The Association of Lipoprotein(A) and Coronary Artery Calcium in Primary Prevention Patients—Data from the STAR-Lp(A) Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Endpoints
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths and Limitations
Clinical Implications and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Cesare, M.; Bixby, H.; Gaziano, T.; Hadeed, L.; Kabudula, C.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Perel, P.; Piñeiro, D.; et al. World Heart Report 2023: Confronting the World’s Number One Killer; World Heart Federation: Geneva, Switzerland, 2023. [Google Scholar]
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: A historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; McClelland, R.L.; Polonsky, T.S.; Burke, G.L.; Sibley, C.T.; O’Leary, D.; Carr, J.J.; Goff, D.C.; Greenland, P.; Herrington, D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012, 308, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary artery calcification and its progression: What does it really mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Sosnowska, B.; Stepinska, J.; Mitkowski, P.; Bielecka-Dabrowa, A.; Bobrowska, B.; Budzianowski, J.; Burchardt, P.; Chlebus, K.; Dobrowolski, P.; Gasior, M.; et al. Recommendations of the Experts of the Polish Cardiac Society (PCS) and the Polish Lipid Association (PoLA) on the diagnosis and management of elevated lipoprotein (a) levels. Arch. Med. Sci. 2024, 20, 8–27. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Maggioni, A.P.; Scicchitano, P.; Zuin, M.; D’elia, E.; Colivicchi, F. Lipoprotein (a), Inflammation, and Atherosclerosis. J. Clin. Med. 2023, 12, 2529. [Google Scholar] [CrossRef]
- Banach, M. Lipoprotein (a): The enemy that we still don’t know how to defeat. Eur. Heart J. Open 2023, 3, oead080. [Google Scholar] [CrossRef]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.L.; Lambert, G.; Mach, F.; et al. Frequent questions and responses on the 2022 lipoprotein (a) consensus statement of the European Atherosclerosis Society. Atherosclerosis 2023, 374, 107–120. [Google Scholar] [CrossRef]
- Vinci, P.; Di Girolamo, F.G.; Panizoni, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Altamura, N.; Pirulli, A.; Zaccari, M.; Biasinutto, C.; et al. Lipoprotein (a) as a Risk Factor for Cardiovascular Diseases: Pathophysiology and Treatment Perspectives. Int. J. Environ. Res. Public Health 2023, 20, 6721. [Google Scholar] [CrossRef]
- Martignoni, F.V.; Júnior, J.E.R.; Marques, I.R.; Gomes, C.; Moreira, V.C.S.; Souza, I.A.F.d.; Miyawaki, I.A.; Silva, C.H.; Neto, A.B.D.A.; Padrão, E.M.H.; et al. The association of lipoprotein (a) and coronary artery calcium in asymptomatic patients: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2024, 31, 732–741. [Google Scholar] [CrossRef]
- Vazirian, F.; Sadeghi, M.; Kelesidis, T.; Budoff, M.J.; Zandi, Z.; Samadi, S.; Mohammadpour, A.H. Predictive value of lipoprotein (a) in coronary artery calcification among asymptomatic cardiovascular disease subjects: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Guerra, R.; Yu, Z.; Marcovina, S.; Peshock, R.; Cohen, J.C.; Hobbs, H.H. Lipoprotein (a) and apolipoprotein (a) isoforms: No association with coronary artery calcification in the Dallas Heart Study. Circulation 2005, 111, 1471–1479. [Google Scholar] [CrossRef]
- Kullo, I.J.; Bailey, K.R.; Bielak, L.F.; Sheedy, P.F.; Klee, G.G.; Kardia, S.L.; Peyser, P.A.; Boerwinkle, E.; Turner, S.T. Lack of association between lipoprotein (a) and coronary artery calcification in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Mayo Clin. Proc. 2004, 79, 1258–1263. [Google Scholar] [CrossRef]
- Mehta, A.; Vasquez, N.; Ayers, C.R.; Patel, J.; Hooda, A.; Khera, A.; Blumenthal, R.S.; Shapiro, M.D.; Rodriguez, C.J.; Tsai, M.Y.; et al. Independent Association of Lipoprotein(a) and Coronary Artery Calcification With Atherosclerotic Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 79, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Burzyńska, M.; Jankowski, P.; Babicki, M.; Banach, M.; Chudzik, M. Prevalence of hyperlipoproteinemia (a) in individuals of European ancestry treated at outpatient cardiology clinics: Results from a cross-sectional STAR-Lp (a) study. Pol. Arch. Intern. Med. 2024, 134, 16860. [Google Scholar] [CrossRef]
- Greif, M.; Arnoldt, T.; von Ziegler, F.; Ruemmler, J.; Becker, C.; Wakili, R.; D’Anastasi, M.; Schenzle, J.; Leber, A.W.; Becker, A. Lipoprotein (a) is independently correlated with coronary artery calcification. Eur. J. Intern. Med. 2013, 24, 75–79. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Matera, M.G.; Rinaldi, B.; Annibale, R.; De Novellis, V.; Cazzola, M. The pharmacological management of asthma in adults: 2023 update. Expert Opin. Pharmacother. 2024, 25, 383–393. [Google Scholar] [CrossRef]
- Terry, P.D.; Dhand, R. The 2023 GOLD Report: Updated Guidelines for Inhaled Pharmacological Therapy in Patients with Stable COPD. Pulm. Ther. 2023, 9, 345–357. [Google Scholar] [CrossRef]
- Myers, L.; Sirois, M.J. Spearman Correlation Coefficients, Differences Between. In Encyclopedia of Statistical Sciences, 2nd ed.; Kotz, S., Balakrishnan, N., Read, C.B., Vidakovic, B., Eds.; John Willey and Sons: Hoboken, NJ, USA, 2006; pp. 7901–7903. [Google Scholar] [CrossRef]
- Obisesan, O.H.; Kou, M.; Wang, F.M.; Boakye, E.; Honda, Y.; Uddin, S.M.I.; Dzaye, O.; Osei, A.D.; Orimoloye, O.A.; Howard-Claudio, C.M.; et al. Lipoprotein (a) and Subclinical Vascular and Valvular Calcification on Cardiac Computed Tomography: The Atherosclerosis Risk in Communities Study. J. Am. Heart Assoc. 2022, 7, e024870. [Google Scholar] [CrossRef]
- Chung, Y.H.; Lee, B.K.; Kwon, H.M.; Min, P.-K.; Choi, E.-Y.; Yoon, Y.W.; Hong, B.-K.; Rim, S.-J.; Kim, J.-Y. Coronary calcification is associated with elevated serum lipoprotein (a) levels in asymptomatic men over the age of 45 years: A cross-sectional study of the Korean national health checkup data. Medicine 2021, 100, e24962. [Google Scholar] [CrossRef] [PubMed]
- Erbel, R.; Lehmann, N.; Churzidse, S.; Möhlenkamp, S.; Moebus, S.; Mahabadi, A.A.; Schmermund, A.; Stang, A.; Dragano, N.; Grönemeyer, D.; et al. Gender-specific association of coronary artery calcium and lipoprotein parameters: The Heinz Nixdorf Recall Study. Atherosclerosis 2013, 229, 531–540. [Google Scholar] [CrossRef]
- Nishino, M.J.; Malloy, J.; Naya-Vigne, J.; Russell, J.; Kane, J.P.; Redberg, R.F. Lack of association of lipoprotein(a) levels with coronary calcium deposits in asymptomatic postmenopausal women. J. Am. Coll. Cardiol. 2000, 35, 314–320. [Google Scholar] [CrossRef][Green Version]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and women’s cardiovascular health: Is it really an obvious relationship? Arch. Med. Sci. 2022, 19, 458–466. [Google Scholar] [CrossRef]
- Qasim, A.N.; Martin, S.S.; Mehta, N.N.; Wolfe, M.L.; Park, J.; Schwartz, S.; Schutta, M.; Iqbal, N.; Reilly, M.P. Lipoprotein (a) is strongly associated with coronary artery calcification in type-2 diabetic women. Int. J. Cardiol. 2011, 150, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, B.; Lewek, J.; Adach, W.; Mierczak, K.; Bielecka-Dąbrowa, A.; Szosland, K.; Zygmunt, A.; Dąbrowski, J.; Banach, M. The prevalence, patients’ characteristics, and hyper-Lp(a)-emia risk factors in the Polish population. The first results from the PMMHRI-Lp(a) Registry. Prog. Cardiovasc. Dis. 2024, 86, 54–61. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.P.; Jacobson, T.A.; Jones, P.H.; Koschinsky, M.L.; McNeal, C.J.; Nordestgaard, B.G.; Orringer, C.E. Use of lipoprotein (a) in clinical practice: A biomarker whose time has come. A scientific statement from the National Lipid Association. J. Clin. Lipidol. 2019, 13, 374–392. [Google Scholar] [CrossRef]
- Patel, A.P.; Wang, M.; Pirruccello, J.P.; Ellinor, P.T.; Ng, K.; Kathiresan, S.; Khera, A.V. Lp (a) (Lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: New insights from a large national biobank. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 465–474. [Google Scholar] [CrossRef]
- Tsimikas, S.; Karwatowska-Prokopczuk, E.; Gouni-Berthold, I.; Tardif, J.-C.; Baum, S.J.; Steinhagen-Thiessen, E.; Shapiro, M.D.; Stroes, E.S.; Moriarty, P.M.; Nordestgaard, B.G.; et al. Lipoprotein (a) reduction in persons with cardiovascular disease. N. Engl. J. Med. 2020, 382, 244–255. [Google Scholar] [CrossRef]
- Dimitriadis, K.; Theofilis, P.; Iliakis, P.; Pyrpyris, N.; Dri, E.; Sakalidis, A.; Soulaidopoulos, S.; Tsioufis, P.; Fragkoulis, C.; Chrysohoou, C.; et al. Management of dyslipidemia in coronary artery disease: The present and the future. Coron. Artery Dis. 2024, 35, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Naami, R.; Miller, D.M.; Al-Kindi, S.; Neeland, I.J. Coronary artery calcium scoring as a tool for risk stratification in patients with an elevated lipoprotein (a) level. Front. Cardiovasc. Med. 2022, 9, 1084814. [Google Scholar] [CrossRef]
- Sheppard, J.P.; Lakshmanan, S.; Lichtenstein, S.J.; Budoff, M.J.; Roy, S.K. Age and the power of zero CAC in cardiac risk assessment: Overview of the literature and a cautionary case. Br. J. Cardiol. 2022, 29, 23. [Google Scholar] [CrossRef]
- Tabbalat, R.A.; Khader, Y.S.; Hammoudeh, A.J.; Alhaddad, I.A. Age and Gender-Based Coronary Artery Calcium Scores in a Middle Eastern Population. Cardiovasc. Imaging Asia 2021, 5, 37–43. [Google Scholar] [CrossRef]
- Grossman, C.; Shemesh, J.; Dovrish, Z.; Morag, N.K.; Segev, S.; Grossman, E. Coronary Artery Calcification Is Associated with the Development of Hypertension. Am. J. Hypertens. 2013, 26, 13–19. [Google Scholar] [CrossRef]
- Schulman-Marcus, J.; Valenti, V.; Hartaigh, B.Ó.; Gransar, H.; Truong, Q.; Giambrone, A.; Callister, T.Q.; Shaw, L.J.; Lin, F.Y.; Min, J.K. Prognostic utility of coronary artery calcium scoring in active smokers: A 15-year follow-up study. Int. J. Cardiol. 2014, 177, 581–583. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, J.T.; Chang, T.I.; Joo, Y.S.; Yoo, T.-H.; Park, S.K.; Chung, W.; Kim, Y.-S.; Kim, S.W.; Oh, K.-H.; et al. Smoking Cessation and Coronary Artery Calcification in CKD. Clin. J. Am. Soc. Nephrol. 2021, 16, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Arad, Y.; Spadaro, L.A.; Roth, M.; Newstein, D.; Guerci, A.D. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: The St. Francis Heart Study randomized clinical trial. J. Am. Coll. Cardiol. 2005, 46, 166–172, Erratum in J. Am. Coll. Cardiol. 2011, 58, 1832. [Google Scholar] [CrossRef]

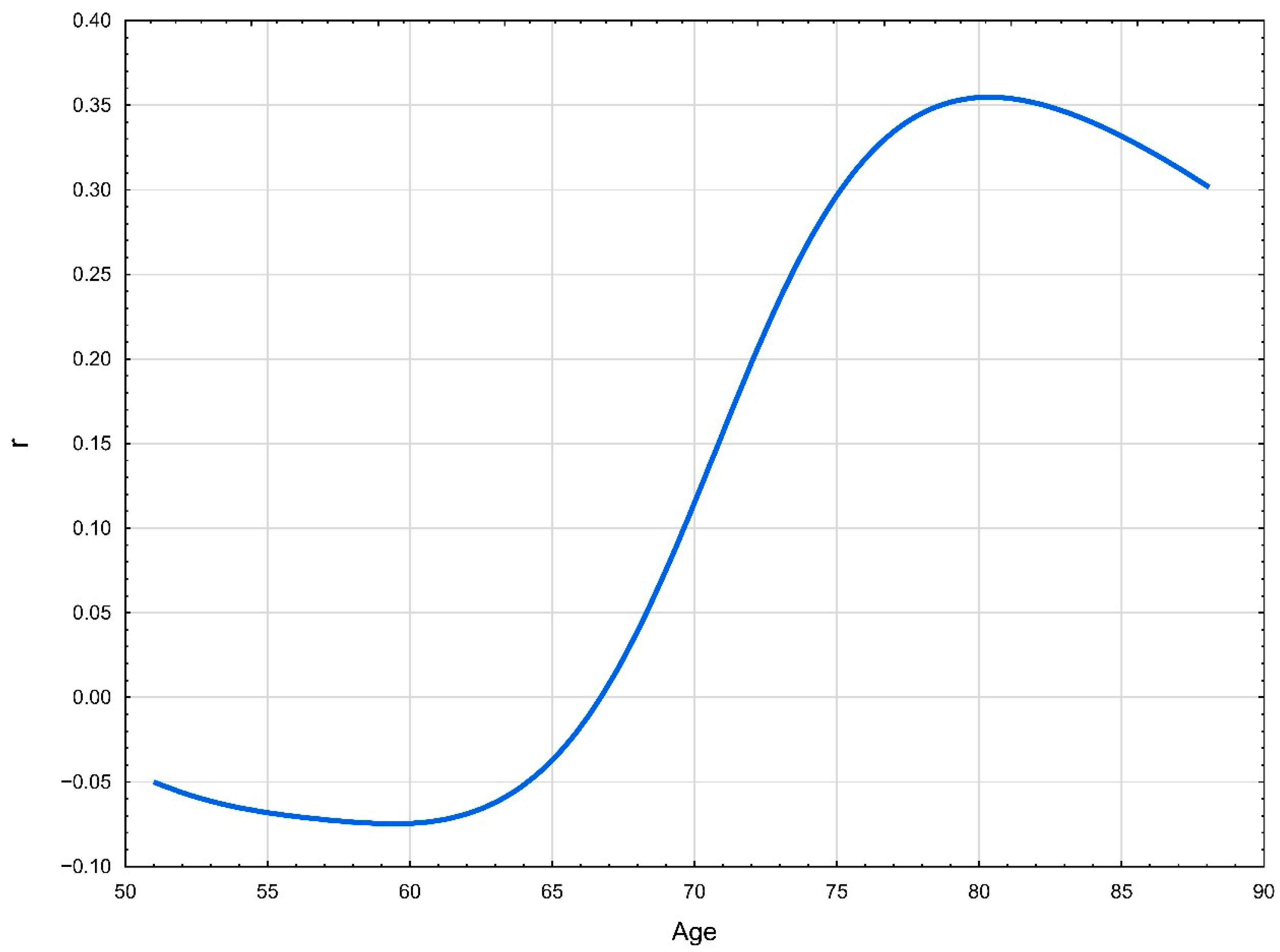
| Variable | All Me (IQR)/N (%) | Lipoprotein(a) Me (IQR)/N(%) | CAC Me (IQR)/N(%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <30 mg/dL N = 390 | 30–50 mg/dL N = 40 | ≥50 mg/dL N = 98 | p | 0 N = 189 | 1–100 N = 159 | >100 N = 180 | p | |||
| Age, years | 68.0 (12.0) | 68.0 (11.0) | 69.0 (13.0) | 68.0 (13.0) | 0.58 | 63.0 (16.0) | 68.0 (10.0) | 71.0 (8.0) | 0.04 | |
| Sex | Male | 179 (33.9) | 138 (35.4) | 15 (37.5) | 26 (26.5) | 0.25 | 44 (23.3) | 57 (35.9) | 78 (43.3) | <0.001 |
| Female | 349 (66.1) | 252 (64.6) | 25 (62.5) | 72 (73.5) | 146 (76.7) | 101 (64.1) | 102 (56.7) | |||
| Hypertension | 350 (66.3) | 260 (66.8) | 21 (52.5) | 69 (70.4) | 0.12 | 106 (56.1) | 105 (66.0) | 139 (77.2) | <0.001 | |
| Diabetes | 119 (22.5) | 95 (24.4) | 7 (17.5) | 17 (17.4) | 0.24 | 36 (19.1) | 35 (22.0) | 48 (26.7) | 0.21 | |
| Hyperlipidemia | 133 (25.2) | 101 (25.9) | 11 (27.5) | 21 (21.4) | 0.62 | 47 (24.9) | 45 (28.3) | 41 (22.8) | 0.50 | |
| Hypothyroidism | 99 (18.6) | 73 (18.7) | 5 (12.5) | 21 (21.4) | 0.48 | 39 (20.6) | 34 (21.4) | 26 (14.4) | 0.19 | |
| Asthma | 54 (10.2) | 41(10.5) | 4 (10.0) | 9(9.2) | 0.93 | 15 (7.9) | 16 (10.1) | 23 (12.8) | 0.31 | |
| COPD | 30 (5.7) | 20 (5.1) | 2 (5.0) | 8 (8.2) | 0.50 | 6 (3.2) | 9 (5.7) | 15 (8.3) | 0.10 | |
| Rheumatoid arthritis | 21 (3.9) | 16 (4.1) | 1 (2.5) | 4 (4.1) | 0.88 | 3 (1.6) | 12 (7.6) | 6 (3.3) | 0.02 | |
| Anxiety disorders | 224 (42.4) | 169 (43.3) | 14 (35.0) | 41 (41.8) | 0.59 | 87 (46.0) | 63 (39.6) | 74 (41.1) | 0.44 | |
| Depression | 270 (51.1) | 208 (53.3) | 18 (45.0) | 44 (44.9) | 0.24 | 103 (54.5) | 82 (51.6) | 85 (47.2) | 0.37 | |
| Sleep disorders | 231 (43.8) | 177 (45.4) | 15 (37.5) | 39 (39.8) | 0.43 | 89 (47.1) | 72 (45.3) | 70 (38.9) | 0.25 | |
| Sleep apnea | 125 (23.7) | 98 (25.1) | 3 (7.5) | 24 (24.5) | 0.04 | 49 (25.9) | 37 (23.3) | 39 (21.7) | 0.62 | |
| Migraine | 54 (10.2) | 42 (10.8) | 1 (2.5) | 11 (11.2) | 0.24 | 30 (15.9) | 15 (9.4) | 9 (5.0) | 0.002 | |
| Erection disorders | 28 (5.3) | 23 (5.9) | 2 (5.0) | 3 (3.1) | 0.53 | 5 (2.7) | 11 (6.9) | 12 (6.7) | 0.13 | |
| Regular physical activity | 202 (38.3) | 154 (39.5) | 9 (22.5) | 39 (39.8) | 0.10 | 70 (37.0) | 66 (41.5) | 66 (36.7) | 0.60 | |
| Smoking | 62 (11.7) | 44 (11.3) | 5 (12.5) | 13 (13.3) | 0.85 | 17 (9.0) | 18 (11.3) | 27 (15.0) | 0.20 | |
| Lipid lowering drugs | 176 (33.3) | 160 (33.6) | 22 (30.0) | 33 (33.7) | 0.90 | 43 (22.8) | 51 (32.1) | 82 (45.6) | <0.001 | |
| BMI kg/m2 | 27.8 (6.1) | 27.8 (6.2) | 26.9 (6.6) | 28.8 (6.1) | 0.45 | 27.3 (6.2) | 28.0 ± 6.2 | 28.0 (6.1) | 0.31 | |
| Creatinine mg/dL | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.24 | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | 0.17 | |
| Glucose mg/dL | 103.0 (24.0) | 102.0 (24.0) | 96.0 (29.0) | 109.0 (23.0) | 0.26 | 99.0 (26.0) | 103 (20.5) | 107.0 (23.0) | 0.02 | |
| Homocysteine µmol/L | 11.8 (4.2) | 11.8 (4.3) | 10.6 (4.4) | 12.0 (4.1) | 0.45 | 11.1 (4.3) | 11.7 (4.0) | 12.4 (3.9) | 0.02 | |
| Hemoglobin A1C (%) | 5.7 (0.6) | 5.6 (0.5) | 5.5 (0.6) | 5.5 (0.5) | 0.30 | 5.5 (0.6) | 5.6 (0.6) | 5.6 (0.6) | 0.01 | |
| TSH µU/mL | 2.0 (1.4) | 1.5 (1.4) | 1.4 (1.6) | 1.5 (1.3) | 0.95 | 1.4 (1.3) | 1.6 (1.6) | 1.5 (1.4) | 0.70 | |
| CAC AU | 23.5 (208.0) | 23.0 (183.0) | 0.0 (242.5) | 35.0 (241.0) | 0.27 | 0.0 (0.0) | 26.0 (38.0) | 391.5 (657.5) | --- | |
| LDL-C (mg/dL) | 105.0 (57.0) | 104.0 (57.0) | 122.0 (75.0) | 104.0 (53.0) | 0.07 | 110.0 (51.0) | 103.5 (64.0) | 100.5 (56.0) | 0.09 | |
| TG (mg/dL) | 116.0 (62.0) | 116.0 (63.0) | 115.0 (52.0) | 118.0 (63.0) | 0.81 | 116.0 (63.5) | 121.5 (68.5) | 114.0 (56.0) | 0.49 | |
| HDL-C (mg/dL) | 52.0 (20.0) | 52.0 (19.0) | 49.5 (18.5) | 52.0 (19.0) | 0.39 | 55.5 (19.0) | 50.5 (21.0) | 52.0 (20.0) | 0.16 | |
| Non-HDL (mg/dL) | 131.0 (59.0) | 129.5 (58.5) | 145.5 (72.5) | 132.0 (58.0) | 0.33 | 132.0 (64.0) | 130.5 (65.0) | 130.5 (53.0) | 0.85 | |
| Lp(a) mg/dL | 10.0 (28.0) | 6.0 (7.8) | 40.0 (9.0) | 80.0 (40.0) | --- | 10.0 (29.5) | 8.0 (21.1) | 10.0 (27.1) | 0.20 | |
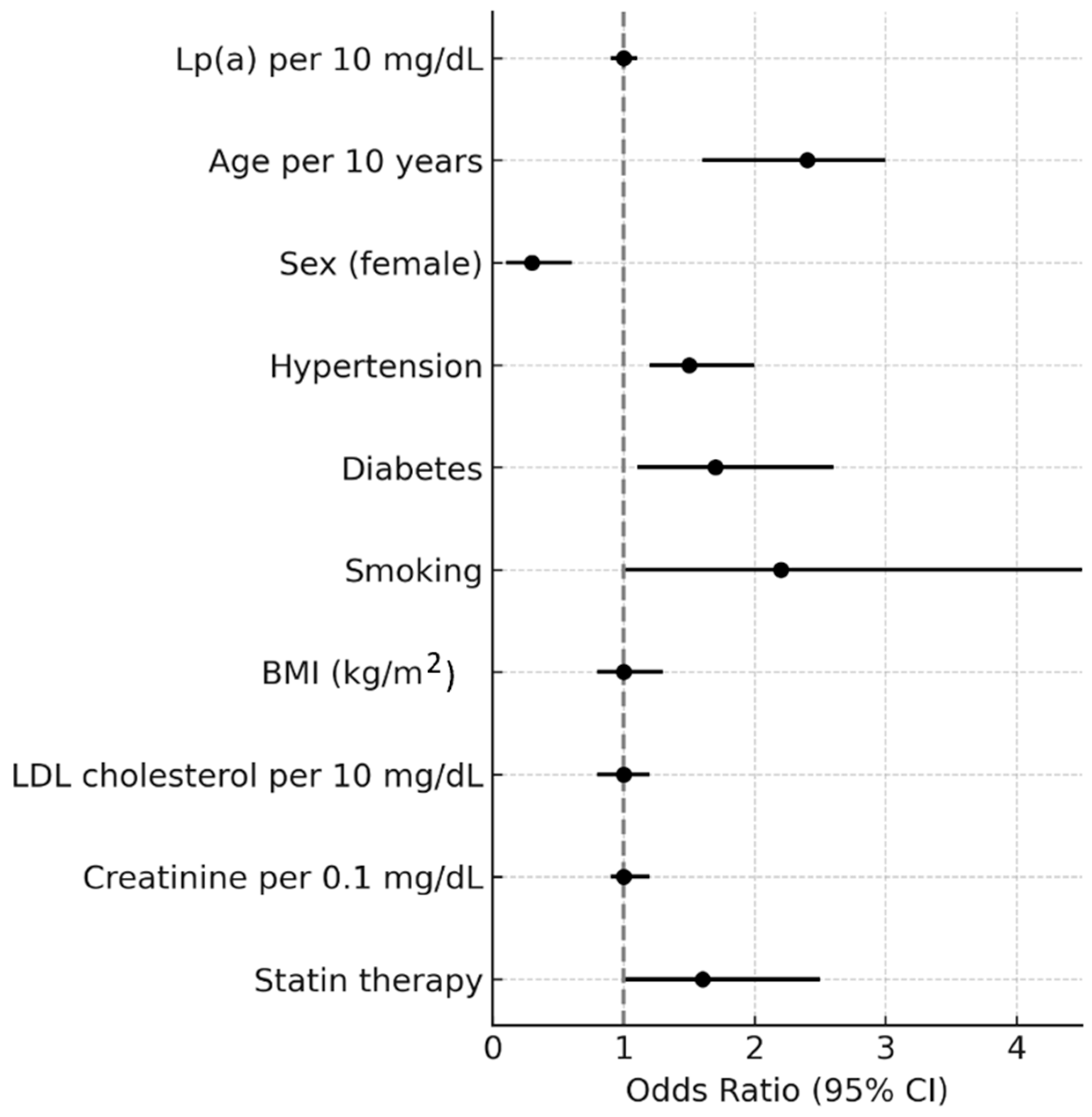
| Variable | OR | 95% CI | p-Value |
|---|---|---|---|
| Lp(a) per 10 mg/dL | 1.02 | 0.97–1.07 | 0.53 |
| Age per 10 years | 2.61 | 2.02–3.38 | <0.001 |
| Sex (female) | 0.21 | 0.12–0.35 | <0.001 |
| Hypertension | 1.40 | 0.88–2.22 | 0.15 |
| Diabetes | 1.43 | 0.84–2.44 | 0.18 |
| Smoking | 2.25 | 1.12–4.53 | 0.02 |
| BMI (kg/m2) | 0.97 | 0.93–1.02 | 0.24 |
| LDL cholesterol per 10 mg/dL | 1.01 | 0.96–1.07 | 0.69 |
| Creatinine per 0.1 mg/dL | 0.97 | 0.88–1.07 | 0.49 |
| Statin therapy | 1.70 | 1.08–2.69 | 0.02 |
| Variable | OR [95Cl] | p |
|---|---|---|
| Sex, female | 4.12 [2.54–6.67] | <0.001 |
| Age, per 10 years | 0.22 [0.14–0.33] | <0.001 |
| Hypertension | 0.59 [0.39–0.91] | 0.02 |
| Lipid lowering drugs | 0.51 [0.33–0.82] | 0.01 |
| Smoking | 0.48 [0.24–0.93] | 0.03 |
| Variable | CAC = 0 | p | CAC = 0–50 | p | CAC > 100 | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lp(a) < 30 N = 137 | Lp(a) = 30–50 N = 21 | Lp(a) > 50 N = 31 | Lp(a) < 30 N = 121 | Lp(a) = 30–50 N = 8 | Lp(a) > 50 N = 30 | Lp(a) < 30 N = 132 | Lp(a) = 30–50 N = 11 | Lp(a) > 50 N = 37 | |||||
| Me (IQR)/n (%) | Me (IQR)/n (%) | Me (IQR)/n (%) | |||||||||||
| Age | 63.0 (16.0) | 67.0 (17.0) | 62.0 (15.0) | 0.88 | 68.0 (9.0) | 71.0 (10.0) | 68.0 (20.0) | 0.61 | 71.0 (8.0) | 73.0 (5.0) | 72.0 (8.0) | 0.64 | |
| Sex | Male | 29 (21.2) | 9 (42.9) | 6 (19.4) | 0.08 | 45 (37.2) | 3 (37.5) | 9 (30.0) | 0.76 | 64 (48.5) | 3 (27.3) | 11 (29.7) | 0.07 |
| Female | 108 (78.8) | 12 (57.1) | 25 (80.6) | 76 (62.8) | 5 (62.5) | 21 (70.0) | 68 (51.5) | 8 (72.7) | 26 (70.3) | ||||
| Hypertension | 76 (55.5) | 9 (42.9) | 21 (67.7) | 0.91 | 82 (67.8) | 3 (37.5) | 20 (60.7) | 0.22 | 102 (77.3) | 9 (81.8) | 28 (75.7) | 0.91 | |
| Diabetes | 26 (18.9) | 5 (23.8) | 5 (16.1) | 0.79 | 31 (25.6) | 0 (0.0) | 4 (13.3) | 0.11 | 38 (28.8) | 2 (18.2) | 8 (21.6) | 0.55 | |
| Hyperlipidemia | 35 (25.6) | 6 (28.6) | 6 (19.4) | 0.71 | 35 (28.9) | 1 (12.5) | 9 (30.0) | 0.59 | 31 (23.5) | 4 (36.4) | 6 (16.2) | 0.35 | |
| Hypothyroidism | 27 (19.7) | 3 (14.3) | 9 (29.0) | 0.38 | 27 (22.3) | 2 (25.0) | 5 (16.7) | 0.77 | 19 (14.4) | 0 (0.0) | 7 (18.9) | 0.29 | |
| Asthma | 13 (9.5) | 1 (4.8) | 1 (3.2) | 0.43 | 13 (10.7) | 0 (0.0) | 3 (10.0) | 0.62 | 15 (11.4) | 3 (27.3) | 5 (13.5) | 0.31 | |
| COPD | 4 (2.9) | 0 (0.0) | 2 (6.5) | 0.41 | 6 (4.9) | 0 (0.0) | 3 (10.0) | 0.44 | 10 (7.6) | 2 (18.2) | 3 (8.1) | 0.47 | |
| RA | 2 (1.5) | 0 (0.0) | 1 (3.2) | 0.64 | 10 (8.3) | 1 (12.5) | 1 (3.3) | 0.57 | 4 (3.0) | 0 (0.0) | 2 (5.4) | 0.63 | |
| Anxiety disorders | 70 (51.1) | 6 (28.6) | 11 (35.5) | 0.07 | 46 (38.0) | 3 (37.5) | 14 (46.7) | 0.68 | 53 (40.2) | 5 (45.5) | 16 (43.2) | 0.90 | |
| Depression | 81 (59.1) | 9 (42.9) | 13 (41.9) | 0.12 | 64 (52.9) | 2 (25.0) | 16 (53.3) | 0.30 | 63 (47.7) | 7 (63.6) | 15 (40.5) | 0.39 | |
| Sleep disorders | 70 (51.1) | 7 (33.3) | 12 (38.7) | 0.19 | 56 (46.3) | 2 (25.0) | 14 (46.7) | 0.49 | 51 (38.6) | 6 (54.6) | 13 (35.1) | 0.51 | |
| Sleep apnea | 40 (29.2) | 1 (4.8) | 8 (25.8) | 0.06 | 33 (27.3) | 0 (0.0) | 4 (13.3) | 0.08 | 25 (18.9) | 2 (18.2) | 12 (32.4) | 0.20 | |
| Migraine | 24 (17.5) | 1 (4.8) | 5 (16.1) | 0.33 | 12 (9.9) | 0 (0.0) | 3 (10.0) | 0.64 | 6 (4.6) | 0 (0.0) | 3 (8.1) | 0.49 | |
| Erection disorders | 4 (2.9) | 0 (0.0) | 1 (3.2) | 0.72 | 9 (7.4) | 0 (0.0) | 2 (6.7) | 0.72 | 10 (7.6) | 2 (18.2) | 0 (0.0) | 0.08 | |
| Regular physical activity | 52 (37.9) | 5 (23.8) | 13 (41.9) | 0.38 | 51 (42.2) | 0 (0.0) | 15 (50.0) | 0.04 | 51 (38.6) | 4 (36.4) | 11 (29.7) | 0.61 | |
| Smoking | 15 (10.9) | 0 (0.0) | 2 (6.5) | 0.23 | 12 (9.9) | 1 (12.5) | 5 (16.7) | 0.58 | 17 (12.9) | 4 (36.4) | 6 (16.1) | 0.11 | |
| Lipid lowering drugs | 33 (24.1) | 3 (14.3) | 7 (22.6) | 0.61 | 38 (31.4) | 2 (25.0) | 11 (36.7) | 0.78 | 60 (45.5) | 7 (63.6) | 15 (40.5) | 0.40 | |
| BMI | 27.6 (6.0) | 25.6 (5.6) | 26.9 (8.3) | 0.43 | 28.5 (6.2) | 29.6 (6.7) | 28.4 (6.4) | 0.77 | 27.7 (6.3) | 27.0 (9.3) | 28.7 (5.8) | 0.38 | |
| Creatinine (mg/dL) | 0.8 (0.2) | 0.8 (0.2) | 0.7 (0.2) | 0.06 | 0.8 (0.2) | 0.9 (0.4) | 0.8 (0.2) | 0.60 | 0.8 (0.3) | 0.8 (0.3) | 0.8 (0.2) | 0.64 | |
| Glucose (mg/dL) | 99.0 (23.0) | 91.0 (21.0) | 110.0 (25.0) | 0.25 | 102.0 (21.0) | 107.5 (23.0) | 101.0 (21.0) | 0.65 | 107.0 (23.0) | 116.5 (21.0) | 109.0 (28.0) | 0.30 | |
| Homocysteine µmol/L | 10.4 (4.7) | 11.2 (2.6) | 10.9 (3.7) | 0.43 | 11.6 (3.8) | 10.9 (4.2) | 12.0 (4.2) | 0.37 | 12.2 (3.9) | 12.7 (7.1) | 13.0 (3.7) | 0.64 | |
| Hemoglobin A1C, % | 5.5 (0.7) | 5.4 (0.6) | 5.4 (0.5) | 0.51 | 5.6 (0.6) | 5.6 (0.5) | 5.6 (0.4) | 0.90 | 5.6 (0.6) | 5.5 (0.2) | 5.6 (0.9) | 0.16 | |
| TSH (µU/mL) | 1.4 (1.2) | 1.3 (0.9) | 1.7 (1.2) | 0.37 | 1.5 (1.7) | 2.0 (1.5) | 1.6 (1.6) | 0.32 | 1.6 (1.3) | 1.6 (1.9) | 1.4 (1.4) | 0.56 | |
| LDL-C (mg/dL) | 109.0 (50.0) | 112.0 (59.0) | 119.0 (54.0) | 0.11 | 103.0 (61.5) | 115.0 (64.5) | 102.5 (59.0) | 0.65 | 101.0 (55.0) | 79.0 (87.0) | 97.0 (49.0) | 0.55 | |
| TG (mg/dL) | 115.0 (73.0) | 119.0 (77.0) | 116.0 (55.0) | 0.30 | 124.0 (70.0) | 114.5 (40.0) | 111.5 (67.0) | 0.34 | 1212.5 (52.0) | 108.0 (28.0) | 128.0 (63.0) | 0.64 | |
| Non-HDL (mg/dL) | 127.5 (60.5) | 151.0 (57.0) | 132.0 (66.0) | 0.42 | 130.0 (65.0) | 139.5 (66.0) | 128.0 (70.0) | 0.44 | 129.5 (53.0) | 162.0 (10.0) | 132.0 (49.0) | 0.31 | |
| HDL (mg/dL) | 56.0 (19.5) | 50.0 (19.0) | 56.0 (23.0) | 0.39 | 50.0 (21.0) | 47.0 (12.0) | 53.5 (19.0) | 0.32 | 53.0 (22.0) | 52.0 (21.0) | 48.0 (17.0) | 0.14 | |
| Lp(a) (mg/dL) | 6.0 (8.8) | 40.0 (5.0) | 78.0 (39.2) | <0.001 | 5.4 (7.0) | 43.9 (9.5) | 80.0 (39.1) | <0.001 | 7.0 (7.9) | 38.0 (8.0) | 84.0 (37.0) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burzyńska, M.; Jankowski, P.; Babicki, M.; Banach, M.; Chudzik, M. The Association of Lipoprotein(A) and Coronary Artery Calcium in Primary Prevention Patients—Data from the STAR-Lp(A) Study. J. Clin. Med. 2025, 14, 6713. https://doi.org/10.3390/jcm14196713
Burzyńska M, Jankowski P, Babicki M, Banach M, Chudzik M. The Association of Lipoprotein(A) and Coronary Artery Calcium in Primary Prevention Patients—Data from the STAR-Lp(A) Study. Journal of Clinical Medicine. 2025; 14(19):6713. https://doi.org/10.3390/jcm14196713
Chicago/Turabian StyleBurzyńska, Monika, Piotr Jankowski, Mateusz Babicki, Maciej Banach, and Michał Chudzik. 2025. "The Association of Lipoprotein(A) and Coronary Artery Calcium in Primary Prevention Patients—Data from the STAR-Lp(A) Study" Journal of Clinical Medicine 14, no. 19: 6713. https://doi.org/10.3390/jcm14196713
APA StyleBurzyńska, M., Jankowski, P., Babicki, M., Banach, M., & Chudzik, M. (2025). The Association of Lipoprotein(A) and Coronary Artery Calcium in Primary Prevention Patients—Data from the STAR-Lp(A) Study. Journal of Clinical Medicine, 14(19), 6713. https://doi.org/10.3390/jcm14196713










