Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim and Study Design
2.2. Therapy and Disease Response Evaluation
2.3. Subgroup Analysis
2.4. Statistical Analyses
3. Results
3.1. Baseline Data
3.2. Induction Therapy and Overall Response
3.3. Measurable Residual Disease (MRD)
3.4. Consolidation Therapy and HSCT
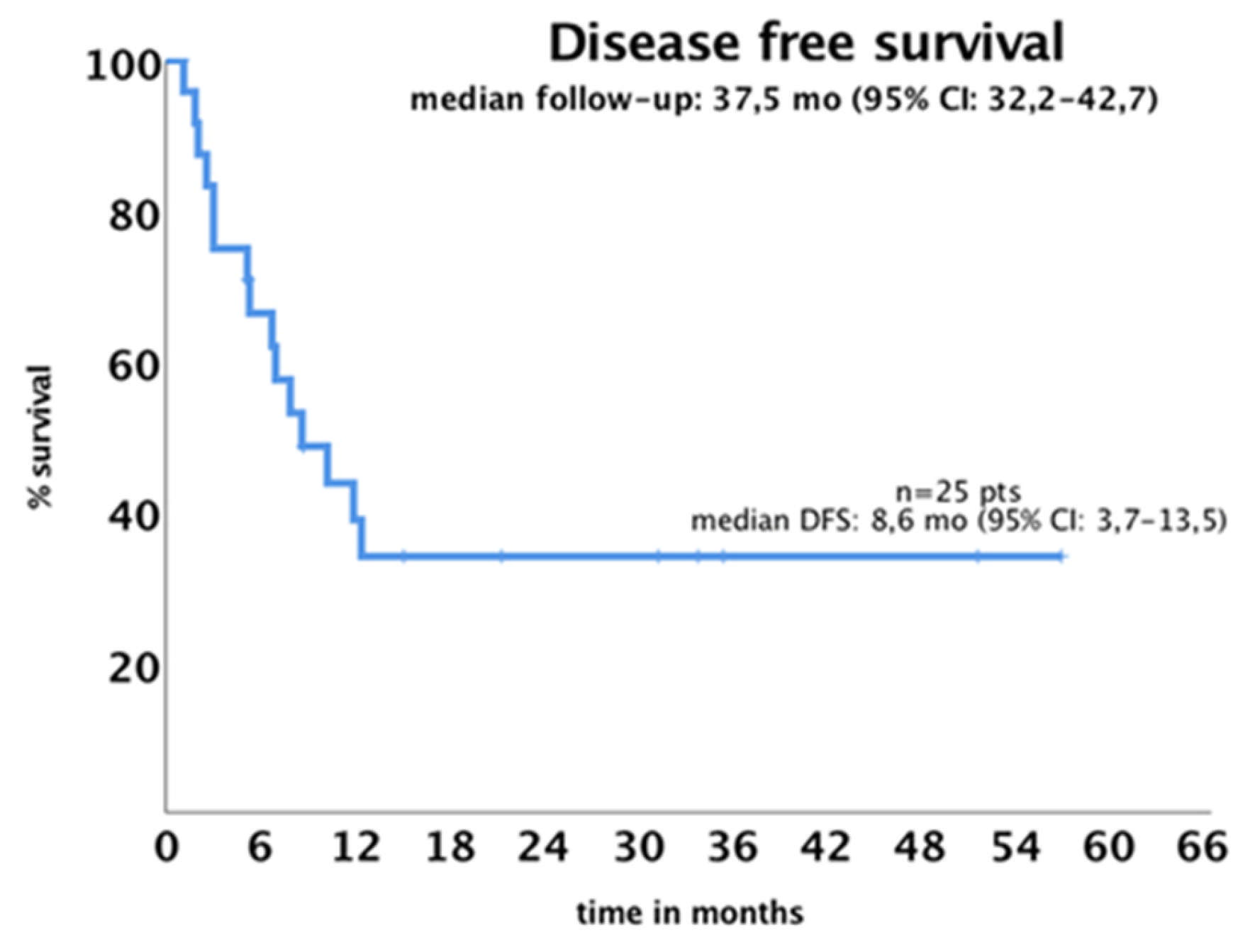
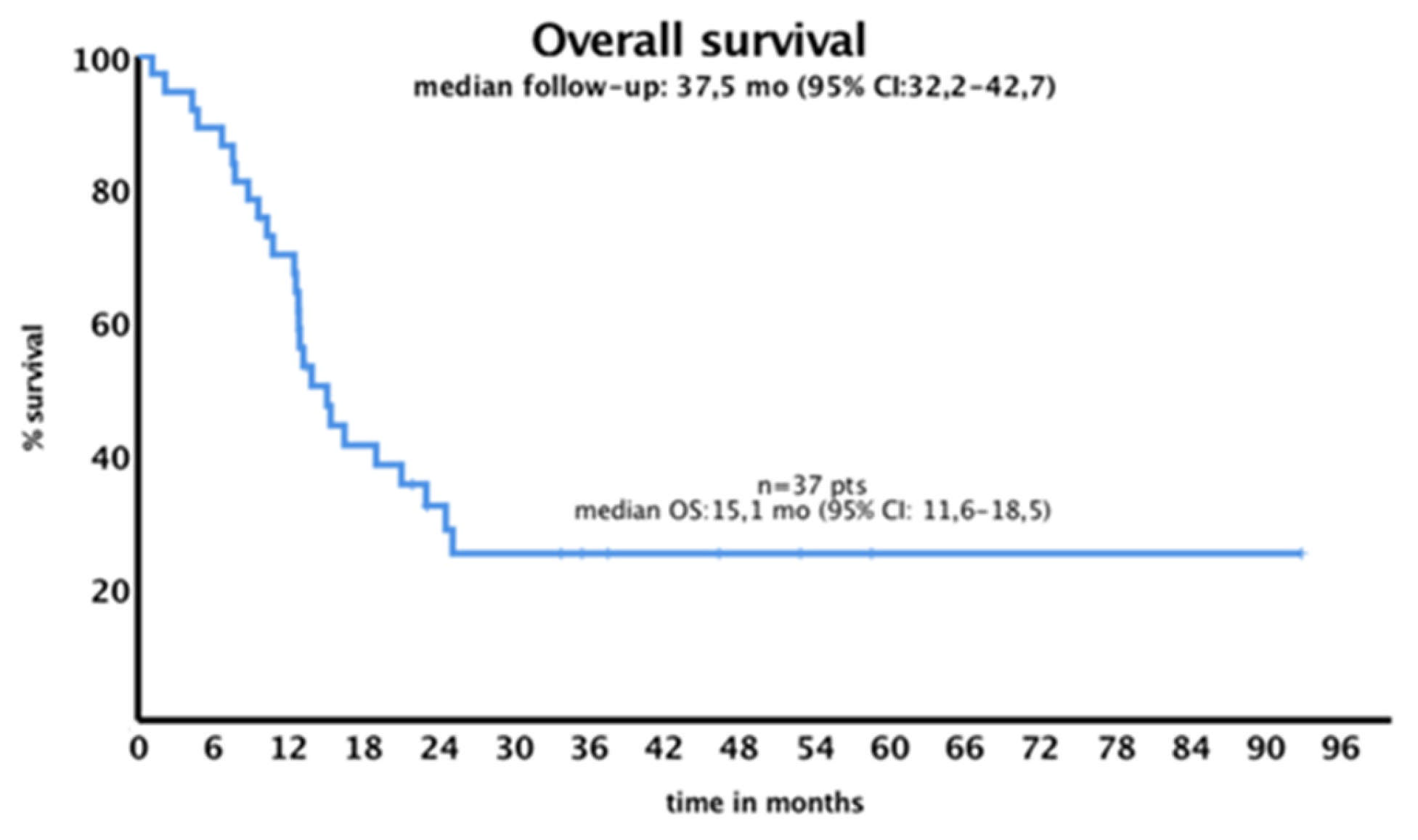
3.5. Survival Outcome
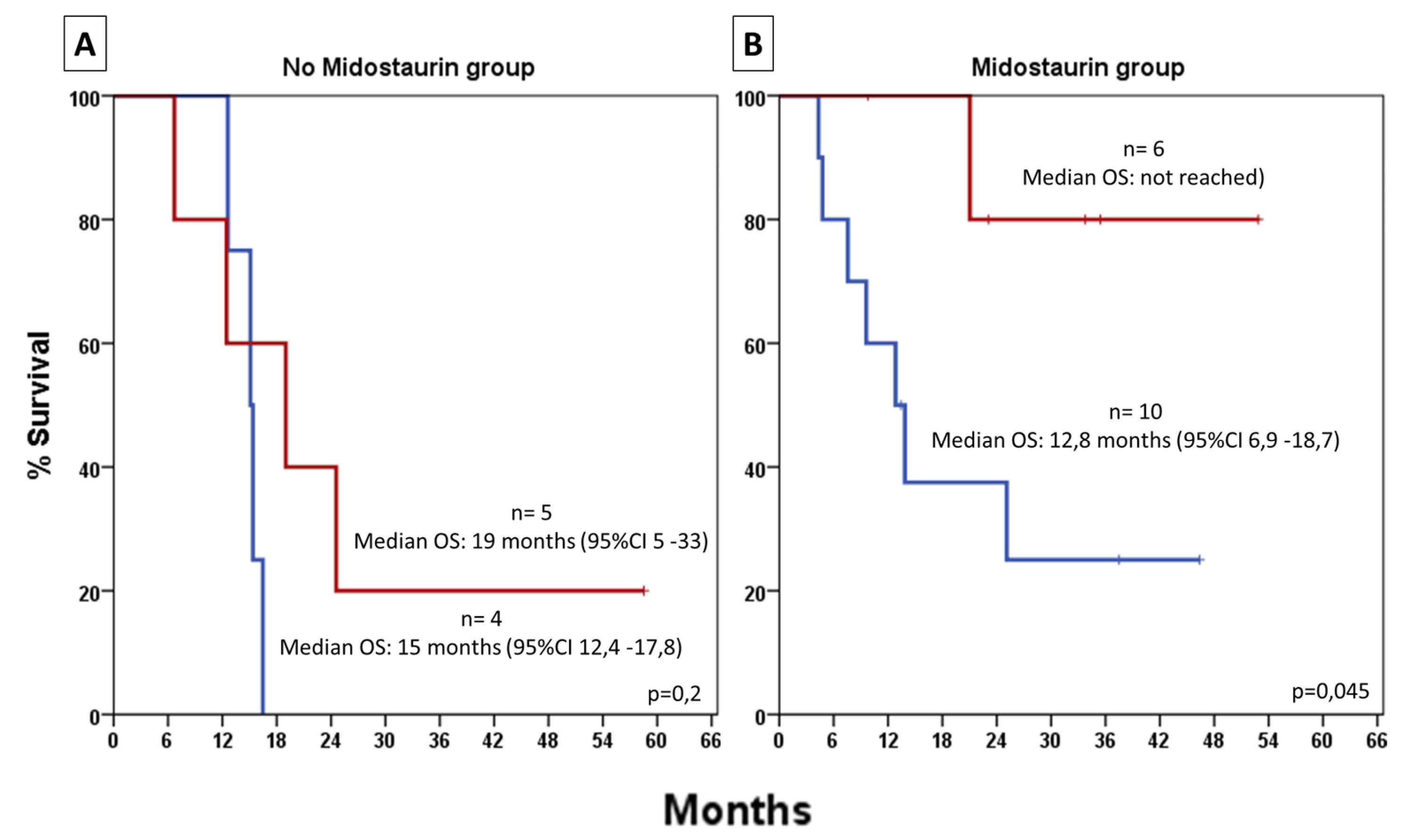
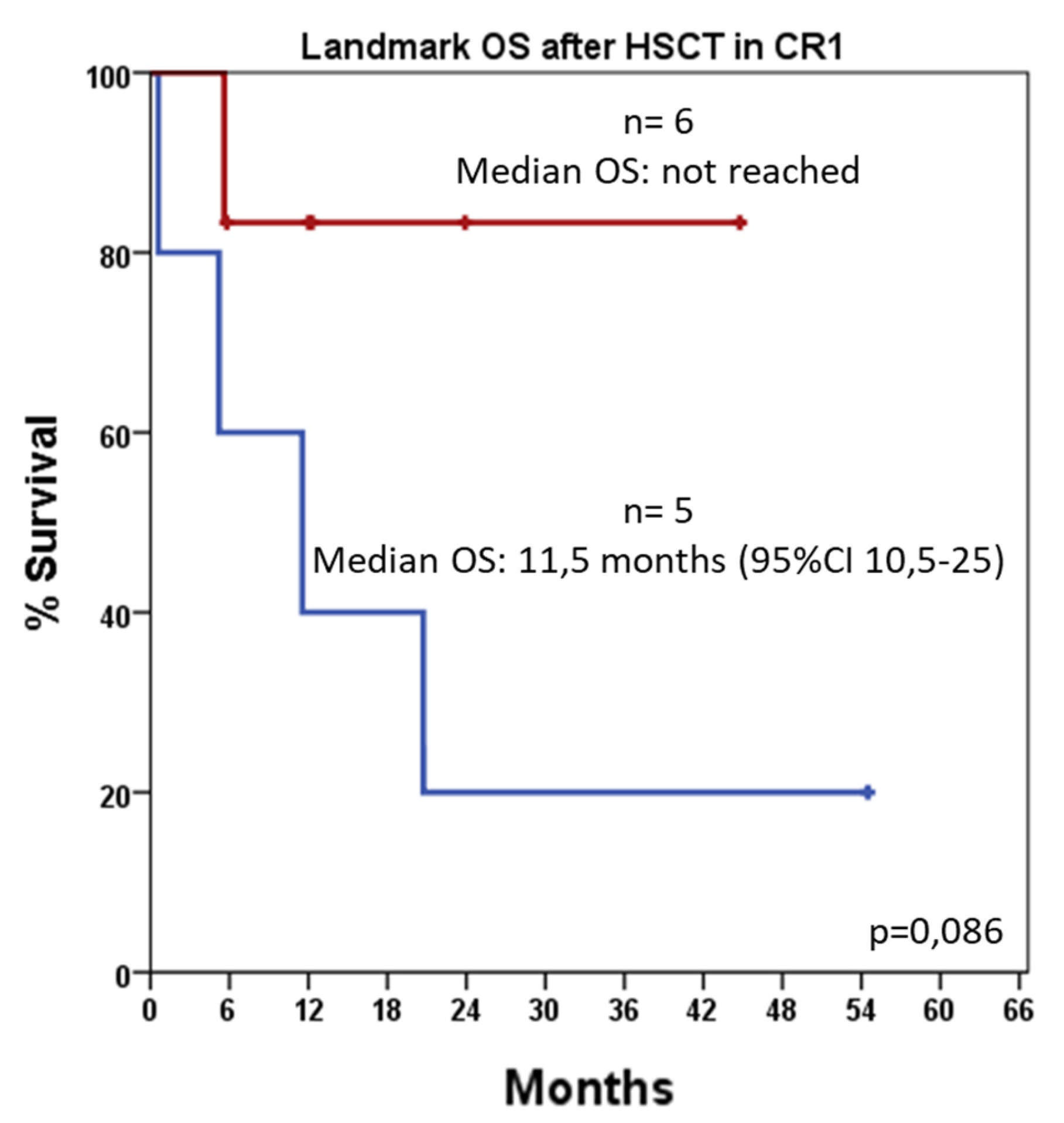
3.6. HSCT-Adjusted Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 Mutations in AML: Review of Current Knowledge and Evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Oran, B.; Cortes, J.; Beitinjaneh, A.; Chen, H.C.; de Lima, M.; Patel, K.; Ravandi, F.; Wang, X.; Brandt, M.; Andersson, B.S.; et al. Allogeneic Transplantation in First Remission Improves Outcomes Irrespective of FLT3-ITD Allelic Ratio in FLT3-ITD–Positive Acute Myelogenous Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 1218–1226. [Google Scholar] [CrossRef]
- de Jonge, H.J.M.; Valk, P.J.M.; de Bont, E.S.J.M.; Schuringa, J.J.; Ossenkoppele, G.; Vellenga, E.; Huls, G. Prognostic Impact of White Blood Cell Count in Intermediate Risk Acute Myeloid Leukemia: Relevance of Mutated NPM1 and FLT3-ITD. Haematologica 2011, 96, 1310–1317. [Google Scholar] [CrossRef]
- Zhao, J.C.; Agarwal, S.; Ahmad, H.; Amin, K.; Bewersdorf, J.P.; Zeidan, A.M. A Review of FLT3 Inhibitors in Acute Myeloid Leukemia. Blood Rev. 2022, 52, 100905. [Google Scholar] [CrossRef] [PubMed]
- Duminuco, A.; Del Fabro, V.; De Luca, P.; Leotta, D.; Limoli, M.C.; Longo, E.; Nardo, A.; Santuccio, G.; Petronaci, A.; Stanzione, G.; et al. Emergencies in Hematology: Why, When and How I Treat? J. Clin. Med. 2024, 13, 7572. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Alotaibi, A.S.; Bücklein, V.; Subklewe, M. T-Cell-Based Immunotherapy of Acute Myeloid Leukemia: Current Concepts and Future Developments. Leukemia 2021, 35, 1843–1863. [Google Scholar] [CrossRef] [PubMed]
- Tsapogas, P.; Mooney, C.J.; Brown, G.; Rolink, A. The Cytokine FLT3-Ligand in Normal and Malignant Hematopoiesis. Int. J. Mol. Sci. 2017, 18, 1115. [Google Scholar] [CrossRef]
- Duminuco, A.; Maugeri, C.; Parisi, M.; Mauro, E.; Fiumara, P.F.; Randazzo, V.; Salemi, D.; Agueli, C.; Palumbo, G.A.; Santoro, A.; et al. Target Therapy for Extramedullary Relapse of FLT3-ITD Acute Myeloid Leukemia: Emerging Data from the Field. Cancers 2022, 14, 2186. [Google Scholar] [CrossRef]
- Duminuco, A.; Raimondo, F.D.; Mauro, E.; Maugeri, C.; Parisi, M.S.; Palumbo, G.A.M.; Fiumara, P.F.; Garibaldi, B.; Vetro, C. Regression of Leukemia Cutis with Gilteritinib in a Case of FLT3-Positive Acute Myeloid Leukemia. Curr. Probl. Cancer Case Rep. 2022, 7, 100184. [Google Scholar] [CrossRef]
- Döhner, K.; Thiede, C.; Jahn, N.; Panina, E.; Gambietz, A.; Larson, R.A.; Prior, T.W.; Marcucci, G.; Jones, D.; Krauter, J.; et al. Impact of NPM1/FLT3-ITD Genotypes Defined by the 2017 European LeukemiaNet in Patients with Acute Myeloid Leukemia. Blood 2020, 135, 371–380. [Google Scholar] [CrossRef]
- Kantarjian, H.; Kadia, T.; DiNardo, C.; Daver, N.; Borthakur, G.; Jabbour, E.; Garcia-Manero, G.; Konopleva, M.; Ravandi, F. Acute Myeloid Leukemia: Current Progress and Future Directions. Blood Cancer J. 2021, 11, 41. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Sargas, C.; Ayala, R.; Larráyoz, M.J.; Chillón, M.C.; Rodriguez-Arboli, E.; Bilbao, C.; Prados de la Torre, E.; Martínez-Cuadrón, D.; Rodríguez-Veiga, R.; Boluda, B.; et al. Comparison of the 2022 and 2017 European LeukemiaNet Risk Classifications in a Real-Life Cohort of the PETHEMA Group. Blood Cancer J. 2023, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Venugopal, S.; Ravandi, F. FLT3 Mutated Acute Myeloid Leukemia: 2021 Treatment Algorithm. Blood Cancer J. 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Gozzo, L.; Nardo, A.; Brancati, S.; Judica, A.; Duminuco, A.; Maugeri, C.; Parisi, M.; Longo, L.; Vitale, D.C.; Ruscica, R.; et al. Severe Gastrointestinal Toxicity Following the Use of Gilteritinib: A Case Series and Analysis of Postmarketing Surveillance Data. Healthcare 2023, 11, 1479. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Thiede, C.; Steudel, C.; Mohr, B.; Schaich, M.; Schäkel, U.; Platzbecker, U.; Wermke, M.; Bornhäuser, M.; Ritter, M.; Neubauer, A.; et al. Analysis of FLT3-Activating Mutations in 979 Patients with Acute Myelogenous Leukemia: Association with FAB Subtypes and Identification of Subgroups with Poor Prognosis: Presented in Part at the 42nd Annual Meeting of the American Society of Hematology, December 1–5, 2000, San Francisco, CA (Abstract 2334). Blood 2002, 99, 4326–4335. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Kayser, S.; Bullinger, L.; Kobbe, G.; Casper, J.; Ringhoffer, M.; Held, G.; Brossart, P.; Lübbert, M.; Salih, H.R.; et al. Differential Impact of Allelic Ratio and Insertion Site in FLT3-ITD–Positive AML with Respect to Allogeneic Transplantation. Blood 2014, 124, 3441–3449. [Google Scholar] [CrossRef]
- Pratcorona, M.; Brunet, S.; Nomdedéu, J.; Ribera, J.M.; Tormo, M.; Duarte, R.; Escoda, L.; Guàrdia, R.; Queipo de Llano, M.P.; Salamero, O.; et al. Favorable Outcome of Patients with Acute Myeloid Leukemia Harboring a Low-Allelic Burden FLT3-ITD Mutation and Concomitant NPM1 Mutation: Relevance to Post-Remission Therapy. Blood 2013, 121, 2734–2738. [Google Scholar] [CrossRef]
- Bazarbachi, A.; Bug, G.; Baron, F.; Brissot, E.; Ciceri, F.; Abou Dalle, I.; Döhner, H.; Esteve, J.; Floisand, Y.; Giebel, S.; et al. Clinical Practice Recommendation on Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia Patients with FLT3-Internal Tandem Duplication: A Position Statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2020, 105, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Wang, E.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; Coutre, S.E.; De Lima, M.; Fathi, A.T.; Fiorella, M.; et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 721–749. [Google Scholar] [CrossRef] [PubMed]
- Bazarbachi, A.; Labopin, M.; Gedde-Dahl, T.; Remenyi, P.; Forcade, E.; Kröger, N.; Socié, G.; Craddock, C.; Bourhis, J.H.; Versluis, J.; et al. Improved Posttransplant Outcomes in Recent Years for AML Patients with FLT3-ITD and Wild-Type NPM1: A Report from the EBMT Acute Leukemia Working Party. Clin. Cancer Res. 2023, 29, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Barosi, G.; Venditti, A.; Angelucci, E.; Gobbi, M.; Pane, F.; Tosi, P.; Zinzani, P.; Tura, S. Consensus-Based Definition of Unfitness to Intensive and Non-Intensive Chemotherapy in Acute Myeloid Leukemia: A Project of SIE, SIES and GITMO Group on a New Tool for Therapy Decision Making. Leukemia 2013, 27, 997–999. [Google Scholar] [CrossRef]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K.; National Cancer Research Institute Adult Leukaemia Working Group. Refinement of Cytogenetic Classification in Acute Myeloid Leukemia: Determination of Prognostic Significance of Rare Recurring Chromosomal Abnormalities among 5876 Younger Adult Patients Treated in the United Kingdom Medical Research Council Trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef]
- Noguera, N.I.; Ammatuna, E.; Zangrilli, D.; Lavorgna, S.; Divona, M.; Buccisano, F.; Amadori, S.; Mecucci, C.; Falini, B.; Lo-Coco, F. Simultaneous Detection of NPM1 and FLT3-ITD Mutations by Capillary Electrophoresis in Acute Myeloid Leukemia. Leukemia 2005, 19, 1479–1482. [Google Scholar] [CrossRef]
- Sapienza, G.; Castronovo, M.; Tringali, S.; Bono, R.; Rotolo, C.; Mulè, A.; Calafiore, V.; Patti, C.; Agueli, C.; Randazzo, V.; et al. Sorafenib Maintenance after Allogeneic Stem Cell Transplantation in Patients with FLT3+ AML Receiving Midostaurin during Induction and Consolidation: A Retrospective Analysis. Front. Oncol. 2024, 14, 1441254. [Google Scholar] [CrossRef]
- Cilloni, D.; Renneville, A.; Hermitte, F.; Hills, R.K.; Daly, S.; Jovanovic, J.V.; Gottardi, E.; Fava, M.; Schnittger, S.; Weiss, T.; et al. Real-Time Quantitative Polymerase Chain Reaction Detection of Minimal Residual Disease by Standardized WT1 Assay to Enhance Risk Stratification in Acute Myeloid Leukemia: A European LeukemiaNet Study. J. Clin. Oncol. 2009, 27, 5195–5201. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Büchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef]
- Kottaridis, P.D.; Gale, R.E.; Langabeer, S.E.; Frew, M.E.; Bowen, D.T.; Linch, D.C. Studies of FLT3 Mutations in Paired Presentation and Relapse Samples from Patients with Acute Myeloid Leukemia: Implications for the Role of FLT3 Mutations in Leukemogenesis, Minimal Residual Disease Detection, and Possible Therapy with FLT3 Inhibitors. Blood 2002, 100, 2393–2398. [Google Scholar] [CrossRef]
- Levis, M. FLT3 Mutations in Acute Myeloid Leukemia: What Is the Best Approach in 2013? Hematology 2013, 2013, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, F.R.; Gundacker, H.; Head, D.R.; Slovak, M.L.; Willman, C.L.; Godwin, J.E.; Anderson, J.E.; Petersdorf, S.H. Age and Acute Myeloid Leukemia. Blood 2006, 107, 3481–3485. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, S.; Bacher, U.; Kern, W.; Alpermann, T.; Haferlach, C.; Haferlach, T. Prognostic Impact of FLT3-ITD Load in NPM1 Mutated Acute Myeloid Leukemia. Leukemia 2011, 25, 1297–1304. [Google Scholar] [CrossRef]
- Bornhäuser, M.; Illmer, T.; Schaich, M.; Soucek, S.; Ehninger, G.; Thiede, C. Improved Outcome after Stem-Cell Transplantation in FLT3/ITD-Positive AML. Blood 2007, 109, 2264–2265. [Google Scholar] [CrossRef]
- Bazzell, B.G.; Marini, B.L.; Benitez, L.L.; Bixby, D.; Burke, P.; Pettit, K.; Perissinotti, A.J. Real World Use of FLT3 Inhibitors for Treatment of FLT3+ Acute Myeloid Leukemia (AML): A Single Center, Propensity-Score Matched, Retrospective Cohort Study. J. Oncol. Pharm. Pract. 2021, 28, 1315–1325. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Fiedler, W.; Salih, H.R.; Wulf, G.; Thol, F.; Kündgen, A.; Kindler, T.; Salwender, H.-J.; Lübbert, M.; Brossart, P.; et al. Impact of Age and Midostaurin-Dose on Response and Outcome in Acute Myeloid Leukemia with FLT3-ITD: Interim-Analyses of the AMLSG 16-10 Trial. Blood 2016, 128, 449. [Google Scholar] [CrossRef]
- Oñate, G.; Pratcorona, M.; Garrido, A.; Artigas-Baleri, A.; Bataller, A.; Tormo, M.; Arnan, M.; Vives, S.; Coll, R.; Salamero, O.; et al. Survival Improvement of Patients with FLT3 Mutated Acute Myeloid Leukemia: Results from a Prospective 9 Years Cohort. Blood Cancer J. 2023, 13, 69. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, Y.; Kang, D.; Kim, H.S.; Lee, J.-M.; Kim, M.; Cho, B.-S. Prognostic Value of Measurable Residual Disease Monitoring by Next-Generation Sequencing before and after Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia. Blood Cancer J. 2021, 11, 109. [Google Scholar] [CrossRef]
- Levis, M.J.; Hamadani, M.; Logan, B.; Jones, R.J.; Singh, A.K.; Litzow, M.; Wingard, J.R.; Papadopoulos, E.B.; Perl, A.E.; Soiffer, R.J.; et al. Gilteritinib as Post-Transplant Maintenance for AML With Internal Tandem Duplication Mutation of FLT3. J. Clin. Oncol. 2024, 42, 1766–1775. [Google Scholar] [CrossRef]
- Smith, C.C.; Wang, Q.; Chin, C.-S.; Salerno, S.; Damon, L.E.; Levis, M.J.; Perl, A.E.; Travers, K.J.; Wang, S.; Hunt, J.P.; et al. Validation of ITD Mutations in FLT3 as a Therapeutic Target in Human Acute Myeloid Leukaemia. Nature 2012, 485, 260–263. [Google Scholar] [CrossRef]
- McMahon, C.M.; Ferng, T.; Canaani, J.; Wang, E.S.; Morrissette, J.J.D.; Eastburn, D.J.; Pellegrino, M.; Durruthy-Durruthy, R.; Watt, C.D.; Asthana, S.; et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019, 9, 1050–1063. [Google Scholar] [CrossRef]
- Erba, H.P.; Montesinos, P.; Kim, H.-J.; Patkowska, E.; Vrhovac, R.; Žák, P.; Wang, P.-N.; Mitov, T.; Hanyok, J.; Kamel, Y.M.; et al. Quizartinib plus Chemotherapy in Newly Diagnosed Patients with FLT3-Internal-Tandem-Duplication-Positive Acute Myeloid Leukaemia (QuANTUM-First): A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 2023, 401, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Shaik, N.; Erba, H.P.; Unni, S.; Petersohn, S.; Westerberg, E.; Gorsh, B.; Nahar, A.; Burns, K.; Kroep, S. Anchored Matching Adjusted Indirect Treatment Comparison of Quizartinib Vs Midostaurin in Newly Diagnosed Patients with FLT3-ITD-Positive Acute Myeloid Leukaemia. Blood 2024, 144, 1509. [Google Scholar] [CrossRef]
- Lee, M.H.; McCall, W.; Cwykiel, J.; Lin, C.; Madarang, E.; Watts, J.M.; Erba, H.P.; Abaza, Y.; Perl, A.E.; Matthews, A.H.; et al. Real-World Treatment Patterns and Effectiveness of Midostaurin Versus Quizartinib in FLT3-ITD Mutated Acute Myeloid Leukemia Undergoing Intensive Induction. Blood 2024, 144, 4282. [Google Scholar] [CrossRef]
| 37 Patients | Low-AR (n. 24) | High-AR (n. 13) | ||
|---|---|---|---|---|
| Karyotype (3 failed) | 1 low risk t(8;21) 2 clonal aberrations (intermediate) 30 normal 1 complex | 18 normal 1 complex 1clonal aberration 1 low risk | 12 normal 1 clonal aberration | p = 0.39 |
| NPM1 mutated (%) | 25/37 (67.6%) | 16/24 (66.7%) | 9/13 (69.2%) | p > 0.99 |
| Average Hb g/dL (±SD) | 8.7 (±1.8) | 8.3 (±1.9) | 9.4 (±1.5) | p = 0.07 |
| Platelets/µL | 91 × 103 (±76 × 103) | 89 × 103 (±59 × 103) | 94 × 103 (±103 × 103) | p = 0.83 |
| WBC/µL | 85 × 103 (±117 × 103) | 71 × 103 (±106 × 103) | 110 × 103 (±138 × 103) | p = 0.35 |
| Chemotherapy “7+3” (yrs 2013–2018) (%) | 15/37 (40%) | 13/24 (54.2%) | 2/13 (15.3%) | p = 0.035 |
| Chemotherapy “7+3” and midostaurin (yrs 2019–2022) (%) | 22/37 (60%) | 11/24 (45.8%) | 11/13 (84.6%) |
| (A) Overall response (CR and CRi) and AR | |||
| n. pts low-AR (24) | n. pts high-AR (13) | ||
|
Responders 25/37 (67.6%) | 16/24 (66.7%) | 9/13 (69.2%) | p > 0.99 |
|
Not responders 12/37 (32.4%) | 8/24 (33.3%) | 4/13 (30.7%) | |
| (B) Overall response and NPM1 mutation | |||
| NPM1 mutated (25) | Wild type NPM1 (12) | ||
|
Responders 25/37 (67.6%) | 19/25 (76%) | 6/12 (50%) | p = 0.14 |
|
Not responders 12/37 (32.4%) | 6/25 (24%) | 6/12 (50%) | |
| (C) Overall response and NPM1 mutation in low-AR patients | |||
| NPM1 mutated in low-AR pts (16) | Wild-type NPM1 in low-AR pts (8) | ||
|
Responders 16/24 (66.7%) | 11/16 (68.7%) | 5/8 (62.5%) | p > 0.99 |
|
Not responders 8/24 (33.3%) | 5/16 (31.3%) | 3/8 (37.5%) | |
| (D) Overall response and NPM1 mutation in high-AR patients | |||
| NPM1 mutated in high AR (9) | Wild type NPM1 in high AR (4) | ||
|
Responders 9/13 (69.2%) | 8/9 (88.8%) | 1/4 (25%) | p = 0.05 |
|
Not responders 4/13 (30.8%) | 1/9 (11.2%) | 3/4 (75%) | |
| N. pts/Total pts (%) 24/37 (64.9%) | N. pts/Total Low-AR (%) 16/24 (66.6%) | N. pts/Total High-AR (%) 8/13 (61.5%) |
|---|---|---|
| N. cycles of consolidation | 1–2 cycles: 8 pts 3–4 cycles: 8 pts | 1–2 cycles: 4 pts 3–4 cycles: 4 pts |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchio, V.; Duminuco, A.; Leotta, S.; Mauro, E.; Maugeri, C.; Parisi, M.; Fiumara, P.F.; Di Raimondo, F.; Palumbo, G.A.; Gozzo, L.; et al. Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort. J. Clin. Med. 2025, 14, 5110. https://doi.org/10.3390/jcm14145110
Vecchio V, Duminuco A, Leotta S, Mauro E, Maugeri C, Parisi M, Fiumara PF, Di Raimondo F, Palumbo GA, Gozzo L, et al. Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort. Journal of Clinical Medicine. 2025; 14(14):5110. https://doi.org/10.3390/jcm14145110
Chicago/Turabian StyleVecchio, Veronica, Andrea Duminuco, Salvatore Leotta, Elisa Mauro, Cinzia Maugeri, Marina Parisi, Paolo Fabio Fiumara, Francesco Di Raimondo, Giuseppe A. Palumbo, Lucia Gozzo, and et al. 2025. "Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort" Journal of Clinical Medicine 14, no. 14: 5110. https://doi.org/10.3390/jcm14145110
APA StyleVecchio, V., Duminuco, A., Leotta, S., Mauro, E., Maugeri, C., Parisi, M., Fiumara, P. F., Di Raimondo, F., Palumbo, G. A., Gozzo, L., Palumbo, F. E., & Vetro, C. (2025). Real-World Outcomes in FLT3-ITD Mutated Acute Myeloid Leukemia: Impact of NPM1 Mutations and Allogeneic Transplantation in a Retrospective Unicentric Cohort. Journal of Clinical Medicine, 14(14), 5110. https://doi.org/10.3390/jcm14145110








