Impact of COVID-19 on Pregnancy Outcomes: A Phase-Based Analysis from a Spanish Tertiary Hospital (2020–2023)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Type
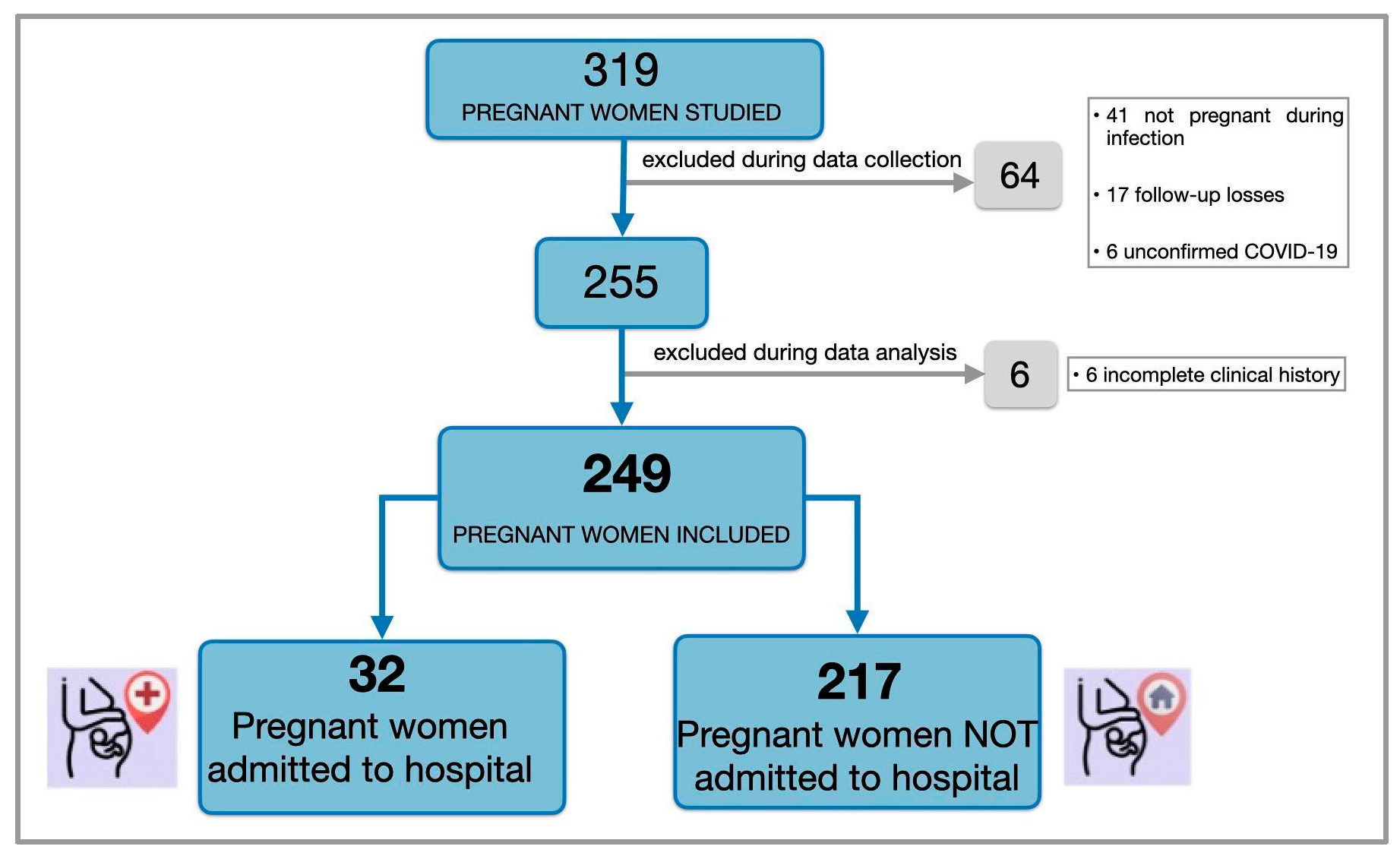
2.2. Study Population
2.3. Study Periods and Phase Definitions
- -
- Period 1 (11 March 2020–28 February 2021): Characterized by the original Wuhan strain and a non-vaccinated population. 28 February 2021 was selected as the cutoff point because it marked the beginning of sustained circulation of the Alpha variant in Spain and the initial rollout of COVID-19 vaccination in pregnant women.
- -
- Period 2 (1 March 2021–31 December 2021): Defined by the predominance of the Delta variant and a partially vaccinated population. During this phase, vaccination coverage among pregnant women began to progressively increase.
- -
- Period 3 (1 January 2022–5 May 2023): Corresponding to the predominance of the Omicron variant and widespread vaccination coverage. This period was associated with high vaccination rates and reduced COVID-19 severity, in line with the evolving behavior of circulating variants and the national public health response.
2.4. Data Collection and Definitions
- Maternal history: Maternal age, gestational age at the time of infection, country of birth, height, weight, body mass index (BMI), pregestational comorbidities (e.g., chronic respiratory disease, chronic hypertension, pregestational diabetes), COVID-19 vaccination status, parity, and history of previous spontaneous abortion.
- COVID-19-related variables: Symptoms (fever, cough, sore throat, dyspnea, myalgia, anosmia), emergency department visits, hospitalization, length of hospital stay, ICU admission and length of stay, treatments received (low molecular weight heparin, corticosteroids, oxygen therapy), and complications (bilateral pneumonia, thromboembolic events, maternal death, use of invasive or non-invasive mechanical ventilation).
- Pregnancy-related variables: Pregnancy outcome (miscarriage, preterm delivery, or term delivery), gestational complications (gestational diabetes, hypertensive disorders, threatened preterm labor), and fetal conditions (intrauterine growth restriction (IUGR)/small for gestational age (SGA), congenital anomalies, or other fetal complications).
- Delivery and perinatal outcomes: Date of delivery, gestational age at delivery, onset of labor (spontaneous or induced), and mode of delivery (vaginal or cesarean).
- Maternal postpartum complications: Postpartum hemorrhage, need for maternal blood transfusion, postpartum fever, ICU admission, and other maternal complications.
- Neonatal outcomes: Birth weight, Apgar scores, arterial and venous umbilical cord pH, NICU admission, perinatal complications, and neonatal death.
2.5. Statistical Analysis
2.6. Ethical Approach
3. Results
3.1. COVID-19 in Pregnant Women
3.2. Obstetric Outcomes: COVID-19 vs. Non-COVID-19 Pregnancies
3.3. Epidemiological and Clinical Characteristics in Pregnant Women with COVID-19: Admitted vs. Non-Admitted
3.4. Obstetric Outcomes in Pregnant Women with COVID-19: Admitted vs. Non-Admitted
3.5. Neonatal Outcomes in Pregnant Women with COVID-19: Admitted vs. Non-Admitted
3.6. Risk Factors for Severe COVID-19 Among Pregnant Women Admitted
3.7. Effect of Vaccination on Hospital Admission in Pregnant Women
4. Discussion
4.1. Prevalence and Incidence of COVID-19
4.2. Obstetric Outcomes: COVID-19 vs. Non-COVID-19 Pregnancies
4.3. Epidemiological Characteristics and Clinical Outcomes: Admitted vs. Non-Admitted Pregnant Women
4.4. Obstetric Outcomes: Admitted vs. Non-Admitted Pregnant Women
4.5. Neonatal Outcomes in Pregnant Women with COVID-19: Admitted vs. Non-Admitted Pregnancies
4.6. Severity of COVID-19 in Hospitalized Pregnant Women
4.7. Vaccination
4.8. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CI | Confidence interval |
| DBGUH | Doctor Balmis General University Hospital |
| ICU | Intensive care unit |
| IMV | Invasive mechanical ventilation |
| IQR | Interquartile range |
| IUGR | Intrauterine growth restriction |
| LMWH | Low-molecular-weight heparin |
| Mdn | Median |
| NICU | Neonatal intensive care unit |
| NIMV | Non-invasive mechanical ventilation |
| OR | Odds ratio |
| RT-PCR | Reverse transcription polymerase chain reaction |
| SD | Standard deviation |
| SGA | Small for gestational age |
| WHO | World Health Organization |
References
- Ellington, S.; Strid, P.; Tong, V.T.; Woodworth, K.; Galang, R.R.; Zambrano, L.D.; Nahabedian, J.; Anderson, K.; Gilboa, S.M. Characteristics of Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status—United States, January 22–June 7, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Infection in Pregnancy. Information for healthcare professionals; Version 16; RCOG: London, UK, 2022. [Google Scholar]
- Lassi, Z.S.; Ana, A.; Das, J.K.; Salam, R.A.; Padhani, Z.A.; Irfan, O.; Bhutta, Z.A. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: Clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J. Glob. Health. 2021, 11, 05018. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.L.; Tsai, T.C.; Dai, D.; Soto, M.; Rosenthal, N.; Orav, E.J.; Figueroa, J.F. Comparison of Pregnancy and Birth Outcomes Before vs During the COVID-19 Pandemic. JAMA Netw. Open. 2022, 5, e2226531. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, L.B.; Metz, T.D. Maternal and neonatal outcomes following SARS-CoV-2 infection. Semin. Fetal Neonatal Med. 2023, 28, 101428. [Google Scholar] [CrossRef] [PubMed]
- Pineles, B.L.; Goodman, K.E.; Pineles, L.; O’HAra, L.M.; Nadimpalli, G.; Magder, L.S.; Baghdadi, J.D.; Parchem, J.G.; Harris, A.D. Pregnancy and the Risk of In-Hospital Coronavirus Disease 2019 (COVID-19) Mortality. Obstet. Gynecol. 2022, 139, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Goldbergerová, A.; Kováč, L.; Marcišová, C.; Borovský, M.; Kotríková, D.; Izáková, Ľ.; Mikas, J.; Námešná, J.; Krištúfková, Z.; Krištúfková, A. Assessing the Impact of COVID-19 on Pregnancy and Maternal Outcomes: A Slovak National Study. Reprod. Med. 2024, 5, 319–334. [Google Scholar] [CrossRef]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. Can. Med Assoc. J. 2021, 193, E540–E548. [Google Scholar] [CrossRef] [PubMed]
- Mullins, E.; Perry, A.; Banerjee, J.; Townson, J.; Grozeva, D.; Milton, R.; Kirby, N.; Playle, R.; Bourne, T.; Lees, C.; et al. Pregnancy and neonatal outcomes of COVID-19: The PAN_COVID study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 276, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Incognito, G.; Distefano, R.E.; Campo, G.; Gulino, F.A.; Gulisano, C.; Gullotta, C.; Gullo, G.; Cucinella, G.; Tuscano, A.; Bruno, M.T.; et al. Comparison of Maternal and Neonatal Outcomes between SARS-CoV-2 Variants: A Retrospective, Monocentric Study. J. Clin. Med. 2023, 12, 6329. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Oakley, E.; Grandner, G.W.; Rukundo, G.; Farooq, F.; Ferguson, K.; Baumann, S.; Waldorf, K.M.A.; Afshar, Y.; Ahlberg, M.; et al. Clinical risk factors of adverse outcomes among women with COVID-19 in the pregnancy and postpartum period: A sequential, prospective meta-analysis. Am. J. Obstet. Gynecol. 2023, 228, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Gallagher, K.; Lo, J.Y.; Lindgren, E.J.; Burris, H.H.; Dysart, K.; Greenspan, J.; Culhane, J.F.; Handley, S.C.M. Coronavirus Disease 2019 (COVID-19) pandemic and pregnancy outcomes in a U. S. population. Obstet. Gynecol. 2021, 138, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Corsi, E.; Maraschini, A.; Salvatore, M.A.; the ItOSS-COVID-19 Working Group. SARS-CoV-2 infection among hospitalised pregnant women and impact of different viral strains on COVID-19 severity in Italy: a national prospective population-based cohort study. BJOG 2022, 129, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Vousden, N.; Ramakrishnan, R.; Bunch, K.; Morris, E.; Simpson, N.; Gale, C.; O’Brien, P.; Quigley, M.; Brocklehurst, P.; Kurinczuk, J.J.; et al. Impact of different SARS-CoV-2 variants on maternal and perinatal outcomes: A population-based study in England. BMJ 2023, 381, e074259. [Google Scholar] [CrossRef]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health. 2021, 9, e759–e772. [Google Scholar] [CrossRef] [PubMed]
- Seaton, C.L.; Cohen, A.; Henninger, E.M.; Gendlina, I.; Hou, W.; Bernstein, P.S.; Duong, T.Q. Coronavirus disease 2019 (COVID-19) perinatal outcomes across the pandemic at an academic medical center in New York City. Obstet. Gynecol. 2023, 141, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Marzan, M.B.; Rolnik, D.L.; Potenza, S.; Pritchard, N.; Said, J.M.; Palmer, K.R.; Whitehead, C.L.; Sheehan, P.M.; Ford, J.; et al. Reductions on stillbirths and preterm birth in COVID-19 vaccinated women: A multicenter cohort study of vaccination uptake and perinatal outcomes. Am. J. Obstet. Gynecol. 2023, 228, e1–e585.e16. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, F.; Bartolus, R.; Mauri, P.A.; Favilli, A.; Gerli, S.; Ferrazzi, E. Delivery in pregnant women infected with SARS-CoV-2: A fast review. Int. J. Gynaecol. Obstet. 2020, 150, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Metz, T.D.; Clifton, R.G.; Hughes, B.L.; Sandoval, G.J.; Grobman, W.A.; Saade, G.R.; Manuck, T.A.; Longo, M.; Sowles, A.; Clark, K.; et al. Association of SARS-Cov-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 2022, 327, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Morán Antolín, E.; Broullón Molanes, J.R.; de la Cruz Conty, M.L.; Pardilla, M.B.E.; Martín, M.d.P.G.; Bueno, J.A.S.; Acebal, L.F.; Recarte, P.P.; Bartolomé, A.Á.; Cendán, J.P.M.; et al. SARS-CoV-2 infection and C-section: A prospective observational study. Virus 2021, 13, 2330. [Google Scholar] [CrossRef] [PubMed]
- Trinh, L.T.T.; Achat, H.M.; Pesce, A. Caesarean sections before and during the COVID-19 pandemic in western Sydney, Australia. J. Obstet. Gynaecol. 2023, 43, 2265668. [Google Scholar] [CrossRef] [PubMed]
- Jering, K.S.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Vardeny, O.; Greene, M.F.; Solomon, S.D. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern. Med. 2021, 181, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Pinargote-Celorio, H.; Moreno-Pérez, Ó.; González-De-La-Aleja, P.; Llenas-García, J.; Pérez-Crespo, P.M.M.; Rodríguez-Díaz, J.-C.; Martínez-López, B.; Gutiérrez, N.M.; Ramos-Rincón, J.-M.; Merino, E. Real-world effectiveness of early anti-SARS therapy in severely immunocompromised COVID-19 outpatients during the SARS-CoV-2 omicron variant era: A propensity score-adjusted retrospective cohort study. J. Antimicrob. Chemother. 2024, 79, 3248–3253. [Google Scholar] [CrossRef] [PubMed]
- Conde-Agudelo, A.; Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2022, 226, 68–89.e3. [Google Scholar] [CrossRef] [PubMed]
- Berumen-Lechuga, M.G.; Leaños-Miranda, A.; Molina-Pérez, C.J.; García-Cortés, L.R.; Palomo-Piñón, S. Risk factors for severe-critical COVID-19 in pregnant women. J. Clin. Med. 2023, 12, 5812. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ma, Y.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Association of infection with different SARS-CoV-2 variants during pregnancy with maternal and perinatal outcomes: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 15932. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, V.L.; Oddo-Sommerfeld, S.; Schermelleh-Engel, K.; Commodari, E. From lockdown to cradle: Navigating the psychological challenges of childbirth during the COVID-19 pandemic in Italy–Evidence from a 3-year analysis. Curr. Psychol. 2024, 43, 35616–35629. [Google Scholar] [CrossRef]
- Giesbrecht, G.F.; Rojas, L.; Patel, S.; Kuret, V.; MacKinnon, A.L.; Tomfohr-Madsen, L.; Lebel, C. Fear of COVID-19, mental health, and pregnancy outcomes in the pregnancy during the COVID-19 pandemic study: Fear of COVID-19 and pregnancy outcomes. J. Affect. Disord. 2022, 299, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Tauqeer, F.; Ceulemans, M.; Gerbier, E.; Passier, A.; Oliver, A.; Foulon, V.; Panchaud, A.; Lupattelli, A.; Nordeng, H. Mental health of pregnant and postpartum women during the third wave of the COVID-19 pandemic: A European cross-sectional study. BMJ Open 2023, 13, e063391. [Google Scholar] [CrossRef] [PubMed]
- Schell, R.C.; Macias, D.A.; Garner, W.H.; White, A.M.; McIntire, D.D.; Pruszynski, J.; Adhikari, E.H. Examining the impact of trimester of diagnosis on COVID-19 disease progression in pregnancy. Am. J. Obstet. Gynecol. MFM. 2022, 4, 100728. [Google Scholar] [CrossRef] [PubMed]
- Engjom, H.; Aabakke, A.J.M.; Klungsoyr, K.; Svanvik, T.; Äyräs, O.; Jonasdottir, E.; Thurn, L.; Jones, E.; Pettersson, K.; Nyfløt, L.T.; et al. COVID-19 in pregnancy-characteristics and outcomes of pregnant women admitted to hospital because of SARS-CoV-2 infection in the Nordic countries. Acta Obstet. Gynecol. Scand. 2021, 100, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Trinchillo, M.G.; Di Girolamo, R.; Raffone, A.; Saccone, G.; Iorio, G.G.; Gabrielli, O.; Maruotti, G.M. COVID-19 vaccine and pregnancy outcomes: A systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 2022, 159, 651–661. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Interim Recommendations for the Use of COVID-19 Vaccines in Pregnant and Lactating Women—March 2024. Available online: https://www.who.int (accessed on 1 November 2024).
- Barros, F.C.; Gunier, R.B.; Rego, A.; Sentilhes, L.; Rauch, S.; Gandino, S.; Teji, J.S.; Thornton, J.G.; Kachikis, A.B.; Nieto, R.; et al. Maternal vaccination against COVID-19 and neonatal outcomes during Omicron: INTERCOVID-2022 study. Am. J. Obstet. Gynecol. 2024, 231, 460.e1–460.e17. [Google Scholar] [CrossRef] [PubMed]
| (a) | ||||||
| Gestational Age at Birth <28 Weeks 28–37 Weeks ≥37 Weeks | ||||||
| Period of Study | Overall % (n/N) a | COVID-19 % (n/N1) | Non-COVID-19 % (n/N2) | p-Value | ||
| 4 March 2020–28 February 2021 | 1.11 (21/1894) 8.08 (153/1894) 90.81 (1720/1894) | 5.56 (2/36) 36.11 (13/36) 58.33 (21/36) | 1.02 (19/1858) 7.53 (140/1858) 91.44 (1699/1858) | <0.001 | ||
| 1 March 2021–31 December 2021 | 0.74 (12/1630) 8.83 (144/1630) 90.43 (1474/1630) | 0 (0/18) 22.22 (4/18) 77.78 (14/18) | 0.74 (12/1612) 8.68 (140/1612) 90.57 (1460/1612) | 0.125 | ||
| 1 January 2022–5 May 2023 | 0.96 (25/2596) 7.82 (203/2596) 91.22 (2368/2596) | 0 (0/186) 13.44 (25/186) 86.56 (161/186) | 1.04 (25/2410) 7.39 (178/2410) 91.58 (2207/2410) | 0.005 | ||
| Overall | 0.95 (58/6120) 8.17 (500/6120) 90.88 (5562/6120) | 0.83 (2/240) 17.5 (42/240) 81.67 (196/240) | 0.95 (56/5880) 7.79 (458/5880) 91.26 (5366/5880) | <0.001 | ||
| (b) | ||||||
| Cesarean Section | Induced Labor | |||||
| Period of study | COVID-19 % (n/N1) | No COVID-19 % (n/N2) | p-value | COVID-19 % (n/N1) | No COVID-19 % (n/N2) | p-value |
| 4 March 2020–28 February 2021 | 36.1 (13/36) | 23.3 (433/1858) | 0.072 | 47.2 (17/36) | 40.3 (749/1858) | 0.441 |
| 1 March 2021–31 December 2021 | 33.3 (6/18) | 23.8 (384/1612) | 0.181 | 66.7 (12/18) | 41.1 (662/1612) | 0.028 |
| 1 January 2022–5 May 2023 | 27.4 (51/186) | 24.1 (582/2410) | 0.321 | 41.4 (77/186) | 34.9 (842/2410) | 0.075 |
| Overall | 29.2 (70/240) | 23.8 (1399/5880) | 0.052 | 44.2 (106/240) | 38.3 (2253/5880) | 0.521 |
| Overall (n = 249) % (n/N) a | Non-Admitted (n = 217) % (n/N) a | Admitted (n = 32) % (n/N) a | p-Value | |
|---|---|---|---|---|
| Age. Mdn (IQR) | 32 (26–36) | 31 (26–36) | 33 (27.5–36.5) | 0.251 |
| Country of birth | ||||
| Spain | 62.7 (156/249) | 64.5 (140/217) | 50 (16/32) | 0.113 |
| South America | 21.7 (54/249) | 20.7 (45/217) | 28.1 (9/32) | |
| Africa | 12.4 (31/249) | 11.1 (24/217) | 21.9 (7/32) | |
| Europe | 2.8 (7/249) | 3.2 (7/217) | 0 (0/32) | |
| Period of study | <0.001 | |||
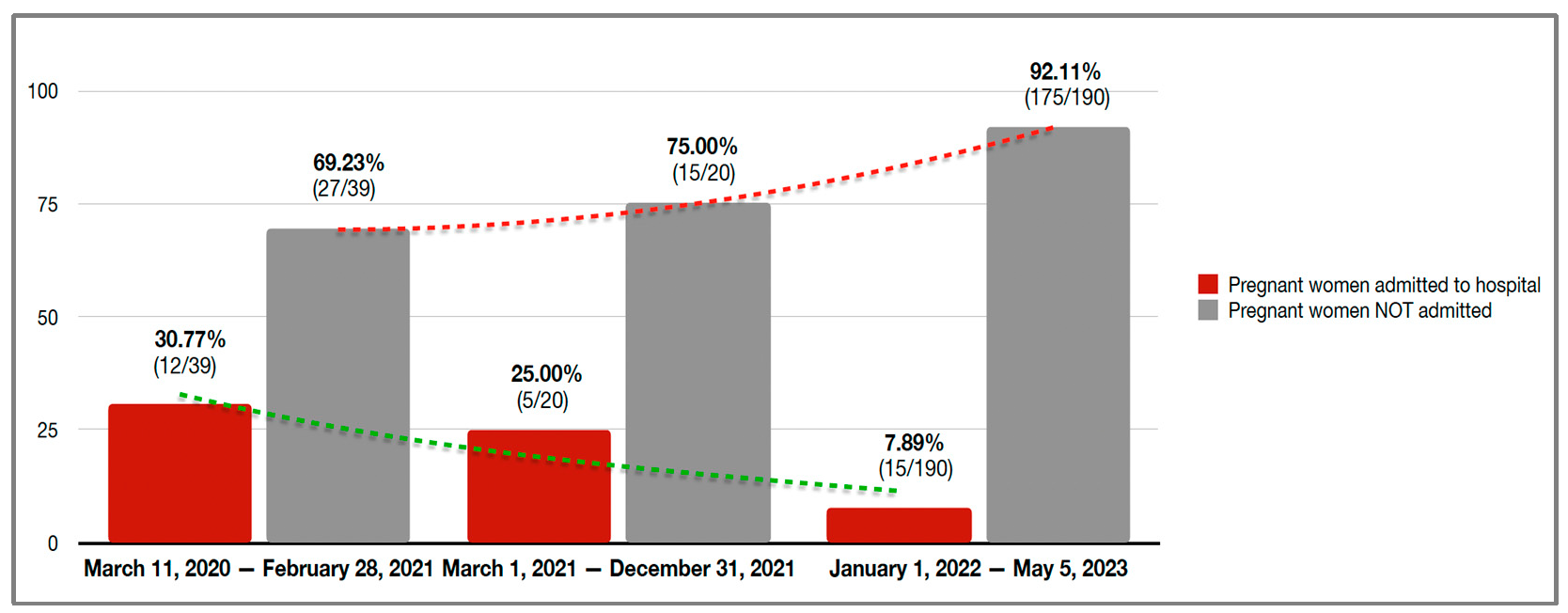
| 4 March 2020–28 February 2021 | 15.7 (39/249) | 12.4 (27/217) | 37.5 (12/32) | |
| 1 March 2021–31 December 2021 | 8.0 (20/249) | 6.9 (15/217) | 15.6 (5/32) | |
| 1 January 2022–5 May 2023 | 76.3 (190/249) | 80.6 (175/217) | 46.9 (15/32) | |
| Comorbidities | ||||
| Obesity * | 20.6 (50/243) | 20.9 (44/211) | 19.4 (6/31) | 0.847 |
| Pulmonary disease | 6.4 (16/249) | 5.1 (11/217) | 15.6 (5/32) | 0.040 |
| Other comorbidities ** | 26.9 (67/249) | 25.8 (56/217) | 34.4 (11/32) | 0.308 |
| Clinical symptoms | ||||
| Fever | 40.5 (94/232) | 36.5 (73/200) | 65.6 (21/32) | <0.001 |
| Cough | 32 (74/231) | 27 (54/200) | 64.5 (20/31) | <0.001 |
| Myalgia | 19.8 (46/232 | 17.5 (35/200) | 34.4 (11/32) | 0.026 |
| Odynophagia | 18.1 (42/232) | 14.5 (29/200) | 40.6 (13/32) | <0.001 |
| Dyspnea | 12 (28/233) | 4.5 (9/201) | 59.4 (19/32) | <0.001 |
| Anosmia | 3.4 (8/233) | 2 (4/201) | 12.5 (4/32) | 0.014 |
| Clinical complications | ||||
| Bilateral pneumonia | 10.0 (25/249) | 0 (0/217) | 25 (8/32) | <0.001 |
| Thromboembolism | 0.4 (1/249) | 0.5 (1/217) | 0 (0/32) | 1.000 |
| Outcomes | ||||
| Admission ICU | 1.2 (3/248) | 0 (0/217) | 9.7 (3/31) | 0.002 |
| NIMV | 0.8 (2/248) | 0 (0/217) | 6.4 (2/31) | 0.015 |
| Death | 0.4 (1/248) | 0 (0/217) | 3.2 (1/31) | 0.120 |
| Overall (n = 249) % (n/N) a | Non-Admitted (n = 217) % (n/N) a | Admitted (n = 32) % (n/N) a | p-Value | |
|---|---|---|---|---|
| Gestational age at the time of infection | ||||
| Mdn (IQR) | 32 (22–38) | 32 (21–38) | 29.50 (23.75–34.5) | 0.160 |
| <24 weeks | 27.6 (68/246) | 28 (60/214) | 25 (8/32) | 0.037 |
| 24–34 weeks | 30.9 (76/246) | 28 (60/214) | 50 (16/32) | |
| ≥35 weeks | 41.5 (102/246) | 43.9 (94/214) | 25 (8/32) | |
| Obstetric history | ||||
| Number of pregnancies, Mdn (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3.75) | 0.657 |
| Number of previous miscarriages | 30.1 (75/249) | 30.9 (67/217) | 25 (8/32) | 0.499 |
| Previous vaginal deliveries | 38.2 (95/249) | 38.7 (84/217) | 34.4 (11/32) | 0.637 |
| Previous cesarean sections | 15.8 (39/247) | 15.3 (33/216) | 19.4 (6/31) | 0.598 |
| Pregnancy complications | ||||
| Gestational diabetes | 11.2 (28/249) | 11.5 (25/217) | 9.4 (3/32) | 1.000 |
| Hypertensive disorders | 9.2 (23/249) | 8.8 (19/217) | 12.5 (4/32) | 0.520 |
| Threatened of preterm labor | 5.4 (13/217) | 5.7 (12/210) | 3.2 (1/31) | 1.000 |
| Fetal pathology * | 5 (12/242) | 4.8 (10/210) | 6.3 (2/32) | 0.663 |
| Delivery outcomes | ||||
| Gestational age at birth, weeks. Mdn. (IQR) | 39 (26–38) | 39 (37–40) | 38 (37–39) | 0.251 |
| <34 weeks | 4.6 (11/240) | 3.8 (8/208) | 9.4 (3/32) | 0.121 |
| ≥34 weeks | 95.4 (229/240) | 96.2 (200/208) | 90.6 (29/32) | 0.168 |
| Term delivery | 86.7 (216/249) | 87.1 (189/217) | 84.4 (27/32) | 0.437 |
| Induced labor | 42.2 (105/249) | 39.6 (86/217) | 59.4 (19/32) | 0.023 |
| Unsatisfactory intrapartum fetal cardiotocography | 11.3 (26/230) | 10.6 (21/199) | 16.1 (5/31) | 0.363 |
| Cesarean section | 27.7 (69/249) | 24.9 (54/217) | 46.9 (15/32) | 0.009 |
| Postpartum hemorrhage | 4.9 (11/224) | 5.1 (10/195) | 3.4 (1/29) | 1.000 |
| Maternal complications | ||||
| Maternal blood transfusion | 0.4 (1/239) | 0.5 (1/207) | 0 (0/32) | 1.000 |
| Maternal admission to ICU | 1.3 (3/238) | 0 (0/207) | 9.7 (3/31) | 0.002 |
| Postpartum fever | 2.5 (6/239) | 2.9 (6/207) | 0 (0/32) | 1.000 |
| Overall % (n/N) a | Non-Admitted % (n/N) a | Admitted % (n/N) a | p-Value | |
|---|---|---|---|---|
| Birth weight; g | ||||
| Mdn (IQR) | 3168 (2920–3501) | 3248 (2937–3542) | 3.090 (2837–3535) | 0.132 |
| <2500 g | 6.3 (15/223) | 5.8 (12/194) | 9.4 (3/29) | 0.433 |
| Apgar 1 score (1st min) | <0.001 | |||
| <7 | 5.0 (12/240) | 2.9 (6/208) | 18.7 (6/32) | |
| ≥7 | 95.0 (228/240) | 97.1 (202/208) | 81.3 (26/32) | |
| Apgar score (5th min) | 0.133 | |||
| <7 | 0.4 (1/240) | 0 | 3.1 (1/32) | |
| ≥7 | 99.6 (239/240) | 100 (208/208) | 96.9 (31/32) | |
| Newborn admitted in NICU | 10.5 (25/238) | 8.2 (17/207) | 25.8 (8/31) | 0.007 |
| Neonatal complications * | 9.7 (23/237) | 7.7 (16/207) | 23.3 (7/30) | 0.015 |
| Umbilical arterial pH | 7.25 (7.24–7.27) | 7.25 (7.24–7.28) | 7.25 (7.23–7.29) | 0.921 |
| Umbilical venous pH | 7.33 (7.27–7.37) | 7.33 (7.28–7.36) | 7.32 (7.28–7.34) | 0.585 |
| Severe COVID-19 % (n/N) a | Non-Severe COVID-19 % (n/N) a | Crude OR | p-Value | |
|---|---|---|---|---|
| Age ≥ 35 years | 0 (0/5) | 38.5 (10/26) | NA | 0.147 |
| Gestational age at delivery, (weeks) | 1.000 | |||
| <35 | 80.0 (4/5) | 74.1 (20/27) | 1 | |
| ≥35 | 20.0 (1/5) | 27.9 (7/26) | 0.71 (0.07–7.6) | |
| Born in Spain | 0 (0/5) | 61.5 (16/26) | NA | 0.018 |
| Comorbidities | ||||
| Obesity * | 60.0 (3/5) | 12.0 (3/25) | 11.0 (1.27–95) | 0.041 |
| Pulmonary diseases | 20.0 (1/5) | 11.5 (3/26) | 1.91 (0.15–23) | 0.525 |
| Hypertension | 0 | 15.4 (4/26) | NA | 1.000 |
| Clinical presentation | ||||
| Fever | 100 (5/5) | 61.5 (16/26) | NA | 0.147 |
| Cough | 75.0 (3/4) | 65.4 (17/26) | 1.58 (0.14–17) | 1.000 |
| Odynophagia | 6.0 (3/5) | 38.5 (10/26) | 2.40 (0.33–17) | 0.625 |
| Anosmia | 0 (0/5) | 15.4 (4/26) | NA | 1.000 |
| Dyspnea | 100 (5/5) | 53.8 (14/26) | NA | 0.128 |
| Myalgia | 60 (3/5) | 30.8 (8/26) | 3.37 (0.46–24) | 0.317 |
| Treatment | ||||
| LMWH | 80 (4/5) | 84.6 (22/26) | 0.77 (0.64–8.31) | 1.000 |
| Steroid | 100 (5/5) | 26.9 (7/26) | NA | 0.005 |
| Complication/outcome | ||||
| Bilateral pneumonia | 100 (5/5) | 11.5 (3/26) | NA | <0.001 |
| Thromboembolism | 0 | 3.8 (1/26) | NA | 1.000 |
| Death | 20 (1/5) | 0 | NA | 0.161 |
| Overall % (n/N) a | Non-Admitted % (n/N) a | Admitted % (n/N) a | p-Value | |
|---|---|---|---|---|
| Vaccination status | ||||
| Not vaccinated | 47.4 (118/249) | 43.8 (95/217) | 71.9 (23/32) | 0.003 |
| Vaccinated (1 or 2 doses) | 52.6 (131/249) | 56.2 (122/217) | 28.1 (9/32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quijada-Cazorla, M.-A.; Simó-Rodríguez, M.-V.; Palacios-Marqués, A.-M.; Peláez-García, M.; Ramos-Rincón, J.-M. Impact of COVID-19 on Pregnancy Outcomes: A Phase-Based Analysis from a Spanish Tertiary Hospital (2020–2023). J. Clin. Med. 2025, 14, 5136. https://doi.org/10.3390/jcm14145136
Quijada-Cazorla M-A, Simó-Rodríguez M-V, Palacios-Marqués A-M, Peláez-García M, Ramos-Rincón J-M. Impact of COVID-19 on Pregnancy Outcomes: A Phase-Based Analysis from a Spanish Tertiary Hospital (2020–2023). Journal of Clinical Medicine. 2025; 14(14):5136. https://doi.org/10.3390/jcm14145136
Chicago/Turabian StyleQuijada-Cazorla, María-Asunción, María-Virgilia Simó-Rodríguez, Ana-María Palacios-Marqués, María Peláez-García, and José-Manuel Ramos-Rincón. 2025. "Impact of COVID-19 on Pregnancy Outcomes: A Phase-Based Analysis from a Spanish Tertiary Hospital (2020–2023)" Journal of Clinical Medicine 14, no. 14: 5136. https://doi.org/10.3390/jcm14145136
APA StyleQuijada-Cazorla, M.-A., Simó-Rodríguez, M.-V., Palacios-Marqués, A.-M., Peláez-García, M., & Ramos-Rincón, J.-M. (2025). Impact of COVID-19 on Pregnancy Outcomes: A Phase-Based Analysis from a Spanish Tertiary Hospital (2020–2023). Journal of Clinical Medicine, 14(14), 5136. https://doi.org/10.3390/jcm14145136







