Assessing the Impact of Metabolic Syndrome on Liver Outcomes in Methotrexate Users: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
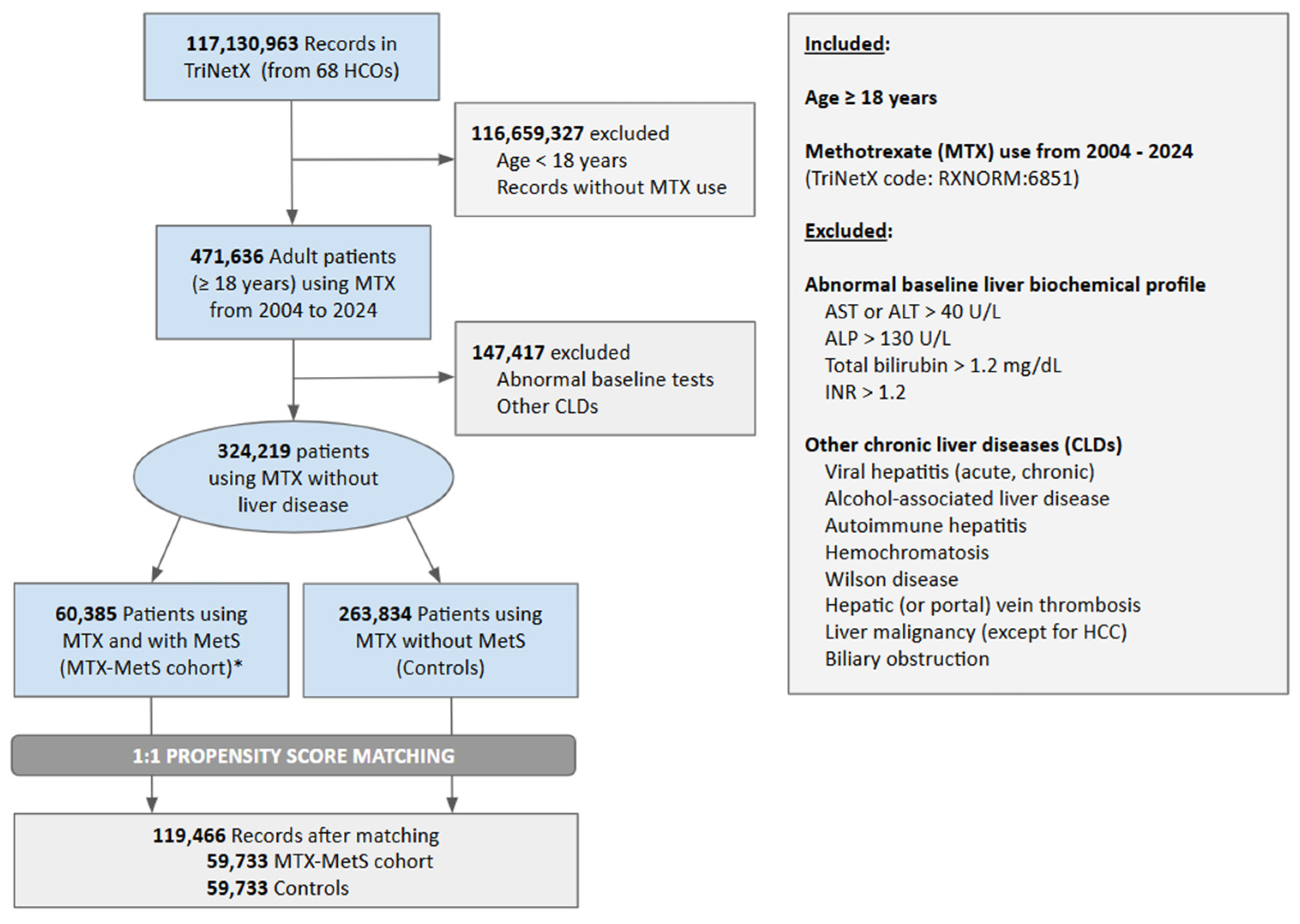
2.2. Study Population and Variables
2.3. Patient and Hospital Characteristics
2.4. Objectives and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Patient Characteristics
3.3. Laboratory Abnormalities
3.4. Liver-Related Outcomes
3.5. Mortality and Healthcare Resource Utilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DILI | Drug-induced liver injury |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MetS | Metabolic syndrome |
| MTX | Methotrexate |
| PA | Psoriatic arthritis |
| RA | Rheumatoid arthritis |
References
- Cipriani, P.; Ruscitti, P.; Carubbi, F.; Liakouli, V.; Giacomelli, R. Methotrexate: An old new drug in autoimmune disease. Expert Rev. Clin. Immunol. 2014, 10, 1519–1530. [Google Scholar] [CrossRef]
- Koźmiński, P.; Halik, P.K.; Chesori, R.; Gniazdowska, E. Overview of Dual-Acting Drug Methotrexate in Different Neurological Diseases, Autoimmune Pathologies and Cancers. Int. J. Mol. Sci. 2020, 21, 3483. [Google Scholar] [CrossRef]
- Schmajuk, G.; Miao, Y.; Yazdany, J.; Boscardin, W.J.; Daikh, D.I.; Steinman, M.A. Identification of risk factors for elevated transaminases in methotrexate users through an electronic health record. Arthritis Care Res. 2014, 66, 1159–1166. [Google Scholar] [CrossRef]
- Whiting-O’Keefe, Q.E.; Fye, K.H.; Sack, K.D. Methotrexate and histologic hepatic abnormalities: A meta-analysis. Am. J. Med. 1991, 90, 711–716. [Google Scholar] [CrossRef]
- Conway, R.; Carey, J.J. Risk of liver disease in methotrexate treated patients. World J. Hepatol. 2017, 9, 1092–1100. [Google Scholar] [CrossRef]
- Atallah, E.; Grove, J.I.; Crooks, C.; Burden-Teh, E.; Abhishek, A.; Moreea, S.; Jordan, K.M.; Ala, A.; Hutchinson, D.; Aspinall, R.J.; et al. Risk of liver fibrosis associated with long-term methotrexate therapy may be overestimated. J. Hepatol. 2023, 78, 989–997. [Google Scholar] [CrossRef]
- Di Martino, V.; Verhoeven, D.W.; Verhoeven, F.; Aubin, F.; Avouac, J.; Vuitton, L.; Lioté, F.; Thévenot, T.; Wendling, D. Busting the myth of methotrexate chronic hepatotoxicity. Nat. Rev. Rheumatol. 2023, 19, 96–110. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.P. Metabolic syndrome. Nat. Rev. Dis. Primers 2024, 10, 77. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar]
- Yip, T.C.; Wong, G.L.; Wong, V.W.; Goh, G.B.; Chan, W.K. Nonalcoholic Fatty Liver Disease: A Unique Entity or Part of the Metabolic Syndrome or Both. Med. Clin. N. Am. 2023, 107, 449–463. [Google Scholar] [CrossRef]
- Shetty, A.; Cho, W.; Alazawi, W.; Syn, W.K. Methotrexate Hepatotoxicity and the Impact of Nonalcoholic Fatty Liver Disease. Am. J. Med. Sci. 2017, 354, 172–181. [Google Scholar] [CrossRef]
- Menter, A.; Gelfand, J.M.; Connor, C.; Armstrong, A.W.; Cordoro, K.M.; Davis, D.M.; Elewski, B.E.; Gordon, K.B.; Gottlieb, A.B.; Kaplan, D.H.; et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J. Am. Acad. Dermatol. 2020, 82, 1445–1486. [Google Scholar] [CrossRef]
- Nakafero, G.; Grainge, M.J.; Card, T.; Mallen, C.D.; Zhang, W.; Doherty, M.; Taal, M.W.; Aithal, G.P.; Abhishek, A. What is the incidence of methotrexate or leflunomide discontinuation related to cytopenia, liver enzyme elevation or kidney function decline? Rheumatology 2021, 60, 5785–5794. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St. CLair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Langman, G.; Hall, P.M.; Todd, G. Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J. Gastroenterol. Hepatol. 2001, 16, 1395–1401. [Google Scholar] [CrossRef]
- Mori, S.; Arima, N.; Ito, M.; Fujiyama, S.; Kamo, Y.; Ueki, Y. Non-alcoholic steatohepatitis-like pattern in liver biopsy of rheumatoid arthritis patients with persistent transaminitis during low-dose methotrexate treatment. PLoS ONE 2018, 13, e0203084. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.; Urwitz, H.; Johannesson, A.; Ros, A.-M.; Lindholm, J.; Kinnman, N.; Hultcrantz, R. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J. Hepatol. 2007, 46, 1111–1118. [Google Scholar] [CrossRef]
- Cheema, H.I.; Haselow, D.; Dranoff, J.A. Review of existing evidence demonstrates that methotrexate does not cause liver fibrosis. J. Investig. Med. 2022, 70, 1452–1460. [Google Scholar] [CrossRef]
- TriNetX. Available online: https://trinetx.com/ (accessed on 2 February 2024).
- Institute of Medicine (US) Committee on Assessing the System for Protecting Human Research Participants. Responsible Research: A Systems Approach to Protecting Research Participants; Federman, D.D., Hanna, K.E., Rodriguez, L.L., Eds.; National Academies Press (US): Washington, DC, USA, 2002. [Google Scholar]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Models Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Hanoodi, M.; Mittal, M. Methotrexate. 2024 Dec 11. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Centers for Disease Control and Prevention. Social Determinants of Health. [updated 2023]. Available online: https://www.cdc.gov/nchs/data/icd/social-determinants-of-health.pdf (accessed on 2 February 2025).
- American Hospital Association. Value Initiative: ICD-10 Codes for Social Determinants of Health. [updated 2022]. Available online: https://www.aha.org/system/files/2018-04/value-initiative-icd-10-code-social-determinants-of-health.pdf (accessed on 2 February 2025).
- Fontana, R.J.; Liou, I.; Reuben, A.; Suzuki, A.; Fiel, M.I.; Lee, W.; Navarro, V. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology 2023, 77, 1036–1065. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
| Before Propensity Matching | After Propensity Matching | |||||
|---|---|---|---|---|---|---|
| Baseline Characteristics | MTX-MetS | Controls | p | MTX-MetS | Controls | p |
| Age (Mean ± SD) | 60.5 ± 13.7 | 53.4 ± 18.0 | <0.001 | 60.5 ± 13.7 | 60.7 ± 13.8 | 0.002 |
| Sex: Female | 68.6% (41,007) | 73.8% (191,128) | 0.113 | 68.6% (41,006) | 68.8% (41,068) | 0.699 |
| Race/Ethnicity | ||||||
| Non-Hispanic White | 64.1% (38,302) | 65.7% (170,143) | 0.032 | 64.1% (38,302) | 64.2% (38,323) | 0.899 |
| Black or African American | 17.2% (10,285) | 13.0% (33,748) | 0.117 | 17.2% (10,284) | 17.2% (10,275) | 0.945 |
| Hispanic or Latino | 7.3% (4354) | 7.6% (19,804) | 0.013 | 7.3% (4354) | 7.2% (4307) | 0.600 |
| Etiology for MTX Use | ||||||
| Seropositive RA | 7.1% (4244) | 4.9% (12,570) | <0.001 | 7.1% (4243) | 6.7% (4018) | 0.010 |
| Seronegative RA | 2.7% (1642) | 1.8% (4535) | <0.001 | 2.7% (1642) | 2.5% (1479) | 0.003 |
| Juvenile idiopathic arthritis | 0.4% (261) | 1.2% (2982) | <0.001 | 0.4% (261) | 0.3% (204) | 0.008 |
| Psoriasis | 8.9% (5306) | 6.4% (16,587) | <0.001 | 8.9% (5306) | 8.6% (5143) | 0.095 |
| Connective tissue diseases | 10.2% (6114) | 8.7% (22,509) | <0.001 | 10.2% (6114) | 10.0% (5990) | 0.234 |
| Hematopoietic malignancy | 2.3% (1394) | 1.6% (4200) | <0.001 | 2.3% (1394) | 2.3% (1360) | 0.512 |
| Any malignancy | 21.6% (12,918) | 14.6% (37,717) | <0.001 | 21.6% (12,917) | 21.6% (12,926) | 0.950 |
| Glucocorticoid therapy | 53.4% (31,876) | 40.4% (104,724) | <0.001 | 53.4% (31,875) | 52.9% (31,582) | 0.089 |
| SDHOs | 1.4% (846) | 1.0% (2462) | <0.001 | 1.4% (846) | 1.4% (819) | 0.512 |
| Before Propensity Matching | After Propensity Matching | ||||||
|---|---|---|---|---|---|---|---|
| Charlson Comorbidities | ICD-10 CM CODES | MTX-MetSα | Controls | p | MTX-MetS | Controls | p |
| HIV/AIDS | B20 | 0.1% (73) | 0.1% (211) | 0.003 | 0.1% (73) | 0.1% (68) | 0.674 |
| Cerebrovascular diseases | I60-I69 | 5.9% (3511) | 2.5% (6565) | <0.001 | 5.9% (3510) | 5.9% (3526) | 0.844 |
| Chronicpulmonary disease | J40-J4A | 16.0% (9538) | 9.3% (24,038) | <0.001 | 16.0% (9537) | 15.9% (9481) | 0.658 |
| Congestive heart failure | I50 | 3.9% (2352) | 1.4% (3752) | <0.001 | 3.9% (2351) | 3.8% (2252) | 0.137 |
| Ischemic heart diseases | I20-I25 | 10.9% (6518) | 4.1% (10,730) | <0.001 | 10.9% (6517) | 10.8% (6465) | 0.629 |
| Dementia | F01-F09 | 1.5% (916) | 0.9% (2258) | <0.001 | 1.5% (916) | 1.5% (893) | 0.586 |
| Peptic ulcer disease | K25 | 0.7% (410) | 0.4% (1025) | <0.001 | 0.7% (410) | 0.7% (397) | 0.646 |
| Peripheral vascular disease | I70-I79 | 8.3% (4951) | 4.8% (12,472) | <0.001 | 8.3% (4950) | 8.2% (4878) | 0.448 |
| Paralytic syndromes * | G80-G83 | 0.8% (503) | 0.4% (1039) | <0.001 | 0.8% (503) | 0.8% (476) | 0.386 |
| Chronic kidney disease | N18 | 4.7% (2812) | 1.7% (4489) | <0.001 | 4.7% (2811) | 4.5% (2684) | 0.079 |
| Connective tissue diseases | M30-M36 | 10.2% (6114) | 8.7% (22,509) | <0.001 | 10.2% (6114) | 10.0% (5990) | 0.234 |
| Neoplasms | C00-D49 | 21.6% (12,918) | 14.6% (37,717) | <0.001 | 21.6% (12,917) | 21.6% (12,926) | 0.950 |
| Incidence % (N) MTX-MetS vs. Controls | PSM Analysis (10-Year Outcomes) aOR * [95%CI] | ||||
|---|---|---|---|---|---|
| Outcomes | 3-Year | 5-Year | 10-Year | MTX-MetS vs. Controls ** | MTX-MetS-MASLD vs. Controls ** |
| Laboratory abnormalities | |||||
| Hepatic-enzyme elevations 1 | 13.3% (7768) vs. 10.3% (6125) | 17.0% (9891) vs. 12.9% (7674) | 20.7% (12,051) vs. 15.5% (9255) | 1.41 [1.38–1.46] | 1.31 [1.17–1.46] |
| Hyperbilirubinemia 2 | 2.5% (1460) vs. 1.8% (1046) | 3.2% (1932) vs. 2.3% (1384) | 4.1% (2470) vs. 3.0% (1787) | 1.40 [1.32–1.49] | 1.57 [1.27–1.95] |
| Prolonged INR 3 | 3.2% (1883) vs. 2.1% (1227) | 4.2% (2500) vs. 2.7% (1636) | 5.4% (3216) vs. 3.5% (2087) | 1.58 [1.49–1.67] | 1.52 [1.24–1.88] |
| Liver-related Outcomes | |||||
| Clinically significant DILI 4 | 3.1% (1835) vs. 2.1% (1282) | 4.1% (2441) vs. 2.9% (1711) | 5.3% (3175) vs. 3.7% (2181) | 1.49 [1.41–1.57] | 1.56 [1.28–1.89] |
| Liver cirrhosis | 1.1% (634) vs. 0.8% (453) | 1.4% (857) vs. 1.0% (576) | 1.9% (1102) vs. 1.3% (748) | 1.48 [1.35–1.63] | 2.21 [1.72–2.84] |
| Other Clinical Outcomes | |||||
| All-cause mortality | 4.5% (2652) vs. 4.0% (2397) | 6.2% (3715) vs. 5.6% (3314) | 8.5% (5045) vs. 7.6% (4514) | 1.13 [1.08–1.18] | 0.88 [0.75–1.03] |
| All cause hospitalizations | 10.5% (5602) vs. 7.4% (4015) | 13.6% (7247) vs. 9.6% (5206) | 17.3% (9171) vs. 12.1% (6550) | 1.43 [1.39–1.47] | 1.36 [1.20–1.54] |
| All-cause ICU admissions | 3.6% (2118) vs. 2.3% (1360) | 4.8% (2819) vs. 3.1% (1826) | 6.4% (3745) vs. 4.1% (2401) | 1.60 [1.52–1.69] | 1.46 [1.22–1.75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilani, Y.; Mosquera, D.A.G.; Fennell, K.; Qureshi, K. Assessing the Impact of Metabolic Syndrome on Liver Outcomes in Methotrexate Users: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 6799. https://doi.org/10.3390/jcm14196799
Kilani Y, Mosquera DAG, Fennell K, Qureshi K. Assessing the Impact of Metabolic Syndrome on Liver Outcomes in Methotrexate Users: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(19):6799. https://doi.org/10.3390/jcm14196799
Chicago/Turabian StyleKilani, Yassine, Daniel Alejandro Gonzalez Mosquera, Kaila Fennell, and Kamran Qureshi. 2025. "Assessing the Impact of Metabolic Syndrome on Liver Outcomes in Methotrexate Users: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 19: 6799. https://doi.org/10.3390/jcm14196799
APA StyleKilani, Y., Mosquera, D. A. G., Fennell, K., & Qureshi, K. (2025). Assessing the Impact of Metabolic Syndrome on Liver Outcomes in Methotrexate Users: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(19), 6799. https://doi.org/10.3390/jcm14196799






