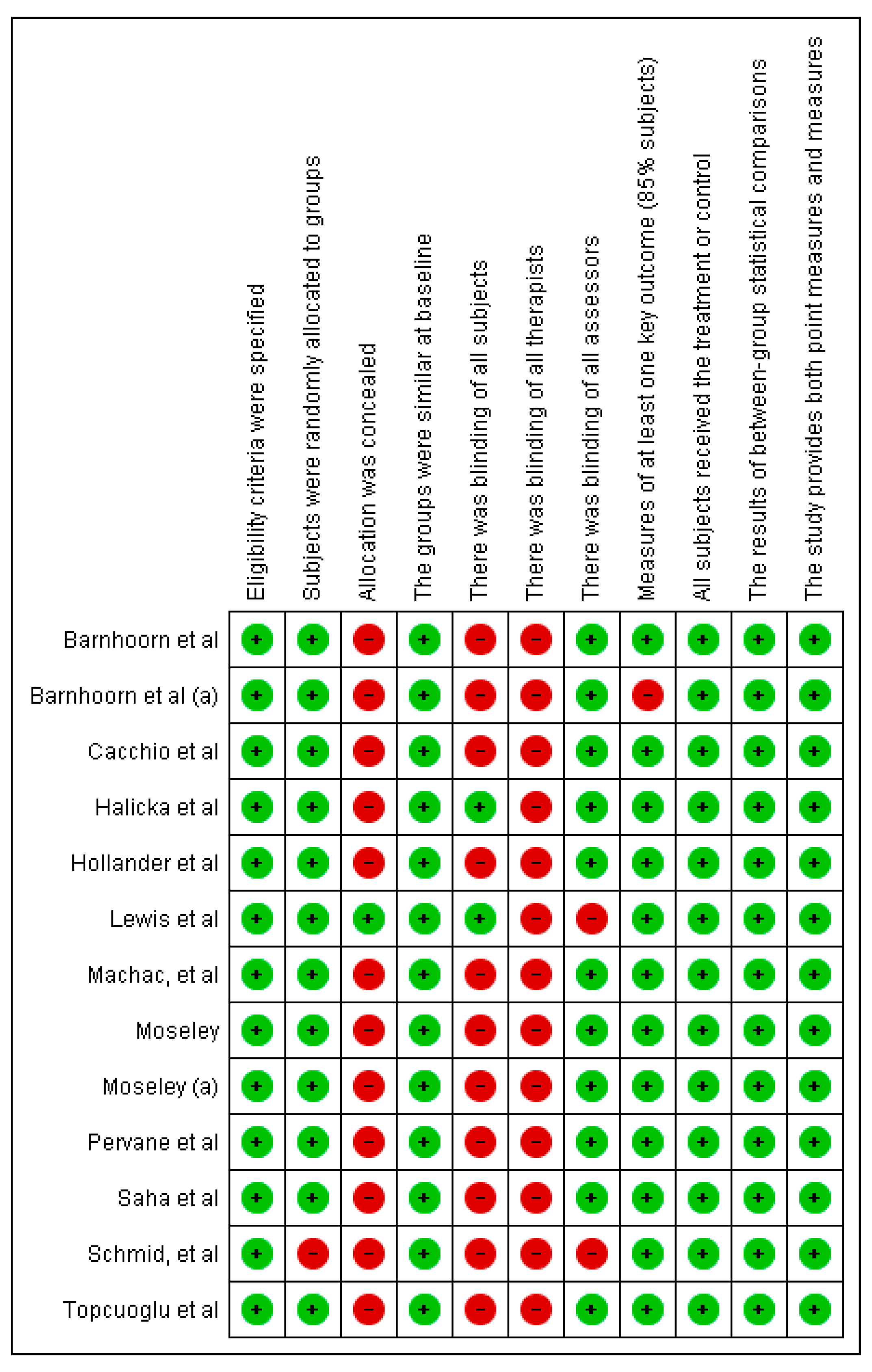
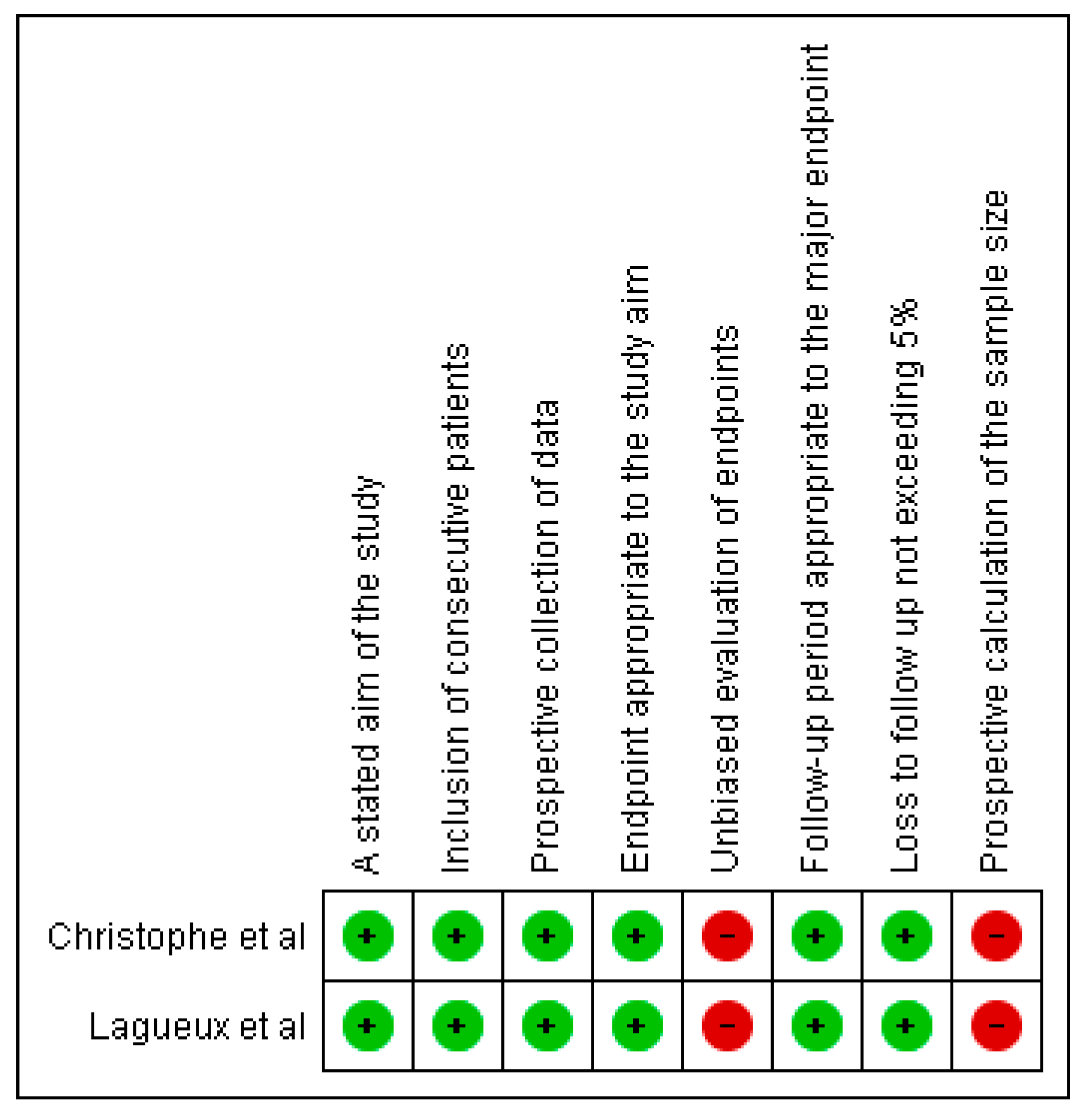
2.5. Article Quality Rating and Risk of Bias
2.6. Data Analysis and Results Synthesis
3. Results
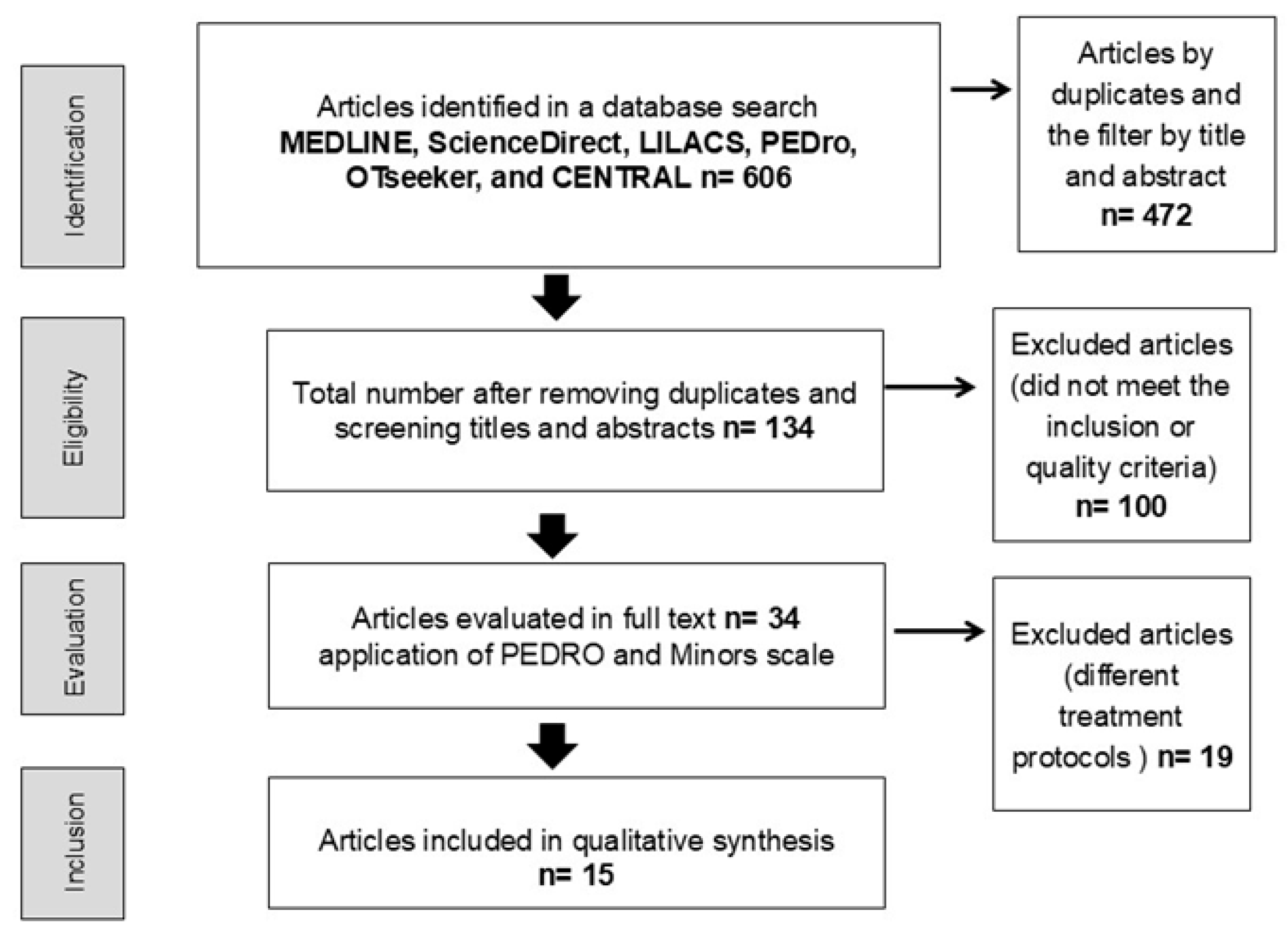
3.1. Selection of Studies
3.2. Characteristics of Excluded Studies
3.3. Characteristics of the Included Studies
3.4. Studies Included and Type of Intervention
3.5. Effects of Physical Exercise on Pain, Physical Function, and Functional Outcomes
3.6. Effects of Neurocognitive Interventions Informed by CBT Principles on Pain
3.7. Effects of Neurocognitive Interventions Informed by CBT Principles on Physical Function
3.8. Effects of Neurocognitive Interventions Informed by CBT Principles on Functional Outcomes or Disability Related to Daily Activities
4. Discussion
4.1. Strengths and Limitations
4.2. Research Priorities
4.3. Public Health Significance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| 1 | “Complex Regional Pain Syndromes” AND “Physical Therapy” |
| 2 | “Complex Regional Pain Syndromes” AND “Exercise” |
| 3 | “Complex Regional Pain Syndromes” AND “Mirror therapy” |
| 4 | “Complex Regional Pain Syndromes” AND “Motor imagery” |
| 5 | “Complex Regional Pain Syndromes” AND “Pain” |
| 6 | “Complex Regional Pain Syndromes” AND “Muscle Strength” |
| 7 | “Complex Regional Pain Syndromes” AND “Range of Motion, Articular” |
| 8 | “Complex Regional Pain Syndromes” AND “Activities of Daily Living” |
| 9 | “Complex Regional Pain Syndromes” AND “Motor function” |
| 10 | “Complex Regional Pain Syndromes” AND “cognitive behavioral therapy” |
| 11 | “Complex Regional Pain Syndromes” AND “physical therapy modalities” |
Appendix B
| DOI/link | Author, Year | Design/Approach | Reason for Exclusion (According to Criteria) | |
|---|---|---|---|---|
| 1 | 10.1186/1471-2377-4-13 | (van de Vusse et al., 2004) [47] | Pharmacological trial | Exclusively pharmacological (no exercise or neurocognitive intervention |
| 2 | 10.1089/jpm.2022.0539 | (Wang et al., 2023) [48] | Case series | Case series—excluded by design. |
| 3 | 10.1016/j.pain.2009.06.023 | (Sigtermans et al., 2009) [49] | Pharmacological RCT | Exclusively pharmacological. |
| 4 | 10.7326/0003-4819-152-3-201002020-00006 | (Goebel et al., 2010) [50] | Pharmacological trial | Exclusively pharmacological. |
| 5 | 10.1016/j.jbspin.2017.03.009 | (Chevreau et al., 2017) [51] | Systematic review/pharmacological meta-analysis | Secondary study and pharmacological; not eligible design/intervention. |
| 6 | 10.1007/s00482-024-00858-2 | (Bernardy et al., 2025) [52] | Review/perspective | Review (not a primary study). |
| 7 | 10.1097/00002508-199909000-00009 | (Sherry et al., 1999) [53] | Pediatric (adolescents) | Pediatric population—excluded. |
| 8 | 10.1186/s12969-016-0090-8 | (Weissmann & Uziel, 2016) [54] | Review (pediatric population) | Review and pediatric—excluded. |
| 9 | 10.1017/S0266462318000429 | (den Hollander et al., 2018) [21] | Economic evaluation (cost-effectiveness) | No therapeutic intervention—excluded. |
| 10 | 10.1186/s13018-022-03461-2 | (Sobeeh et al., 2023) [55] | Systematic review (mechanisms/sensitization) | Not a primary intervention study—excluded. |
| 11 | 10.1007/s00482-021-00559-0 | (Storz & Kraft, 2021) [56] | Summary article/guideline | No estudio primario de intervención excluido. |
| 12 | 10.3390/medicina57111262 | (Moretti et al., 2021) [57] | Scoping review | Review, no primary intervention—excluded. |
| 13 | 10.3233/BMR-220081 | (Kavka, 2023) [58] | Narrative review | Revisión (no elegible por diseño). |
| 14 | 10.1016/j.jstrokecerebrovasdis.2020.104995 | (Altas et al., 2020) [59] | Retrospective observational study (risk factors) | No therapeutic intervention—excluded. |
| 15 | 10.1080/09638288.2019.1650295 | (Mouraux et al., 2021) [60] | Cohort/observational | No therapeutic intervention—excluded. |
| 16 | 10.1080/09593985.2024.2393213 | (Batalla & Lewis, 2025) [61] | Case series | Case series (not RCT/cohort/cross-sectional)—excluded. |
| 17 | 10.1067/mpd.2002.124380 | (Lee et al., 2002) [12] | Pediatric observational study | Pediatric—excluded. |
| 18 | 10.1097/AJP.0000000000001089 | (Shafiee et al., 2023) [62] | Systematic review/meta-analysis | Secondary study (not primary)—excluded. |
| 19 | PubMed 24084413 | (Oral et al., 2013) [63] | Narrative review | Review, not a primary study—excluded. |
References
- Shim, H.; Rose, J.; Halle, S.; Shekane, P. Complex regional pain syndrome: A narrative review for the practising clinician. Br. J. Anaesth. 2019, 123, e424–e433. [Google Scholar] [CrossRef]
- de Mos, M.; de Bruijn, A.G.J.; Huygen, F.J.P.M.; Dieleman, J.P.; Stricker, C.h.B.H.; Sturkenboom, M.C.J.M. The incidence of complex regional pain syndrome: A population-based study. Pain 2007, 129, 12–20. [Google Scholar] [CrossRef]
- Sandroni, P.; Benrud-Larson, L.M.; McClelland, R.L.; Low, P.A. Complex regional pain syndrome type I: Incidence and prevalence in Olmsted county, a population-based study. Pain 2003, 103, 199–207. [Google Scholar] [CrossRef]
- Ott, S.; Maihöfner, C. Signs and Symptoms in 1,043 Patients with Complex Regional Pain Syndrome. J. Pain 2018, 19, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.L.; Chidambaram, S.; Ma, D. Complex regional pain syndrome: A recent update. Burn. Trauma 2017, 5, 2. [Google Scholar] [CrossRef]
- Rodríguez-López, M.J.; Fernández-Baena, M.; Yáñez-Santos, J.A. Complex regional pain syndrome in children: Treatment possibilities. J. Span. Pain Soc. 2014, 21, 138–145. [Google Scholar] [CrossRef]
- Hernández-Porras, B.C.; Plancarte-Sánchez, R.; Alarcón-Barrios, S.; Sámano-García, M. Complex regional pain syndrome: A review. Cir. Cir. 2017, 85, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Misidou, C.; Papagoras, C. Complex Regional Pain Syndrome: An update. Mediterr. J. Rheumatol. 2019, 30, 16–25. [Google Scholar] [CrossRef]
- Zangrandi, A.; Allen Demers, F.; Schneider, C. Complex Regional Pain Syndrome. A Comprehensive Review on Neuroplastic Changes Supporting the Use of Non-invasive Neurostimulation in Clinical Settings. Front. Pain Res. 2021, 2, 732343. [Google Scholar] [CrossRef]
- Forouzanfar, T.; Köke, A.J.A.; van Kleef, M.; Weber, W.E.J. Treatment of complex regional pain syndrome type I. Eur. J. Pain 2002, 6, 105–122. [Google Scholar] [CrossRef]
- Smith, T.O. How effective is physiotherapy in the treatment of complex regional pain syndrome type I? A review of the literature. Musculoskelet. Care 2005, 3, 181–200. [Google Scholar] [CrossRef]
- Lee, B.H.; Scharff, L.; Sethna, N.F.; McCarthy, C.F.; Scott-Sutherland, J.; Shea, A.M.; Sullivan, P.; Meier, P.; Zurakowski, D.; Masek, B.J.; et al. Physical therapy and cognitive-behavioral treatment for complex regional pain syndromes. J. Pediatr. 2002, 141, 135–140. [Google Scholar] [CrossRef]
- Smart, K.M.; Wand, B.M.; O’Connell, N.E. Physiotherapy for pain and disability in adults with complex regional pain syndrome (CRPS) types I and II. Cochrane Database Syst. Rev. 2016, 2016, CD010853. [Google Scholar] [CrossRef] [PubMed]
- Fenn, K.; Byrne, M. The key principles of cognitive behavioural therapy. InnovAiT Educ. Inspir. Gen. Pract. 2013, 6, 579–585. [Google Scholar] [CrossRef]
- Al Sayegh SAl Filén, T.; Johansson, M.; Sandström, S.; Stiewe, G.; Butler, S. Mirror therapy for Complex Regional Pain Syndrome (CRPS)-A literature review and an illustrative case report. Scand. J. Pain 2013, 4, 200–207. [Google Scholar] [CrossRef]
- Donati, D.; Boccolari, P.; Giorgi, F.; Berti, L.; Platano, D.; Tedeschi, R. Breaking the Cycle of Pain: The Role of Graded Motor Imagery and Mirror Therapy in Complex Regional Pain Syndrome. Biomedicines 2024, 12, 2140. [Google Scholar] [CrossRef]
- Barnhoorn, K.J.; Oostendorp, R.A.B.; van Dongen, R.T.M.; Klomp, F.P.; Samwel, H.; van der Wilt, G.J.; Adang, E.; Groenewoud, H.; van de Meent, H.; Frölke, J.P.M. The effectiveness and cost evaluation of pain exposure physical therapy and conventional therapy in patients with complex regional pain syndrome type 1. Rationale and design of a randomized controlled trial. BMC Musculoskelet Disord. 2012, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Halicka, M.; Vittersø, A.D.; Proulx, M.J.; Bultitude, J.H. Pain reduction by inducing sensory-motor adaptation in Complex Regional Pain Syndrome (CRPS PRISMA): Protocol for a double-blind randomized controlled trial. BMC Neurol. 2020, 20, 62. [Google Scholar] [CrossRef]
- Halicka, M.; Vittersø, A.D.; McCullough, H.; Goebel, A.; Heelas, L.; Proulx, M.J.; Bultitude, J.H. Prism adaptation treatment for upper-limb complex regional pain syndrome: A double-blind randomized controlled trial. Pain 2021, 162, 471–489. [Google Scholar] [CrossRef]
- Donegan, T.; Ryan, B.E.; Sanchez-Vives, M.V.; Świdrak, J. Altered bodily perceptions in chronic neuropathic pain conditions and implications for treatment using immersive virtual reality. Front. Hum. Neurosci. 2022, 16, 1024910. [Google Scholar] [CrossRef]
- den Hollander, M.; Heijnders, N.; de Jong, J.R.; Vlaeyen, J.W.S.; Smeets, R.J.E.M.; Goossens, M.E.J.B. In Vivo Exposure Versus Pain-Contingent Physical Therapy in Complex Regional Pain Syndrome Type I: A Cost-Effectiveness Analysis. Int. J. Technol. Assess. Health Care 2018, 34, 400–409. [Google Scholar] [CrossRef]
- Li, T.S.; Wang, R.; Su, X.; Wang, X.Q. Effect and mechanisms of exercise for complex regional pain syndrome. Front. Mol. Neurosci. 2023, 16, 1167166. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Moseley, L.G. Graded motor imagery is effective for long-standing complex regional pain syndrome: A randomised controlled trial. Pain 2004, 108, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L. Graded motor imagery for pathologic pain. Neurology 2006, 67, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Cacchio, A.; De Blasis, E.; De Blasis, V.; Santilli, V.; Spacca, G. Mirror Therapy in Complex Regional Pain Syndrome Type 1 of the Upper Limb in Stroke Patients. Neurorehabil. Neural Repair 2009, 23, 792–799. [Google Scholar] [CrossRef]
- Lagueux, E.; Charest, J.; Lefrançois-Caron, E.; Mauger, M.E.; Mercier, E.; Savard, K.; Tousignant-Laflamme, Y. Modified graded motor imagery for complex regional pain syndrome type 1 of the upper extremity in the acute phase. Int. J. Rehabil. Res. 2012, 35, 138–145. [Google Scholar] [CrossRef]
- Pervane Vural, S.; Nakipoglu Yuzer, G.F.; Sezgin Ozcan, D.; Demir Ozbudak, S.; Ozgirgin, N. Effects of Mirror Therapy in Stroke Patients with Complex Regional Pain Syndrome Type 1: A Randomized Controlled Study. Arch. Phys. Med. Rehabil. 2016, 97, 575–581. [Google Scholar] [CrossRef]
- Barnhoorn, K.J.; Staal, J.B.; van Dongen, R.T.; Frölke, J.P.; Klomp, F.P.; van de Meent, H.; Samwel, H.; Nijhuis-van der Sanden, M.W. Are Pain-Related Fears Mediators for Reducing Disability and Pain in Patients with Complex Regional Pain Syndrome Type 1? An Explorative Analysis on Pain Exposure Physical Therapy. PLoS ONE 2015, 10, e0123008. [Google Scholar] [CrossRef]
- Topcuoglu, A.; Gokkaya, N.K.O.; Ucan, H.; Karakuş, D. The effect of upper-extremity aerobic exercise on complex regional pain syndrome type I: A randomized controlled study on subacute stroke. Top. Stroke Rehabil. 2015, 22, 253–261. [Google Scholar] [CrossRef]
- Barnhoorn, K.J.; van de Meent, H.; van Dongen, R.T.M.; Klomp, F.P.; Groenewoud, H.; Samwel, H.; der Sanden, M.W.G.N.-V.; Frölke, J.P.M.; Staal, J.B. Pain exposure physical therapy (PEPT) compared to conventional treatment in complex regional pain syndrome type 1: A randomised controlled trial. BMJ Open 2015, 5, e008283. [Google Scholar] [CrossRef]
- Christophe, L.; Chabanat, E.; Delporte, L.; Revol, P.; Volckmann, P.; Jacquin-Courtois, S.; Rossetti, Y. Prisms to Shift Pain Away: Pathophysiological and Therapeutic Exploration of CRPS with Prism Adaptation. Neural. Plast. 2016, 2016, 1694256. [Google Scholar] [CrossRef]
- den Hollander, M.; Goossens, M.; de Jong, J.; Ruijgrok, J.; Oosterhof, J.; Onghena, P.; Smeets, R.; Vlaeyen, J.W.S. Expose or protect? A randomized controlled trial of exposure in vivo vs pain-contingent treatment as usual in patients with complex regional pain syndrome type 1. Pain 2016, 157, 2318–2329. [Google Scholar] [CrossRef]
- Schmid, A.C.; Schwarz, A.; Gustin, S.M.; Greenspan, J.D.; Hummel, F.C.; Birbaumer, N. Pain reduction due to novel sensory-motor training in Complex Regional Pain Syndrome I—A pilot study. Scand. J. Pain 2017, 15, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Machač, S.; Chasáková, L.; Kakawand, S.; Kozák, J.; Štěpánek, L.; Vejvalka, J.; Kolář, P.; Černý, R. Mirror visual feedback as therapeutic modality in unilateral upper extremity complex regional pain syndrome type I: Randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2024, 60, 280–291. [Google Scholar] [CrossRef]
- Lewis, J.S.; Newport, R.; Taylor, G.; Smith, M.; McCabe, C.S. Visual illusions modulate body perception disturbance and pain in Complex Regional Pain Syndrome: A randomized trial. Eur. J. Pain 2021, 25, 1551–1563. [Google Scholar] [CrossRef]
- Saha, S.; Sur, M.; Ray Chaudhuri, G.; Agarwal, S. Effects of mirror therapy on oedema, pain and functional activities in patients with poststroke shoulder-hand syndrome: A randomized controlled trial. Physiother. Res. Int. 2021, 26, e1902. [Google Scholar] [CrossRef] [PubMed]
- Harden, R.N.; McCabe, C.S.; Goebel, A.; Massey, M.; Suvar, T.; Grieve, S.; Bruehl, S. Complex Regional Pain Syndrome: Practical Diagnostic and Treatment Guidelines, 5th Edition. Pain Med. 2022, 23 (Suppl. S1), S1–S53. [Google Scholar] [CrossRef] [PubMed]
- Duong, S.; Bravo, D.; Todd, K.J.; Finlayson, R.J.; Tran, D.Q. Treatment of complex regional pain syndrome: An updated systematic review and narrative synthesis. Can. J. Anesth./J. Can. D’anesthésie 2018, 65, 658–684. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Correa, H.R.; Sanchez-Montoya, L.J.; Daza-Arana, J.E.; Ordoñez-Mora, L.T. Aerobic Physical Exercise for Pain Intensity, Aerobic Capacity, and Quality of Life in Patients with Chronic Pain: A Systematic Review and Meta-Analysis. J. Phys. Act. Health 2021, 18, 1126–1142. [Google Scholar] [CrossRef]
- Méndez-Rebolledo, G.; Gatica-Rojas, V.; Torres-Cueco, R.; Albornoz-Verdugo, M.; Guzmán-Muñoz, E. Update on the effects of graded motor imagery and mirror therapy on complex regional pain syndrome type 1: A systematic review. J. Back Musculoskelet. Rehabil. 2017, 30, 441–449. [Google Scholar] [CrossRef]
- Schubert, C. Therapie des komplexen regionalen Schmerzsyndroms im Handbereich aus Sicht der Physiotherapie. Unfallchirurg 2021, 124, 456–464. [Google Scholar] [CrossRef]
- Johnson, S.; Cowell, F.; Gillespie, S.; Goebel, A. Complex regional pain syndrome what is the outcome?—A systematic review of the course and impact of CRPS at 12 months from symptom onset and beyond. Eur. J. Pain 2022, 26, 1203–1220. [Google Scholar] [CrossRef]
- Kraft, E.; Storz, C.; Ranker, A. Physikalische Therapie in der Behandlung des komplexen regionalen Schmerzsyndroms. Der Schmerz 2021, 35, 363–372. [Google Scholar] [CrossRef]
- van de Vusse, A.C.; Stomp-van den Berg, S.G.; Kessels, A.H.; Weber, W.E. Randomised controlled trial of gabapentin in Complex Regional Pain Syndrome type 1 [ISRCTN84121379]. BMC Neurol. 2004, 4, 13. [Google Scholar] [CrossRef]
- Wang, A.T.; Wang, E.J.; Smith, T.J.; Razzak, R.; Christo, P.J. Scrambler Therapy for Patients with Complex Regional Pain Syndrome: A Case Series. J. Palliat. Med. 2023, 26, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Sigtermans, M.J.; van Hilten, J.J.; Bauer, M.C.R.; Arbous, S.M.; Marinus, J.; Sarton, E.Y.; Dahan, A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain 2009, 145, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Goebel, A.; Baranowski, A.; Maurer, K.; Ghiai, A.; McCabe, C.; Ambler, G. Intravenous Immunoglobulin Treatment of the Complex Regional Pain Syndrome. Ann. Intern. Med. 2010, 152, 152–158. [Google Scholar] [CrossRef]
- Chevreau, M.; Romand, X.; Gaudin, P.; Juvin, R.; Baillet, A. Bisphosphonates for treatment of Complex Regional Pain Syndrome type 1: A systematic literature review and meta-analysis of randomized controlled trials versus placebo. Jt. Bone Spine 2017, 84, 393–399. [Google Scholar] [CrossRef]
- Bernardy, K.; Wicking, M.; Michelka, R.; Schwarzer, A. Kognitive Verhaltenstherapie beim komplexen regionalen Schmerzsyndrom. Der Schmerz 2025, 39, 67–77. [Google Scholar] [CrossRef]
- Sherry, D.D.; Wallace, C.A.; Kelley, C.; Kidder, M.; Sapp, L. Short- and Long-term Outcomes of Children with Complex Regional Pain Syndrome Type I Treated with Exercise Therapy. Clin. J. Pain 1999, 15, 218–223. [Google Scholar] [CrossRef]
- Weissmann, R.; Uziel, Y. Pediatric complex regional pain syndrome: A review. Pediatr. Rheumatol. 2016, 14, 29. [Google Scholar] [CrossRef]
- Sobeeh, M.G.; Hassan, K.A.; da Silva, A.G.; Youssef, E.F.; Fayaz, N.A.; Mohammed, M.M. Pain mechanisms in complex regional pain syndrome: A systematic review and meta-analysis of quantitative sensory testing outcomes. J. Orthop. Surg. Res. 2023, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Storz, C.; Kraft, E. Ergotherapie bei komplexem regionalem Schmerzsyndrom. Der Schmerz 2021, 35, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Palomba, A.; Paoletta, M.; Liguori, S.; Toro, G.; Iolascon, G. Complex Regional Pain Syndrome in Athletes: Scoping Review. Medicina 2021, 57, 1262. [Google Scholar] [CrossRef]
- Kavka, T. Harmful or safe? Exposure and pain provocation during physiotherapy of complex regional pain syndrome I: A narrative review. J. Back Musculoskelet. Rehabil. 2023, 36, 565–576. [Google Scholar] [CrossRef]
- Altas, E.U.; Onat, Ş.Ş.; Konak, H.E.; Polat, C.S. Post-stroke complex regional pain syndrome and related factors: Experiences from a tertiary rehabilitation center. J. Stroke Cerebrovasc. Dis. 2020, 29, 104995. [Google Scholar] [CrossRef] [PubMed]
- Mouraux, D.; Lenoir, C.; Tuna, T.; Brassinne, E.; Sobczak, S. The long-term effect of complex regional pain syndrome type 1 on disability and quality of life after foot injury. Disabil. Rehabil. 2021, 43, 967–975. [Google Scholar] [CrossRef]
- Batalla, M.A.P.; Lewis, J.S. Cognitive Multisensory Rehabilitation, a novel approach for Complex Regional Pain Syndrome: Case series. Physiother. Theory Pract. 2025, 41, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, E.; MacDermid, J.; Packham, T.; Walton, D.; Grewal, R.; Farzad, M. The Effectiveness of Rehabilitation Interventions on Pain and Disability for Complex Regional Pain Syndrome. Clin. J. Pain 2023, 39, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Oral, A.; Ilieva, E.M.; Küçükdeveci, A.A.; Varela, E.; Valero, R.; Berteanu, M.; Christodoulou, N. Generalised and regional soft tissue pain syndromes. The role of physical and rehabilitation medicine physicians. The European perspective based on the best evidence. A paper by the UEMS-PRM Section Professional Practice Committee. Eur. J. Phys. Rehabil. Med. 2013, 49, 535–549. [Google Scholar] [PubMed]
| Studio | Country | Type of Study | Type of Cprs/Body Segment | Sample | Intervention | Sample Genre | Comparison | Sample Genre | Results |
|---|---|---|---|---|---|---|---|---|---|
| Moseley et al., 2004 [26] | Australia | RCT/12 months of follow-up | CPRSt/upper extremity | Adult patients after wrist fracture | Motor Imaging Program | 7:5 women/2 men | CT | 6:4 women/2 men | The treatment group showed a effect size of 25 points on the neuropathic pain scale. |
| Moseley et al., 2006 [27] | England | RCT/6 months follow-up | CPRSt1/upper extremity | Adult patient | Motor imagery program | 25 | CT | 25 | A decrease in pain between pre- and post-treatment (100 mm visual analog scale) of 23.4 mm (from 16.2 to 30.4 mm) for the motor imagery group and 10.5 mm (from 1.9 to 19.2 mm) for the control group. |
| Cacchio et al., 2009 [28] | Rome | RCT/6-month follow-up | CPRSt1/upper extremity | Stroke patients | MT + CT | 24:13 women/11 men | CT | 24:13 women/11 men | Reduction in visual analog scale score of pain at rest, on movement and brush-induced tactile allodynia in the mirror group (p < 0.001). |
| Lagueux et al., 2012 [29] | Canada | Pre-experimental pretest–posttest design | CPRSt1/upper extremity | Adult patients | GMI | 7:6 women/1 man | No comparison | Decrease in pain experienced in the last 7 days (visual analog scale; p = 0.046), improvement of the affected limb. grip strength (p = 0.042), and the patient’s overall impression of change (p = 0.015). | |
| Pervane et al., 2015 [30] | Turkey | RCT | CPRSt1/upper extremity | Stroke patients | MT + CT | 15:6 women/9 men | CT | 15:7 women/8 men | Both groups had significant improvements in pain and motor function, but the MT group improved more (p < 0.001, and p = 0.03, respectively). |
| Barnhoorn et al., 2015 [31] | Netherlands | RCT/3, 6 and 9 month follow-ups | CPRSt1/upper and lower extremities | Adult patients | PEPT | 35:29 women/6 men | CT | 21:16 women/5 men | The experimental group had a significantly greater decrease in disability of 7.77 points (95% CI 1.09 to 14.45) and pain of 1.83 points (95% CI 0.44 to 3.23) over 9 months than the control group. |
| Topcuoglu et al., 2015 [32] | Turkey | RCT | CPRSt1/upper extremity | Stroke patients | Aerobic exercise + CT | 20:9 women/11 men | CT | 20:9 women/11 men | The exercise group showed less daytime pain and pain on movement in the shoulder (p = 0.009, p = 0.012) and less daytime pain, nighttime pain and pain on movement in the hand (p = 0.041, p = 0.001). |
| Barnhoorn et al., 2015 [33] | Netherlands | RCT/9 months of follow-up | CPRSt1/Upper and lower extremity | Adult patients | PEPT | 28:24 women/4 men | CT | 28:21 women/7 men | Decreased pain and improved active range of motion and % muscle strength in the PEPT group compared to CT, but the difference between groups was only significant in active range of motion (p = 0.02). |
| Christoph e et al., 2016 [34] | France | Intervention study | CPRSt1, CPRSt2/upper extremity | Adult patient | PA | 7:6 women/1 man | No comparison | - | Improvement in quality of life after the 10-point intervention on the SIP scale. The preglobal score was 38.7 ± 5.76 points (mean ± SEM), while the postglobal score was only 28.6 ± 4.64 points. |
| Hollander et al., 2016 [35] | Netherlands | RCT/6-month follow-up | CPRSt1/upper and lower extremities | Adult patients | In vivo exposure (EXP) | 23:18 women/5 men | Pain-dependent treatment | 23:19 women/4 men | EXP was superior to TAU in the reduction of upper and lower extremity disability from pretreatment to post-treatment and from pre-treatment to 6-month follow-up (p < 0.001). |
| Schmid et al., 2017 [36] | Germany | Pilot study | CPRSt1/upper extremity | Adult patients | Sensorimotor training | 10:3 men/7 women | No comparison | - | Patients showed a significant reduction in pain after the 2-week training period. Changes in pre-post VAS had a mean of 1.0 ± 1.19 (mean ± SD) with a range between −1 and +3, which represented a statistically significant decrease(t(9) = 2.748, p = 0.023). |
| Halicka et al., 2021 [19] | England | RCT/3 and 6 months follow-up | CPRSt1/upper extremity | Adult patients | PA | 23:19 women/4 men | CT | 26:22 women/4 men | There is no evidence that primary or secondary outcomes differed between the prism adaptation and sham treatment groups when assessed at any of the post-treatment time points. |
| Machač et al., 2024 [37] | Czech Republic | RCT | CPRSt1/upper extremity | Adult patient | MT | 13 | No treatment | 14 | Changes in the Visual Analog Scale (VAS) pre-post had a mean of −2.9 ± 1.4 (mean ± SD) with a range between −1.5 and −5.3, which represented a statistically significant decrease = 3.82, p = 0.002). |
| Lewis et al., 2021 [38] | Reuno United | RCT | CPRSt1/upper extremity | Adult patient | MVR | 23 | No actual image manipulation | 22 | In the experimental group, changes in NRS were from 5.6 ± 3.0 to 4.4 ± 2.5 (p = 0.037), while in the control group no significant changes were observed (p > 0.05). In addition, body perception improved in the experimental group (38.7 ± 5.76 to 28.6 ± 4.64, p = 0.036), but not in the control group (p = 0.056). |
| Saha et al., 2021 [39] | India | RCT | CPRSt1/upper extremity | Adult patient | MT | 15 | CT | 15 | The results of the study showed that mirror therapy combined with postictus rehabilitation produced a significant reduction in pain and an improvement in functional independence compared to non-mirror rehabilitation. |
| Studio | Intervention (Time/Type) | Measure | Scale | Intervention | Comparison | p-Value |
|---|---|---|---|---|---|---|
| Moseley et al., 2004 [26] | Motor imagery program 6 weeks | Pain | NPS | Baseline: Total NPS: 46 (4.2) NPS intensity item: 6.6 (0.5) Post-treatment 6 weeks: Total NPS: 20 (10.1–29.9) Intensity point NPS: 3 (2.6–5.4) Post-treatment 12 weeks: Total NPS: 20 (10.1–29.9) Intensity point NPS: 3 (2.6–5.4) | Baseline: Total NPS: 44 (4.3) Intensity item NPS: 6.0 (1.1) Post-treatment: No change | <0.001 |
| Moseley et al., 2006 [27] | Motor imagery program 2 weeks | Pain | VAS | 2.34 (1.2 a 3.04) 6 months: 3.21 (2.38–4.03) | 1.05 (0.9–1.92) 6 months: 1.16 (0.24–2.07) | 0.002 |
| Cacchio et al., 2009 [28] | Mirror therapy 4 weeks | Pain | VAS | Pain at rest: Pre-treatment 7.6 ± 1.2 Post-treatment 4.3 ± 2.5 Pain on movement: Pretreatment 8.7 ± 0.6 Post-treatment 5.1 ± 2.6 | Pain at rest: Pretreatment 7.5 ± 1.1 Post-treatment 7.2 ± 2.2 Pain on movement: Pretreatment 8.3 ± 0.7 Post-treatment 8.2 ± 1.4 | 0.828 <0.001 0.04 <0.001 |
| Physical function | WMFT | Pretreatment 3.5 ± 1.2 Post-treatment 1.5 ± 0.7 | Pre-treatment 3.6 ± 0.7 Post-treatment 3.4 ± 0.9 | 0.726 <0.001 | ||
| Activities of daily living | MAL | Pretreatment 1.4 ± 0.4 Post-treatment 3.6 ± 1.5 | Pretreatment 1.3 ± 0.5 Post-treatment 1.2 ± 0.8 | 0.468 <0.001 | ||
| Lagueux et al., 2012 [29] | Graded motor imagery 4–12 weeks | Pain | SF-MPQ/VAS | Start: 4.39 (2.24) Post-treatment: 2.05 (2.33) | No comparison | 0.046 |
| Physical function | Grip strength | Onset: 11.78 (9.02) mmHg Post-treatment: 28.90 (13.10) mmHg | No comparison | 0.042 | ||
| Activities of daily living | DASH | Home: 40.97 (12.29) Post-treatment: 31.98 (16.97) | No comparison | 0.138 | ||
| Pervane et al., 2015 [30] | Mirror therapy 4 weeks | Pain | VAS | Start: 6 (2–9) Post-treatment: 3 (1–6) | Start: 5 (3–9) Post-treatment: 5 (2–8) | <0.001 |
| Physical function | FMA | Hand: Baseline: 7 (2–13) Post-treatment: 11 (5–14) Wrist: Baseline: 5 (2–6) Post-treatment: 8 (4–9) | Hand: Baseline: 5 (3–13) Post-treatment: 5 (3–13) Wrist: Baseline: 4 (1–8) Post-treatment: 5 (1–8) | <0.001 <0.001 | ||
| Activities of daily living | FIM | Start: 41 (20–83) Post-tratamiento: 44 (20–83) | Home: 32 (14–86) Post-treatment: 39 (15–86) | 0.09 | ||
| Barnhoorn et al., 2015 [31] | Pain exposure physiotherapy 5 sessions | Pain | VAS | Home: 6.23 (2.40) 3 months: 3.97 (2.47) 6 months: 3.90 (2.62) 9 months: 3.24 (2.31) | Home: 7.33 (1.96) 3 months: 6.24 (3.21) 6 months: 5.65 (3.42) 9 months: 5.89 (3.16) | 0.01 |
| Activities of daily living | PDI | Base: 33.91 (13.00) 3 months: 19.87 (13.56) 6 months: 12.48 (11.96) 9 months: 10.40 (9.54) | Base: 37.17 (13.06) 3 months: 28.18 (16.38) 6 months: 22.58 (16.23) 9 months: 23.48 (18.81) | 0.02 | ||
| Topcuoglu et al., 2015 [32] | Aerobic exercise 4 weeks | Pain | VAS | VAS Shoulder Daytime: 0.50 ± 1.4 Night: 2.00 ± 2.4 Movement: 4.30 ± 2.2 VAS Hand Daytime hours: 0.45 ± 1.1 Night: 1.40 ± 1.9 Movement: 3.35 ± 2.0 | VAS Shoulder Daytime: 2.40 ± 2.7 Night: 2.70 ± 2.5 Movement: 6.00 ± 1.8 VAS Hand Daytime: 2.2 ± 2.3 Night: 2.7 ± 1.9 Movement: 5.4 ± 1.6 | 0.009 0.376 0.012 0.004 0.041 0.03 |
| Physical function | Brunnstrom Stage | Not communicated | Not communicated | 0.059 | ||
| Activities of daily living | FIM | Not communicated | Not communicated | 0.001 | ||
| Barnhoorn et al., 2015 [33] | Pain exposure physiotherapy 5 sessions | Pain | VAS | Start: 6.18 (2.50) 3 months: 4.41 (2.85) 6 months: 4.31 (2.81) 9 months: 3.52 (2.69) | Home: 7.11 (2.01) 3 months: 5.35 (3.09) 6 months: 4.92 (3.34) 9 months: 4.96 (3.02) | 0.36 |
| Physical function | Muscle strength (%) + | Home: 61.90 (22.96) ++ 3 months: 36.80 (27.86) ++ 6 months: 27.50 (26.52) ++ 9 months: 25.83 (27.39) ++ | Home: 67.14 (23.16) ++ 3 months: 46.10 (26.16) ++ 6 months: 38.25 (27.20) ++ 9 months: 32.50 (27.22) ++ | 0.02 | ||
| Active range of motion | Home: 4.71 (2.16) ++ 3 months: 3.11 (1.26) ++ 6 months: 3.35 (1.67) ++ 9 months: 2.89 (1.22) ++ | Home: 4.93 (1.98) ++ 3 months: 4.04 (1.95) ++ 6 months: 3.52 (1.26) ++ 9 months: 3.32 (0.95) ++ | 0.25 | |||
| Activities of daily living | EQ-5D (EuroQol-5D) | Home: 0.53 (0.26) 3 months: 0.63 (0.22) 6 months: 0.77 (0.19) 9 months: 0.76 (0.20) | Home: 0.47 (0.29) 3 months: 0.64 (0.26) 6 months: 0.67 (0.32) 9 months: 0.74 (0.25) | 0.79 | ||
| Christophe et al., 2016 [34] | Prism adaptation 8 sessions | Pain | VAS | Start: 5.88 ± 1.26 Post-treatment: 3.8 ± 0.5 | No comparison | <0.0006 |
| Activities of daily living | SIP | Onset: 38.7 ± 5.76 Post-treatment: 28.6 ± 4.64 | No comparison | <0.05 | ||
| Hollander et al., 2016 [35] | In vivo exposure (EXP) 17 weeks | Pain | NPS | Pretreatment 5.48 (2.22) Post-treatment 2.79 (2.25) | Pretreatment 5.63 (1.63) Post-treatment 4.98 (2.16) | 0.001 |
| Activities of daily living | WAQ | Pretreatment 6.97 (2.48) Post-treatment 1.44 (2.15) | Pretreatment 6.99 (1.76) Post-treatment 4.57 (2.90) | 0.054 | ||
| RASQ | Pretreatment 3.14 (0.89) Post-treatment 1.86 (0.91) | Pre-treatment 3.49 (0.96) Posttreatment 3.02 (1.19) | <0.001 | |||
| Schmid et al., 2017 [36] | Sensorimotor training 2 weeks | Pain | VAS | Onset: 4.5 ± 1.52 Post-treatment: 3.5 ± 2.09 | No comparison | 0.023 |
| Halicka et al., 2021 [19] | PA: Prism adaptation 2 weeks | Pain | NPRS | Home: 5.96 (5.02 to 6.80) Post-treatment: 5.43 (4.55 to 6.24) 3 months: 5.62 (4.69 to 6.48) 6 months: 5.59 (4.69 to 6.41) | Start: 6.15 (5.26 to 7.00) Posttreatment: 5.84 (4.82 to 6.74) 3 months: 6.04 (5.12 to 6.80) 6 months: 5.95 (5.07 to 6.73) | 0.126 |
| Machač et al., 2024 [37] | Mirror Therapy 6 weeks | Pain | VAS | Resting Start: 4.1 ± 2.9 42 days: 1.2 ± 0.4 72 days: 0.9 ± 0.1 Movement Onset: 5.9 ± 5.0 42 days: 2.3 ± 1.0 72 days: 2.0 ± 0.7 | Rest Onset: 4.4 ± 3.4 42 days: 3.6 ± 2.6 72 days: 2.6 ± 1.6 Movement Onset: 6.7 ± 5.6 42 days 5.6 ± 4.2 72 days: 4.0 ± 3.1 | <0.001 |
| SIP | Baseline: 38.7 ± 5.76 Post-treatment: 28.6 ± 4.64 | Home: 37.1 ± 5.89 Post-treatment: 34.2 ± 4.92 | p < 0.05 | |||
| Lewis et al., 2021 [38] | Mediated Virtual Reality 4 weeks of intervention + 2 weeks of follow-up | Pain | NPRS | Onset: 5.6 ± 3.0 After 4 weeks: 5.2 ± 2.7 Follow-up at 2 weeks: 4.4 ± 2.5 | Onset: 5.7 ± 3.0 After 4 weeks: 5.7 ± 3.0 Follow-up at 2 weeks: 5.7 ± 3.0 | p < 0.05 |
| Saha et al., 2021 [39] | Mirror Therapy 4 weeks of intervention + 2 weeks of follow-up | Pain | NPRS | Start: 6.07 ± 1.58 Post-treatment: 3.93 ± 1.39 Follow-up at 2 weeks: 3.47 ± 1.30 | Start: 6.60 ± 1.06 Post-treatment: 5.33 ± 0.98 Follow-up at 2 weeks: 5.33 ± 1.23 | p < 0.05 |
| FIM | Start: 72.47 ± 17.41 Post-treatment: 88.33 ± 18.72 Follow-up at 2 weeks: 92.07 ± 17.21 | Start: 62.88 ± 15.40 Post-treatment: 66.38 ± 15.33 Follow-up at 2 weeks: 66.25 ± 15.45 | p < 0.05 |
| Intervention (Studies) | No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Final Certainty |
|---|---|---|---|---|---|---|---|
| Mirror therapy (MT) [28,30,37,39] | 4 RCTs | Moderate (blinding difficult) | Not serious (consistent effect) | 2 in post-stroke/not serious | Small samples | Possible | Moderate |
| GMI [26,27,29] | 2 RCTs + 1 pre–post | Moderate–high | Serious (heterogeneity) | Direct | Small | Possible | Very low |
| PEPT (exposure) [31,33,35] | 3 RCTs | Moderate | Not serious/minor | Direct | Moderate | Possible | Moderate |
| Prism adaptation [19,34] | 1 pilot + 1 RCT | Moderate | Serious (one positive, one null) | Direct | Small | Possible | Low |
| Virtual reality [38] | 1 RCT | Moderate | N/A | Direct | Moderate | Possible | Low |
| Aerobic exercise [32] | 1 RCT | Moderate | N/A | Direct | Small | Possible | Low |
| Sensorimotor training [36] | 1 pilot | High | N/A | Direct | Very small | Probable | Very low |
| Intervention (Studies) | No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Final Certainty |
|---|---|---|---|---|---|---|---|
| Mirror therapy (MT) [28,30] | 2 RCTs | Moderate | Not serious (improvement in WMFT and Fugl–Meyer) | 1 in post-stroke/minor | Small | Possible | Moderate |
| GMI [26,29] | 1 RCT + 1 pre–post | High | Serious (heterogeneous) | Direct | Very small | Possible | Very low |
| PEPT [33,35] | 2 RCTs | Moderate | Minor (ROM improved; mixed strength results) | Direct | Moderate | Possible | Moderate |
| Aerobic exercise [32] | 1 RCT | Moderate | N/A | Direct | Small; effect on Brunnstrom unclear | Possible | Very low |
| Intervention (Studies) | No. of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Final Certainty |
|---|---|---|---|---|---|---|---|
| Mirror therapy (MT) [28,30,37,39] | 4 RCTs | Moderate | Not serious (MAL/FIM/SIP improved) | 2 in post-stroke/minor | Small | Possible | Moderate |
| PEPT (exposure) [31,33,35] | 3 RCTs | Moderate | Minor (PDI/EQ-5D/WAQ/RAQ mixed in magnitude) | Direct | Moderate | Possible | Moderate |
| GMI [29] | 1 pre–post (DASH) | High | N/A | Direct | Very small | Possible | Very low |
| Prism adaptation [34] | 1 pilot | Moderate | N/A | Direct | Very small | Possible | Very low |
| Aerobic exercise [32] | 1 RCT | Moderate | N/A | Direct | Small; incomplete reporting | Possible | Very low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordoñez-Mora, L.T.; Quintero-López, D.S.; Morales-Osorio, M.A.; Gómez-Gómez, J.F.; Rivas-Tafurt, G.P.; Serna-Orozco, M.F. Neurocognitive Interventions Informed by Cognitive–Behavioral Therapy (CBT) Principles and Physical Exercise for Complex Regional Pain Syndrome: A Systematic Review. J. Clin. Med. 2025, 14, 6820. https://doi.org/10.3390/jcm14196820
Ordoñez-Mora LT, Quintero-López DS, Morales-Osorio MA, Gómez-Gómez JF, Rivas-Tafurt GP, Serna-Orozco MF. Neurocognitive Interventions Informed by Cognitive–Behavioral Therapy (CBT) Principles and Physical Exercise for Complex Regional Pain Syndrome: A Systematic Review. Journal of Clinical Medicine. 2025; 14(19):6820. https://doi.org/10.3390/jcm14196820
Chicago/Turabian StyleOrdoñez-Mora, Leidy Tatiana, Daihana Stefany Quintero-López, Marco Antonio Morales-Osorio, Juan Fernando Gómez-Gómez, Giovanna Patricia Rivas-Tafurt, and María Fernanda Serna-Orozco. 2025. "Neurocognitive Interventions Informed by Cognitive–Behavioral Therapy (CBT) Principles and Physical Exercise for Complex Regional Pain Syndrome: A Systematic Review" Journal of Clinical Medicine 14, no. 19: 6820. https://doi.org/10.3390/jcm14196820
APA StyleOrdoñez-Mora, L. T., Quintero-López, D. S., Morales-Osorio, M. A., Gómez-Gómez, J. F., Rivas-Tafurt, G. P., & Serna-Orozco, M. F. (2025). Neurocognitive Interventions Informed by Cognitive–Behavioral Therapy (CBT) Principles and Physical Exercise for Complex Regional Pain Syndrome: A Systematic Review. Journal of Clinical Medicine, 14(19), 6820. https://doi.org/10.3390/jcm14196820







