Results of Combined Penetrating Keratoplasty and Pars Plana Vitrectomy Performed for Infectious Keratitis with Endophthalmitis Compared to Other Non-Infectious Indications: Series of 129 Eyes
Abstract
1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Video Online
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frisina, R.; Besozzi, G.; Gius, I.; Greggio, A.; De Salvo, G.; Meduri, A. Pole to Pole Surgery in Ocular Trauma: Standardizing Surgical Steps. Ophthalmol. Ther. 2022, 11, 1951–1959. [Google Scholar] [CrossRef]
- Ikeda, T. Pars plana vitrectomy combined with penetrating keratoplasty. Semin. Ophthalmol. 2001, 16, 119–125. [Google Scholar] [CrossRef]
- Mayalı, H.; Kayıkçıoğlu, Ö.; Altınışık, M.; Bıçak, F.; Kurt, E. Clinical results in patients with combined penetrating keratoplasty and vitreoretinal surgery using landers wide-field temporary keratoprosthesis. Turk. J. Ophthalmol. 2019, 49, 270–276. [Google Scholar] [CrossRef]
- Durand, M.L. Endophthalmitis. Clin. Microbiol. Infect. 2013, 19, 227–234. [Google Scholar] [CrossRef]
- Lu, X.; Ng, D.S.C.; Zheng, K.; Peng, K.; Jin, C.; Xia, H.; Chen, W.; Chen, H. Risk factors for endophthalmitis requiring evisceration or enucleation. Sci. Rep. 2016, 6, 28100. [Google Scholar] [CrossRef]
- Alfaro Rangel, R.; Szentmáry, N.; Lepper, S.; Milioti, G.; Daas, L.; Langenbucher, A.; Seitz, B. Large-diameter penetrating keratoplasties are mostly due to very severe infectious keratitis and cannot always prevent secondary enucleation. Klin. Monatsblätter Für Augenheilkd. 2022, 239, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- McGwin, G.; Xie, A.; Owsley, C. Rate of Eye Injury in the United States. Arch. Ophthalmol. 2005, 123, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Gelston, C.D.; Deitz, G.A. Eye Emergencies. Am. Fam. Physician 2020, 102, 539–545. [Google Scholar] [PubMed]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Author Response: Numerical Imputation for Low Vision States Numerical Imputation for Low Vision States. IOVS Invest. Ophthalmol. Vis. Sci. 2007, 47, 1236–1240. [Google Scholar]
- Schulze-Bonsel, K.; Feltgen, N.; Burau, H.; Hansen, L.; Bach, M. Visual Acuities “Hand Motion” and “Counting Fingers” Can Be Quantified with the Freiburg Visual Acuity Test. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1236–1240. [Google Scholar] [CrossRef]
- Finger, R.P.; Fimmers, R.; Holz, F.G.; Scholl, H.P.N. Incidence of Blindness and Severe Visual Impairment in Germany: Projections for 2030. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4381–4389. [Google Scholar] [CrossRef]
- Roters, S.; Hamzei, P.; Szurman, P.; Hermes, S.; Thumann, G.; Bartz-Schmidt, K.; Kirchhof, B. Combined penetrating keratoplasty and vitreoretinal surgery with silicone oil: A 1-year follow-up. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 24–33. [Google Scholar] [CrossRef]
- Fiorentzis, M.; Morinello, E.; Viestenz, A.; Zuche, H.; Seitz, B.; Viestenz, A. Muscle relaxants as a risk factor for vis-à-tergo during penetrating keratoplasty: A prospective interventional study. Adv. Ther. 2017, 34, 2674–2679. [Google Scholar] [CrossRef]
- Garcia-Valenzuela, E.; Blair, N.P.; Shapiro, M.J.; Gieser, J.P.; Resnick, K.I.; Solomon, M.J.; Sugar, J. Outcome of vitreoretinal surgery and penetrating keratoplasty using temporary keratoprosthesis. Retina 1999, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.J. Endophthalmitis. Korean J. Ophthalmol. 2017, 31, 283. [Google Scholar] [CrossRef] [PubMed]
- Bové Álvarez, M.; Arumí, C.G.; Distéfano, L.; Güell, J.L.; Gris, Ó.; Mateo, C.; Corcóstegui, B.; García-Arumí, J. Comparative study of penetrating keratoplasty and vitreoretinal surgery with Eckardt temporary keratoprosthesis in ocular trauma versus non-trauma patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 2547–2558. [Google Scholar] [CrossRef] [PubMed]
- Zapata Cuevas, M.A.; García de Oteyza, G.; Alvarado-Villacorta, R.; Hernández, L.M.Q.; Ordoñez-Ranz, G.; de Wit Carter, G.; García-Albisua, A.M. Pars plana vitrectomy and therapeutic keratoplasty in endophthalmitis and infectious keratitis. Eur. J. Ophthalmol. 2023, 33, 207–215. [Google Scholar] [CrossRef]
- Cooney, T.; Kinast, R.; Juratli, L.; Pedreira, P.N.; Saxe, S.; Musch, D.C.; Mian, S.I. Combined Penetrating Keratoplasty and Vitreoretinal Surgery With Temporary Keratoprosthesis. Cornea Open 2023, 2, e0012. [Google Scholar] [CrossRef]
- Dave, A.; Acharaya, M.; Agarwal, M.; Dave, P.A.; Singh, M.; Mathur, U. Outcomes of combined keratoplasty and pars plana vitrectomy for endophthalmitis with compromised corneal clarity. Clin. Exp. Ophthalmol. 2019, 47, 49–56. [Google Scholar] [CrossRef]
- Velez-Montoya, R.; Rivera-Cortes, M.A.; Ledesma-Gil, G.; Carranza-Casas, M.; Martinez, J.D.; Levine, H.; Yanuzzi, N.A.; Amescua, G.; Ahmed, I.; Beatson, B.; et al. Combined Therapeutic Penetrating Keratoplasty and Pars Plana Vitrectomy for the Treatment of Infectious Keratitis Endophthalmitis: Mexican Endophthalmitis Study Group Protocol 4. Cornea 2023, 42, 805–814. [Google Scholar] [CrossRef]
- Saad, S.; Abdelmassih, Y.; Saad, R.; Guindolet, D.; el Khoury, S.; Doan, S.; Cochereau, I.; Gabison, E.E. Neurotrophic keratitis: Frequency, etiologies, clinical management and outcomes. Ocul. Surf. 2020, 18, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Open globe injury. Am. J. Emerg. Med. 2023, 64, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.H.; Xu, M.N.; Lin, K.; Ren, M.-X.; Wen, H.; Feng, K.-M.; Zhou, H.-J.; Moonasar, N.; Lin, Z. Inner limiting membrane peeling prevents secondary epiretinal membrane after vitrectomy for proliferative diabetic retinopathy. Int. J. Ophthalmol. 2022, 15, 1496. [Google Scholar] [CrossRef]
- Dong, X.; Wang, W.; Xie, L.; Chiu, A.M.C. Long-term outcome of combined penetrating keratoplasty and vitreoretinal surgery using temporary keratoprosthesis. Eye 2006, 20, 59–63. [Google Scholar] [CrossRef]
- Yu, J.; Shalaby, W.S.; Shiuey, E.J.; Rapuano, C.J.; Yonekawa, Y.; Hammersmith, K.M.; Nagra, P.K.; Syed, Z.A. Graft Outcomes After Temporary Keratoprosthesis in Combined Penetrating Keratoplasty and Vitreoretinal Surgery. Cornea 2023, 42, 584–589. [Google Scholar] [CrossRef] [PubMed]
| Total (129 Eyes) | IKE-Group a (65 Eyes) | Non-Infection Group (64 Eyes) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Total (64 eyes) | Retinal membrane (16 eyes) | Retinal detachment (16 eyes) | IOL luxation (7 eyes) | Trauma (25 eyes) | ||||
| Age b (yrs) Mean ± SD a | 61 ± 22 | 68 ± 22 | 54 ± 22 | 54 ± 21 | 56 ± 27 | 63 ± 14 | 50 ± 17 | <0.001 c |
| Gender (Male–Female) | 83:46 | 34:31 | 49:15 | 11:5 | 6:1 | 11:5 | 21:4 | 0.04 d |
| Lens status Pseudophakic–Phakic–Aphakic | 16:78:35 | 9:46:10 | 7:32:25 | 0:14:2 | 4:9:3 | 0:0:7 | 3:9:13 | |
| Ocular surgical history | ||||||||
| Pars plana vitrectomy | 40 (31%) | 12 (18%) | 28 (44%) | 8 (50%) | 4 (24%) | 1 (14%) | 15 (66%) | 0.02 d |
| Penetrating keratoplasty | 75 (58%) | 37 (57%) | 38 (59%) | 10 (63%) | 12 (71%) | 5 (71%) | 11 (44%) | 0.84 d |
| Glaucoma surgical treatment | 22 (17%) | 8 (12%) | 14 (22%) | 7 (44%) | 5 (29%) | 1 (14%) | 1 (4%) | 0.33 d |
| Amnion membrane transplantation | 45 (35%) | 32 (49%) | 13 (20%) | 4 (25%) | 7 (41%) | 0 | 2 (8%) | <0.001 d |
| Total (107 Eyes) | IKE-Group a (50 Eyes) | Non-Infection Group (57 Eyes) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Total (57 eyes) | Epiretinal membrane (15 eyes) | Retinal detachment (13 eyes) | IOL luxation (6 eyes) | Trauma (23 eyes) | ||||
| BCVA a at baseline visit | 2.3 ± 0.5 | 2.3 ± 0.3 | 2.2 ± 0.5 | 2.0 ± 0.5 | 2.4 ± 0.3 | 1.8 ± 0.8 | 2.3 ± 0.5 | 0.16 c |
| BCVA at last visit | 2.0 ± 0.7 | 2.0 ± 0.7 | 2.1 ± 0.6 | 1.7 ± 0.6 | 2.3 ± 0.5 | 1.8 ± 0.5 | 2.2 ± 0.6 | 0.3 c |
| p-value b | <0.001 | <0.001 | 0.02 | 0.01 | 0.6 | 1 | 0.16 | |
| Significant BCVA improvement a | 12 (13%) | 9 (19%) | 3 (6%) | 2 (15%) | 0 | 1 (20%) | 0 | 0.04 d |
| Postoperative BCVA < 1.3 | 15 (14%) | 9 (19%) | 6 (11%) | 4 (27%) | 0 | 1 (16%) | 1 (4%) | 0.26 d |
| Change in BCVA after PKPVR a: Better–same–worse | 53:34:20 | 30:11:9 | 23:23:11 | 7:7:1 | 5.3:5 | 3:2:1 | 8:11:4 | |
| Anatomical results | ||||||||
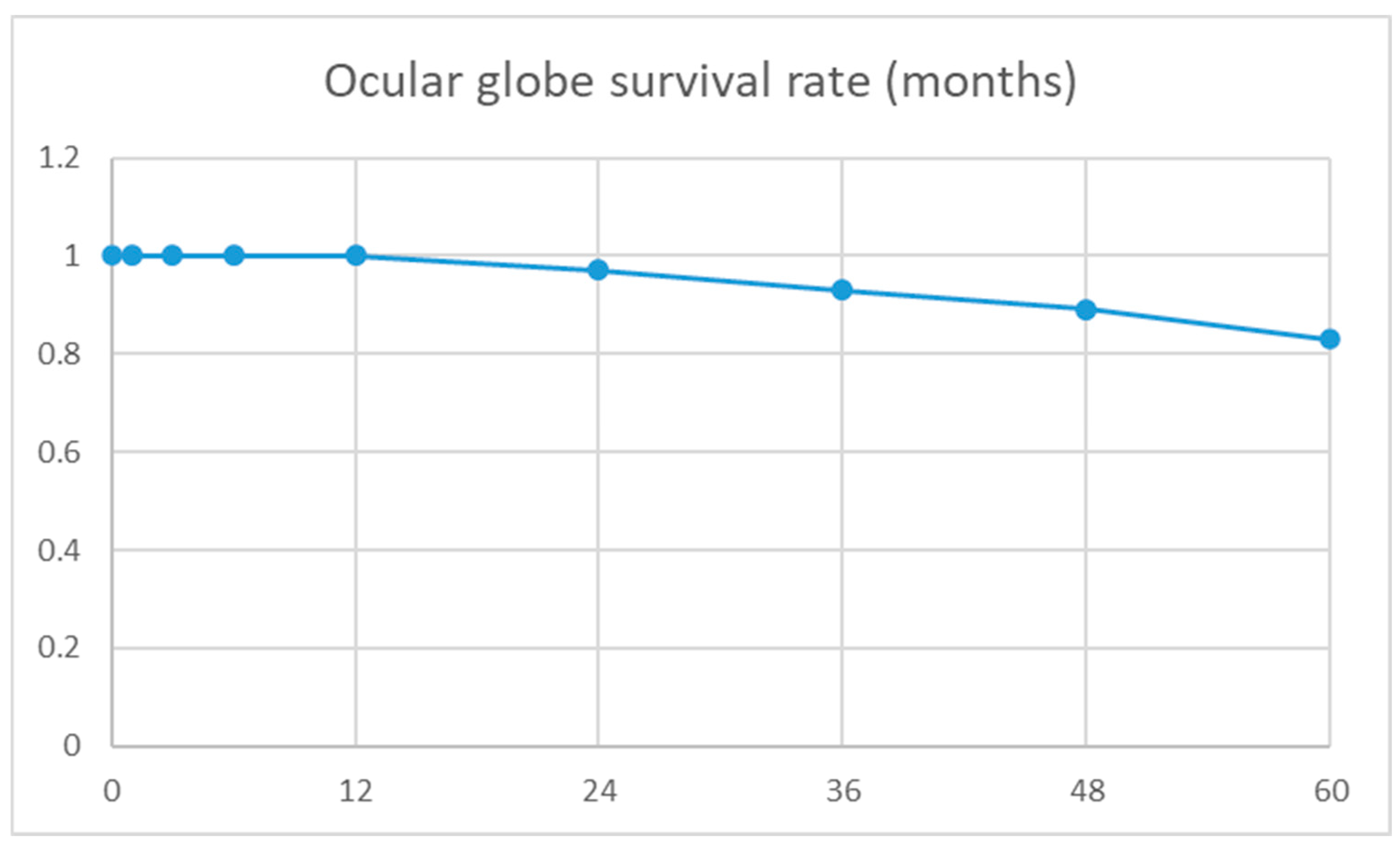
| Enucleation | 9 | 7 | 2 | 0 | 1 | 0 | 1 | 0.05 d |
| Phthisis bulbi | 5 | 2 | 3 | 0 | 1 | 0 | 2 | 0.67 d |
| Clear corneal graft | 61/93 (66%) | 30/41 (73%) | 31/52 (60%) | 11/15 (73%) | 6/11 (55%) | 4/6 (66%) | 10/20 (50%) | 0.17 d |
| Retinal attachment | 92/93 (99%) | 40/41 (98%) | 52/52 (100%) | 15/15 (100%) | 11/11 (100%) | 6/6 (100%) | 20/20 (100%) | |
| Eyes filled with oil at last visit | 13/93 (14%) | 4/41 (9%) | 9/52 (17%) | 0 | 0 | 5/15 (33%) | 4/20 (20%) | |
| Normal IOP at last visit | 77/93 (83%) | 35/41 (85%) | 42/52 (81%) | 12/15 (80%) | 9/11 (82%) | 6/6 (100%) | 15/20 (75%) | |
| Anatomical success | 53/93 (57%) | 27/41 (66%) | 26/52 (50%) | 9/15 (60%) | 5/11 (45%) | 4/6 (66%) | 8/20 (40%) | 0.12 d |
| Postoperative surgical procedures | ||||||||
| Pars plana vitrectomy | 32/107 (30%) | 18 (36%) | 28 (49%) | 3 (20%) | 4 (31%) | 1 (17%) | 6 (26%) | 0.17 d |
| Penetrating keratoplasty | 18 (17%) | 15 (30%) | 3 (5%) | 1 (7%) | 0 | 0 | 2 (9%) | <0.001 d |
| Glaucoma surgical treatment | 18 (17%) | 3 (6%) | 15 (26%) | 7 (47%) | 1 (8%) | 2 (33%) | 5 (22%) | 0.005 d |
| Amnion membrane transplantation | 30 (28%) | 19 (38%) | 11 (19%) | 3 (20%) | 3 (23%) | 0 | 5 (22%) | 0.03 d |
| PKPVR | 16 (15%) | 11 (22%) | 6 (11%) | 3 (20%) | 0 | 0 | 3 (13%) | 0.1 d |
| Significant BCVA Improvement (9 Eyes) | Non-Significant BCVA Improvement (41 Eyes) | p-Value | |
|---|---|---|---|
| Preoperative surgical procedures | |||
| Pars plana vitrectomy | 0 | 11 (27%) | 0.07 b |
| Penetrating keratoplasty | 5 (56%) | 28 (68%) | 0.49 b |
| Glaucoma surgical treatment | 0 | 8 (20%) | 0.14 b |
| Amnion membrane transplantation | 4 (44%) | 26 (63%) | 0.09 |
| Intraoperative retinal pathologies (retinal infiltration, bleeding, and/or ischemia) | 0 | 15 (37%) | 0.03 b |
| Postoperative surgical procedures | |||
| Pars plana vitrectomy | 3 (30%) | 15 (38%) | 0.16 b |
| Penetrating keratoplasty | 1 (11%) | 14 (34%) | 0.17 b |
| Glaucoma surgical treatment | 1 (11%) | 2 (5%) | 0.4 b |
| Amnion membrane transplantation | 4 (44%) | 15 (37%) | 0.64 b |
| PKPVR a | 3 (33%) | 8 (19%) | 0.37 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suffo, S.; Daas, L.; Abdin, A.D.; Qozat, I.; Munteanu, C.; Seitz, B.; Abu Dail, Y. Results of Combined Penetrating Keratoplasty and Pars Plana Vitrectomy Performed for Infectious Keratitis with Endophthalmitis Compared to Other Non-Infectious Indications: Series of 129 Eyes. J. Clin. Med. 2025, 14, 6748. https://doi.org/10.3390/jcm14196748
Suffo S, Daas L, Abdin AD, Qozat I, Munteanu C, Seitz B, Abu Dail Y. Results of Combined Penetrating Keratoplasty and Pars Plana Vitrectomy Performed for Infectious Keratitis with Endophthalmitis Compared to Other Non-Infectious Indications: Series of 129 Eyes. Journal of Clinical Medicine. 2025; 14(19):6748. https://doi.org/10.3390/jcm14196748
Chicago/Turabian StyleSuffo, Shady, Loay Daas, Alaa Din Abdin, Ibrahim Qozat, Cristian Munteanu, Berthold Seitz, and Yaser Abu Dail. 2025. "Results of Combined Penetrating Keratoplasty and Pars Plana Vitrectomy Performed for Infectious Keratitis with Endophthalmitis Compared to Other Non-Infectious Indications: Series of 129 Eyes" Journal of Clinical Medicine 14, no. 19: 6748. https://doi.org/10.3390/jcm14196748
APA StyleSuffo, S., Daas, L., Abdin, A. D., Qozat, I., Munteanu, C., Seitz, B., & Abu Dail, Y. (2025). Results of Combined Penetrating Keratoplasty and Pars Plana Vitrectomy Performed for Infectious Keratitis with Endophthalmitis Compared to Other Non-Infectious Indications: Series of 129 Eyes. Journal of Clinical Medicine, 14(19), 6748. https://doi.org/10.3390/jcm14196748







