Impact of Obstructive Sleep Apnea in Surgical Patients: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
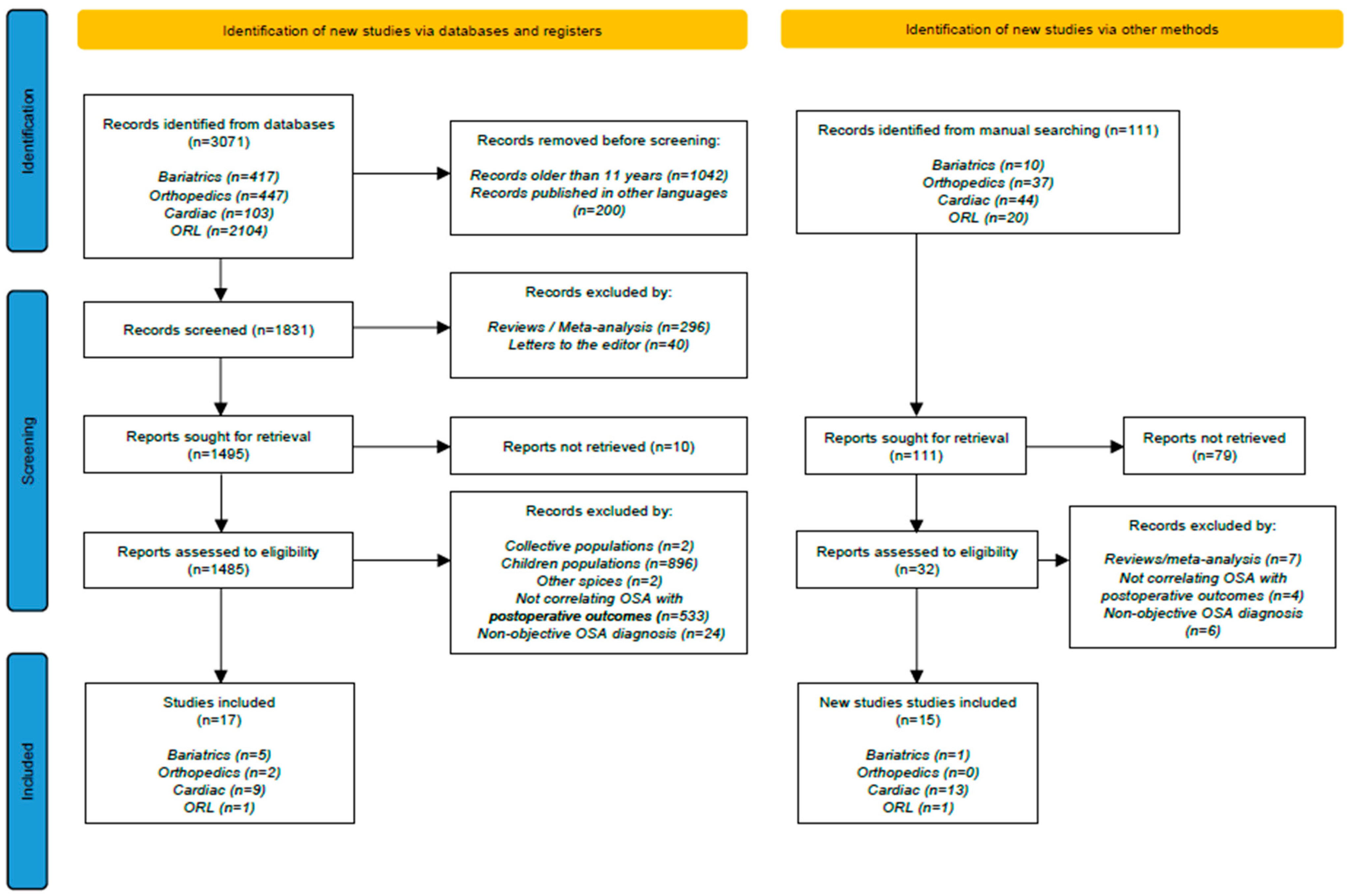
2.1. Literature Research
2.2. Study Selection Criteria
2.3. Synthesis Methods
2.4. Risk of Bias and Reporting Bias Assessment
2.5. Certainty Assessment
3. Results
3.1. Perioperative Risks and Outcomes of OSA in Bariatric Surgery
3.2. Perioperative Risks and Outcomes of OSA in Orthopedic Surgery
3.3. Perioperative Risks and Outcomes of OSA in Cardiac Surgery
3.4. Perioperative Risks and Outcomes of OSA in Otorhinolaryngologic Surgery
4. Discussion
4.1. Is Obstructive Sleep Apnea a Risk Factor for the Surgical Patient?
4.2. Does Obstructive Sleep Apnea Have a Significant Incidence in Surgical Populations to Request a Preoperative Screening?
4.3. Does CPAP Have a Role in Postoperative Complications Incidence in Patients with Obstructive Sleep Apnea?
4.4. Are There Validated Protocols for the Perioperative Management of Surgical Patients with OSA?
4.5. What Is the Cost-Effectiveness of Managing OSA in Surgical Patients?
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of sleep apnea. Physiol. Rev. 2010, 90, 47–112, Erratum in Physiol. Rev. 2010, 90, 797–798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drager, L.F.; Togeiro, S.M.; Polotsky, V.Y.; Lorenzi-Filho, G. Obstructive sleep apnea: A cardiometabolic risk in obesity and the metabolic syndrome. J. Am. Coll. Cardiol. 2013, 62, 569–576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.L.; Moon, T.S.; Schumann, R. Bariatric surgery in patients with obstructive sleep apnea. Int. Anesthesiol. Clin. 2022, 60, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N.E.; Farrag, N.S.; Zaki, N.F.W.; Badawy, A.Y.; Abdelhafez, S.A.; El-Gilany, A.H.; El Shafey, M.M.; Pandi-Perumal, S.R.; Spence, D.W.; BaHammam, A.S. Obstructive sleep apnea: Personal, societal, public health, and legal implications. Rev. Environ. Health 2019, 34, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Waldman, L.T.; Parthasarathy, S.; Villa, K.F.; Bron, M.; Bujanover, S.; Brod, M. Understanding the burden of illness of excessive daytime sleepiness associated with obstructive sleep apnea: A qualitative study. Health Qual. Life Outcomes 2020, 18, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Udholm, N.; Rex, C.E.; Fuglsang, M.; Lundbye-Christensen, S.; Bille, J.; Udholm, S. Obstructive sleep apnea and road traffic accidents: A Danish nationwide cohort study. Sleep Med. 2022, 96, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav. Sleep Med. 2020, 18, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.C.; Borzan, C.; Vesa, C.S.; Todea, D.A. Obstructive sleep apnea syndrome and the quality of life. Clujul. Med. 2016, 89, 390–395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, M.; Nagappa, M.; Guluzade, N.; Saripella, A.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire as a preoperative screening tool for obstructive sleep apnea: A systematic review and meta-analysis. BMC Anesthesiol. 2022, 22, 366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pivetta, B.; Nagappa, M.; Saripella, A.; Islam, S.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire for screening of obstructive sleep apnea in the general population and commercial drivers: A systematic review and meta-analysis. Sleep Breath. 2021, 25, 1741–1751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smith, B.; Phillips, B.A. Truckers drive their own assessment for obstructive sleep apnea: A collaborative approach to online self-assessment for obstructive sleep apnea. J. Clin. Sleep Med. 2011, 7, 241–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veugen, C.C.A.F.M.; Teunissen, E.M.; den Otter, L.A.S.; Kos, M.P.; Stokroos, R.J.; Copper, M.P. Prediction of obstructive sleep apnea: Comparative performance of three screening instruments on the apnea-hypopnea index and the oxygen desaturation index. Sleep Breath. 2021, 25, 1267–1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenthal, L.D.; Dolan, D.C. The Epworth sleepiness scale in the identification of obstructive sleep apnea. J. Nerv. Ment. Dis. 2008, 196, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Osman, E.Z.; Osborne, J.; Hill, P.D.; Lee, B.W. The Epworth Sleepiness Scale: Can it be used for sleep apnoea screening among snorers? Clin. Otolaryngol. Allied Sci. 1999, 24, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Kiciński, P.; Przybylska-Kuć, S.M.; Tatara, K.; Dybała, A.; Zakrzewski, M.; Mysliński, W.; Mosiewicz, J.; Jaroszyński, A.J. Reliability of the Epworth Sleepiness Scale and the Berlin Questionnaire for screening obstructive sleep apnea syndrome in the context of the examination of candidates for drivers. Med. Pr. 2016, 67, 721–728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Małolepsza, A.; Kudrycka, A.; Karwowska, U.; Hoshino, T.; Wibowo, E.; Pál Böjti, P.; Białas, A.; Kuczyński, W. The role of screening questionnaires in the assessment of risk and severity of obstructive sleep apnea—Polysomnography versus polygraphy. Adv. Respir. Med. 2021, 89, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Gozal, D.; Ramirez, H.M.; Bandla, H.P.; Kheirandish-Gozal, L. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep 2014, 37, 255–260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ioan, I.; Renard, E.; Da Mota, S.; Bonabel, C.; Tiotiu, A.; Franco, P.; Coutier, L.; Schweitzer, C. Unattended home sleep studies for the diagnosis of obstructive sleep apnea in a population of French children. Sleep Med. 2023, 102, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Asghari, A.; Mohammadi, F. Is Apnea-Hypopnea Index a proper measure for Obstructive Sleep Apnea severity? Med. J. Islam. Repub. Iran. 2013, 27, 161–162. [Google Scholar] [PubMed] [PubMed Central]
- Vasu, T.S.; Doghramji, K.; Cavallazzi, R.; Grewal, R.; Hirani, A.; Leiby, B.; Markov, D.; Reiter, D.; Kraft, W.K.; Witkowski, T. Obstructive sleep apnea syndrome and postoperative complications: Clinical use of the STOP-BANG questionnaire. Arch. Otolaryngol. Head. Neck Surg. 2010, 136, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Ravesloot, M.J.; van Maanen, J.P.; Hilgevoord, A.A.; van Wagensveld, B.A.; de Vries, N. Obstructive sleep apnea is underrecognized and underdiagnosed in patients undergoing bariatric surgery. Eur. Arch. Otorhinolaryngol. 2012, 269, 1865–1871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Opperer, M.; Cozowicz, C.; Bugada, D.; Mokhlesi, B.; Kaw, R.; Auckley, D.; Chung, F.; Memtsoudis, S.G. Does Obstructive Sleep Apnea Influence Perioperative Outcome? A Qualitative Systematic Review for the Society of Anesthesia and Sleep Medicine Task Force on Preoperative Preparation of Patients with Sleep-Disordered Breathing. Anesth. Analg. 2016, 122, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Fouladpour, N.; Jesudoss, R.; Bolden, N.; Shaman, Z.; Auckley, D. Perioperative Complications in Obstructive Sleep Apnea Patients Undergoing Surgery: A Review of the Legal Literature. Anesth Analg. 2016, 122, 145–151, Erratum in Anesth. Analg. 2016, 122, 1228–1229. [Google Scholar] [CrossRef] [PubMed]
- American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: An updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 2014, 120, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Borsoi, L.; Armeni, P.; Donin, G.; Costa, F.; Ferini-Strambi, L. The invisible costs of obstructive sleep apnea (OSA): Systematic review and cost-of-illness analysis. PLoS ONE 2022, 17, e0268677. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Oxford: Oxford Centre for Evidence-Based Medicine; 2011. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 3 May 2025).
- Zaremba, S.; Shin, C.H.; Hutter, M.M.; Malviya, S.A.; Grabitz, S.D.; MacDonald, T.; Diaz-Gil, D.; Ramachandran, S.K.; Hess, D.; Malhotra, A.; et al. Continuous Positive Airway Pressure Mitigates Opioid-induced Worsening of Sleep-disordered Breathing Early after Bariatric Surgery. Anesthesiology 2016, 125, 92–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, W.T.; Chopra, S.; Kopf, M.; Morales, C.; Khan, S.; Zuccala, K.; Choi, L.; Chronakos, J. Perioperative Risks of Untreated Obstructive Sleep Apnea in the Bariatric Surgery Patient: A Retrospective Study. Obes. Surg. 2016, 26, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Goucham, A.B.; Coblijn, U.K.; Hart-Sweet, H.B.; de Vries, N.; Lagarde, S.M.; van Wagensveld, B.A. Routine Postoperative Monitoring after Bariatric Surgery in Morbidly Obese Patients with Severe Obstructive Sleep Apnea: ICU Admission is not Necessary. Obes. Surg. 2016, 26, 737–742. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.; Doherty, L.; O’Boyle, C. How Relevant Is Pre-operative Obstructive Sleep Apnoea in the Asymptomatic Bariatric Surgery Patient? Obes. Surg. 2020, 30, 969–974. [Google Scholar] [CrossRef] [PubMed]
- de Raaff, C.A.; Bindt, D.M.; de Vries, N.; van Wagensveld, B.A. Positional obstructive sleep apnea in bariatric surgery patients: Risk factor for postoperative cardiopulmonary complications? Sleep Breath. 2016, 20, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mokhlesi, B.; Hovda, M.D.; Vekhter, B.; Arora, V.M.; Chung, F.; Meltzer, D.O. Sleep-disordered breathing and postoperative outcomes after bariatric surgery: Analysis of the nationwide inpatient sample. Obes. Surg. 2013, 23, 1842–1851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, J.; Doherty, H.R.; Singh, M.; Choi, S.; Siddiqui, N.; Lam, D.; Liyanage, N.; Tomlinson, G.; Chung, F. The prevention of delirium in elderly surgical patients with obstructive sleep apnea (PODESA): A randomized controlled trial. BMC Anesthesiol. 2022, 22, 290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bai, J.W.; Singh, M.; Short, A.; Bozak, D.; Chung, F.; Chan, V.W.S.; Bhatia, A.; Perlas, A. Intrathecal Morphine and Pulmonary Complications after Arthroplasty in Patients with Obstructive Sleep Apnea: A Retrospective Cohort Study. Anesthesiology 2020, 132, 702–712. [Google Scholar] [CrossRef] [PubMed]
- van Oosten, E.M.; Hamilton, A.; Petsikas, D.; Payne, D.; Redfearn, D.P.; Zhang, S.; Hopman, W.M.; Baranchuk, A. Effect of preoperative obstructive sleep apnea on the frequency of atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Fein, A.S.; Shvilkin, A.; Shah, D.; Haffajee, C.I.; Das, S.; Kumar, K.; Kramer, D.B.; Zimetbaum, P.J.; Buxton, A.E.; Josephson, M.E.; et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J. Am. Coll. Cardiol. 2013, 62, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Uchôa, C.H.G.; Danzi-Soares, N.J.; Nunes, F.S.; de Souza, A.A.L.; Nerbass, F.B.; Pedrosa, R.P.; César, L.A.M.; Lorenzi-Filho, G.; Drager, L.F. Impact of OSA on cardiovascular events after coronary artery bypass surgery. Chest 2015, 147, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.P.; Kofidis, T.; Lim, T.W.; Chan, S.P.; Ong, T.H.; Tan, H.C.; Lee, C.H. Sleep apnea is associated with new-onset atrial fibrillation after coronary artery bypass grafting. J. Crit. Care 2015, 30, 1418.e1–1418.e5. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Ni, B.Q.; Wang, H.; Ding, W.X.; Xue, R.; Lin, W.; Kai, Z.; Zhang, S.J.; Zhang, X.L. Obstructive Sleep Apnea Increases the Perioperative Risk of Cardiac Valve Replacement Surgery: A Prospective Single-Center Study. J. Clin. Sleep Med. 2016, 12, 1331–1337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Roggenbach, J.; Klamann, M.; von Haken, R.; Bruckner, T.; Karck, M.; Hofer, S. Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: A prospective cohort study. Crit. Care 2014, 18, 477. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, S.; Ren, C.; Yu, J.; Wei, Z.; Ma, H.; Lai, Y. Obstructive sleep apnea is associated with postoperative dialysis in patients who underwent coronary artery bypass grafting. Ann. Palliat. Med. 2021, 10, 6307–6315. [Google Scholar] [CrossRef] [PubMed]
- Kua, J.; Zhao, L.-P.; Kofidis, T.; Chan, S.-P.; Yeo, T.-C.; Tan, H.-C.; Lee, C.-H. Sleep apnoea is a risk factor for acute kidney injury after coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2016, 49, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Tafelmeier, M.; Weizenegger, T.; Ripfel, S.; Fauser, M.; Floerchinger, B.; Camboni, D.; Zausig, Y.; Wittmann, S.; Drzymalski, M.A.; Zeman, F.; et al. Postoperative complications after elective coronary artery bypass grafting surgery in patients with sleep-disordered breathing. Clin. Res. Cardiol. 2018, 107, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Wolf, C.; Cattermole, T.C.; Rando, H.J.; DeNino, W.F.; Iribarne, A.; Ross, C.S.; Ramkumar, N.; Gelb, D.J.; Bourcier, B.; et al. Cardiac Surgery Outcomes: A Case for Increased Screening and Treatment of Obstructive Sleep Apnea. Ann. Thorac. Surg. 2021, 113, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, S.; Schultze, T.; Nachtmann, A.; Rastan, A.J.; Doenst, T.; Schwab, M.; Witte, O.W.; Rohe, S.; Zwacka, I.; Hoyer, H. Impact of sleep disordered breathing on short-term post-operative outcome after elective coronary artery bypass graft surgery: A prospective observational study. Eur. Respir. J. 2017, 49, 1601486. [Google Scholar] [CrossRef] [PubMed]
- Utriainen, K.T.; Airaksinen, J.K.; Polo, O.; Laitio, R.; Pietilä, M.J.; Scheinin, H.; Vahlberg, T.; Leino, K.A.; Kentala, E.S.; Jalonen, J.R.; et al. Sleep apnoea is associated with major cardiac events in peripheral arterial disease. Eur. Respir. J. 2014, 43, 1652–1660. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lv, S.; Yu, X.; Yao, L.; Mokhlesi, B.; Wei, Y. Treatment of OSA reduces the risk of repeat revascularization after percutaneous coronary intervention. Chest 2015, 147, 708–718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teo, Y.H.; Yong, C.L.; Ou, Y.H.; Tam, W.W.; Teo, Y.N.; Koo, C.Y.; Kojodjojo, P.; Lee, C.H. Obstructive sleep apnea and temporal changes in cardiac repolarization in patients undergoing coronary artery bypass grafting. J. Clin. Sleep Med. 2024, 20, 49–55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.H.; Sethi, R.; Li, R.; Ho, H.H.; Hein, T.; Jim, M.H.; Loo, G.; Koo, C.Y.; Gao, X.F.; Chandra, S.; et al. Obstructive Sleep Apnea and Cardiovascular Events After Percutaneous Coronary Intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Fan, K.; Yu, W.; Liu, H.; Wei, Y.; Yu, Y. The effects of high-sensitivity C-reactive protein on the clinical outcomes in obstructive sleep apnea patients undergoing off-pump coronary artery bypass grafting. BMC Cardiovasc. Disord. 2021, 21, 366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hunt, T.E.; Traaen, G.M.; Aakerøy, L.; Bendz, C.; Øverland, B.; Akre, H.; Steinshamn, S.; Loennechen, J.P.; Hegbom, F.; Broch, K.; et al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: A randomized controlled trial. Heart Rhythm. 2022, 19, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, F.M.; Filipiak, K.J.; Platek, A.E.; Hrynkiewicz-Szymanska, A.; Kotkowski, M.; Kozluk, E.; Kiliszek, M.; Sierdzinski, J.; Opolski, G. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations—A long-term prospective, cross-sectional cohort study. Sleep Breath. 2015, 19, 849–856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neilan, T.G.; Farhad, H.; Dodson, J.A.; Shah, R.V.; Abbasi, S.A.; Bakker, J.P.; Michaud, G.F.; van der Geest, R.; Blankstein, R.; Steigner, M.; et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J. Am. Heart Assoc. 2013, 2, e000421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaw, R.; El Zarif, S.; Wang, L.; Bena, J.; Blackstone, E.H.; Mehra, R. Obesity as an Effect Modifier in Sleep-Disordered Breathing and Postcardiac Surgery Atrial Fibrillation. Chest 2017, 151, 1279–1287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foldvary-Schaefer, N.; Kaw, R.; Collop, N.; Andrews, N.D.; Bena, J.; Wang, L.; Stierer, T.; Gillinov, M.; Tarler, M.; Kayyali, H. Prevalence of Undetected Sleep Apnea in Patients Undergoing Cardiovascular Surgery and Impact on Postoperative Outcomes. J. Clin. Sleep Med. 2015, 11, 1083–1089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Passeri, L.A.; Choi, J.G.; Kaban, L.B.; Lahey, E.T., 3rd. Morbidity Mortality Rates After Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. J. Oral Maxillofac. Surg. 2016, 74, 2033–2043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kandasamy, T.; Wright, E.D.; Fuller, J.; Rotenberg, B.W. The incidence of early post-operative complications following uvulopalatopharyngoplasty: Identification of predictive risk factors. J. Otolaryngol. Head Neck Surg. 2013, 42, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beydoun, H.A.; Beydoun, M.A.; Cheng, H.; Khan, A.; Eid, S.M.; Alvarez-Garriga, C.; Anderson-Smits, C.; Zonderman, A.B.; Marinac-Dabic, D. Complications associated with surgical treatment of sleep-disordered breathing among hospitalized U.S. adults. J. Cranio-Maxillofac. Surg. 2018, 46, 1303–1312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gouveia, C.J.; Cramer, J.D.; Liu, S.Y.; Capasso, R. Sleep Surgery in the Elderly: Lessons from the National Surgical Quality Improvement Program. Otolaryngol. Head Neck Surg. 2017, 156, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Baugh, R.; Burke, B.; Fink, B.; Garcia, R.; Kominsky, A.; Yaremchuk, K. Safety of outpatient surgery for obstructive sleep apnea. Otolaryngol. Head Neck Surg. 2013, 148, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Dalmar, A.; Singh, M.; Pandey, B.; Stoming, C.; Heis, Z.; Ammar, K.A.; Jan, M.F.; Choudhuri, I.; Chua, T.Y.; Sra, J.; et al. The beneficial effect of weight reduction on adverse cardiovascular outcomes following bariatric surgery is attenuated in patients with obstructive sleep apnea. Sleep 2018, 41, zsy028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dalmar, A.; Singh, M.; Heis, Z.; Cumpian, T.L.; Ceretto, C.; Mortada, M.E.; Bhatia, A.; Niazi, I.; Chua, T.Y.; Sra, J.; et al. Risk of Atrial Fibrillation and Stroke After Bariatric Surgery in Patients with Morbid Obesity with or without Obstructive Sleep Apnea. Stroke 2021, 52, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.B., 2nd; Cancienne, J.M.; Werner, B.C. Obstructive sleep apnea affects complication rates following knee arthroscopy but use of continuous positive airway pressure is not protective against complications. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Cancienne, J.M.; Brockmeier, S.F.; Deasey, M.J.; Werner, B.C. Obstructive Sleep Apnea and Arthroscopic Rotator Cuff Repair-Are Complication Rates Really Increased? J. Am. Acad. Orthop. Surg. 2019, 27, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Dubin, J.A.; Bains, S.S.; Hameed, D.; Chen, Z.; Mayassi, H.A.; Nace, J.; Delanois, R.E. The use of preoperative continuous positive airway pressure in patients with obstructive sleep apnea following total knee arthroplasty: A propensity score matched analysis. Arch. Orthop. Trauma. Surg. 2024, 144, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Cozowicz, C.; Poeran, J.; Olson, A.; Mazumdar, M.; Mörwald, E.E.; Memtsoudis, S.G. Trends in Perioperative Practice and Resource Utilization in Patients with Obstructive Sleep Apnea Undergoing Joint Arthroplasty. Anesth. Analg. 2017, 125, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.Y.; Rabiei, A.H.; Maltenfort, M.G.; Restrepo, C.; Viscusi, E.R.; Parvizi, J.; Rasouli, M.R. Perioperative Complications in Patients with Sleep Apnea Undergoing Total Joint Arthroplasty. J. Arthroplast. 2017, 32, 2680–2683. [Google Scholar] [CrossRef] [PubMed]
- Pichler, L.; Weinstein, S.M.; Cozowicz, C.; Poeran, J.; Liu, J.; Poultsides, L.A.; Saleh, J.N.; Memtsoudis, S.G. Perioperative impact of sleep apnea in a high-volume specialty practice with a strong focus on regional anesthesia: A database analysis. Reg. Anesth. Pain Med. 2019, 44, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Wortham, T.C.; Rice, A.N.; Gupta, D.K.; Goode, V. Implementation of an Obstructive Sleep Apnea Protocol in the Postanesthesia Care Unit for Patients Undergoing Spinal Fusion Surgery. J. Perianesth. Nurs. 2019, 34, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Jules-Elysée, K.M.; Desai, N.A.; Ma, Y.; Zhang, W.; Luu, T.H.; Memtsoudis, S.G.; Liguori, G.A. Clinical Indicators of the Need for Telemetry Postoperative Monitoring in Patients With Suspected Obstructive Sleep Apnea Undergoing Total Knee Arthroplasty. Reg. Anesth. Pain Med. 2018, 43, 43–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, F.; Memtsoudis, S.G.; Ramachandran, S.K.; Nagappa, M.; Opperer, M.; Cozowicz, C.; Patrawala, S.; Lam, D.; Kumar, A.; Joshi, G.P.; et al. Society of Anesthesia and Sleep Medicine Guidelines on Preoperative Screening and Assessment of Adult Patients with Obstructive Sleep Apnea. Anesth. Analg. 2016, 123, 452–473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nadler, J.W.; Evans, J.L.; Fang, E.; Preud’Homme, X.A.; Daughtry, R.L.; Chapman, J.B.; Bolognesi, M.P.; Attarian, D.E.; Wellman, S.S.; Krystal, A.D. A randomised trial of peri-operative positive airway pressure for postoperative delirium in patients at risk for obstructive sleep apnoea after regional anaesthesia with sedation or general anaesthesia for joint arthroplasty. Anaesthesia 2017, 72, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Meurgey, J.H.; Brown, R.; Woroszyl-Chrusciel, A.; Steier, J. Peri-operative treatment of sleep-disordered breathing and outcomes in bariatric patients. J. Thorac. Dis. 2018, 10 (Suppl. S1), S144–S152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarasiuk, A.; Reuveni, H. The economic impact of obstructive sleep apnea. Curr. Opin. Pulm. Med. 2013, 19, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Mutter, T.C.; Chateau, D.; Moffatt, M.; Ramsey, C.; Roos, L.L.; Kryger, M. A matched cohort study of postoperative outcomes in obstructive sleep apnea: Could preoperative diagnosis and treatment prevent complications? Anesthesiology 2014, 121, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, R.M.; Cohen-Levy, W.B.; Vakharia, A.M.; Donnally CJ3rd Law, T.Y.; Roche, M.W. Sleep Apnea Increases Ninety-Day Complications and Cost Following Primary Total Joint Arthroplasty. J. Arthroplast. 2019, 34, 959–964.e1. [Google Scholar] [CrossRef] [PubMed]
- Memtsoudis, S.G.; Stundner, O.; Rasul, R.; Sun, X.; Chiu, Y.L.; Fleischut, P.; Danninger, T.; Mazumdar, M. Sleep apnea and total joint arthroplasty under various types of anesthesia: A population-based study of perioperative outcomes. Reg. Anesth. Pain Med. 2013, 38, 274–281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mörwald, E.E.; Olson, A.; Cozowicz, C.; Poeran, J.; Mazumdar, M.; Memtsoudis, S.G. Association of opioid prescription and perioperative complications in obstructive sleep apnea patients undergoing total joint arthroplasties. Sleep Breath. 2018, 22, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.; Marques, S.; Huang, A.; De Moraes, T.P.; Previdelli, I.; Cruz, J.A.W.; Al-Sayed, A.A.; Capasso, R. Value of Surgical and Nonsurgical Treatment for Sleep Apnea: A Closer Look at Health Care Utilization. Otolaryngol. Head Neck Surg. 2023, 168, 1228–1237. [Google Scholar] [CrossRef] [PubMed]
- Kempfle, J.S.; BuSaba, N.Y.; Dobrowski, J.M.; Westover, M.B.; Bianchi, M.T. A cost-effectiveness analysis of nasal surgery to increase continuous positive airway pressure adherence in sleep apnea patients with nasal obstruction. Laryngoscope 2017, 127, 977–983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, J.H. Cost-Effectiveness Analysis of Uvulopalatopharyngoplasty Versus Positive Airway Pressure in Patient eith Obstructive Sleep Apnea in South Korea. J. Rhinol. 2023, 30, 15–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Inclusion and Exclusion Criteria |
|---|
| Inclusion Criteria Studies involving adult surgical patients (≥18 years) with confirmed OSA through RP or PSG. Studies published in English. Longitudinal study designs include retrospective and prospective cohort studies, as well as randomized controlled trials (RCTs) and randomized crossover trials. |
| Exclusion Criteria Studies with clinically suspected OSA. Studies involving pediatric patients or pregnant women. Articles published as abstracts, conference proceedings, book chapters, letters to the editor, reviews, or meta-analyses. Animal studies or studies involving in vitro models. Studies involving collective populations where outcomes specific to OSA patients were not clearly distinguishable. Studies that did not explicitly mention OSA or its correlation with postoperative outcomes. |
| Author | Design | Type of Surgery | Sample Size | OSA Diagnosis | Effect Size | Effect Type | Complications | Significance | Oxford Level of Evidence | Grade of Recommendation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OSA | Non-OSA | ||||||||||
| O’Reilly et al., 2020 [33] | RS | bariatric surgery | 300 | 210 | RP/PSG | Logistic regression | Aspiration, atelectasis, pneumonia, hypoxia, respiratory failure, atrial fibrillation, ICU admission, persistent significant OSA | Pulmonary complication: p = 0.117 Cardiac complication: p = 0.607 Readmission: p = 0.933 | C | 4 | |
| Zaremba et al., 2016 [30] | RCT | laparoscopic Roux-en-Y gastric bypass, laparoscopic partial vertical gastrectomy, laparoscopic sleeve, or revision of the gastric band to gastric bypass | 16 | 29 | PSG | CPAP reduced AHI and ODI by 69% (95% CI 7.3–29.8) | Logistic regression | Sleep apnea during daytime Respiratory depression from opioids | p = 0.002 | B | 2b |
| Kong et al., 2016 [31] | RS | laparoscopic gastric bypass, laparoscopic gastric banding, sleeve gastrectomy, open gastric bypass, retrocolic retrogastric gastrojejunostomy. | 352 | 196 | PSG | Pulmonary complications: OR 5.76 All causes complications: OR 1.88 | Logistic regression | Non-CPAP group: higher rate of pneumonia, atelectasis, hypoxemia, pneumomediastinum CPAP-group: Lower rate of pneumonia, atelectasis, pneumonitis | Pulmonary complications: p = 0.0002 All causes complications: p = 0.21 | B | 2b |
| de Raaff et al., 2015 [34] | RS | laparoscopic Roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy | 277 | 1254 | RP/PSG | OR 0.401 | Logistic regression | Respiratory insufficiency and cardiac asthma, pneumonia, sinus tachycardia | p = 0.589 | B | 2b |
| Goucham et al., 2015 [32] | PS | Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, or laparoscopic sleeve gastrectomy | 121 | 794 | PSG | Logistic regression | Desaturations | p = 0.023 for BMI ≥ 60 and severe desaturation < 85% | B | 2b | |
| Mokhlesi et al., 2013 [35] | RS | bariatric surgery | 33,196 | 57,832 | RP/PSG | Emergent endotracheal intubation and mechanical ventilation: OR 4.35, 95% CI 3.97–4.77 CPAP/NIV: OR 14.12, 95% CI 12.09–16.51 AF: OR 1.25, 95% CI 1.11–1.41 | Logistic regression | Emergent endotracheal intubation and mechanical ventilation, CPAP/NIV, and AF | p < 0.001 | B | 2b |
| Author | Design | Type of Surgery | Sample Size | OSA Diagnosis | Effect Size | Effect Type | Complications | Significance | Oxford Level of Evidence | Grade of Recommendation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OSA | Non-OSA | ||||||||||
| Wong et al., 2022 [36] | RCT | hip or knee arthroplasty | 234 | 240 | home sleep apnea test (HSAT) | risk reduction 3.4%; 95% CI: −1.1% to 8.7% | Logistic regression | Delirium | p = 0.21 | B | 2b |
| Bai et al., 2020 [37] | RS | elective total hip or knee arthroplasty | 1326 | 1031 | STOP-BANG questionnaire/PSG | AOR 0.60, 95% CI, 0.24–1.67 | Logistic regression | Respiratory depression, ICU readmission | p = 0.308 | B | 2b |
| Author | Design | Type of Surgery | Sample Size | OSA Diagnosis | Effect Size | Effect Type | Complications | Significance | Oxford Level of Evidence | Grade of Recommendation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OSA | Non-OSA | ||||||||||
| Teo et al., 2024 [51] | RS | CABG | 513 | 494 | RP | HR 0.997, 95% CI: 0.994–1.000 | ΔQTc change | MACCEs | p = 0.032 | B | 2b |
| Wolf et al., 2021 [47] | RS | CABG, isolated valve surgery or CABG with valve surgery | 1555 | 10,450 | PSG | PE: OR 2.89, 95% CI 1.11–7.52 Pneumonia: OR 1.51, 95% CI 1.14–2.02 ICU readmission: OR 1.49, 95% CI 1.17–1.90 Surgical site infection: OR 2.04, 95% CI 1.38–3.02 Renal failure: OR 1.57, 95% CI 1.09–2.27 | Logistic regression | PE, pneumonia, ICU readmission, surgical site infection, renal failure, delayed extubation | PE: p = 0.03 Pneumonia: p = 0.005 ICU readmission: p = 0.001 Surgical site infection: p < 0.001 Renal failure: p = 0.015 | B | 2b |
| Hunt et al., 2022 [54] | RCTs open-label parallel-group | Cryoballoon PVI, RF ablation | 243 | 336 | RP | OR 1.0, 95% CI 0.42–2.4 | Logistic regression | AF, pericardical tamponade, cerebral ischemic event | AF: p = 1.00 | B | 2b |
| Guo et al., 2021 [44] | PS | CABG | 142 | 36 | PSG | eGFR: OR 0.94, 95% CI 0.89–0.99 AHI: OR 1.07, 95% CI 1.01–1.13 | Logistic regression | AKI requiring dialysis, prolonged postoperative ventilation time | AKI requiring dialysis: p = 0.02 Prolonged postoperative ventilation time: p = 0.05 | B | 2b |
| Tafelmeier et al., 2018 [46] | RS | CABG | 23 | 77 | RP | Prolonged LOS > 9 days: OR 3.34, 95% CI 1.24–9.01 Prolonged need of vasopressors > 48 h: OR 2.94, 95% CI 1.05–8.23 | Logistic regression | Prolonged LOS > 9 days, prolonged need of vasopressors > 48 h, tracheostomy requirement | Prolonged LOS > 9 days: p = 0.017 Prolonged need of vasopressors > 48 h: p = 0.04 Tracheostomy: p = 0.028 | B | 2b |
| Kaw et al., 2017 [57] | RS | CABG and/or valve replacement | 132 | 58 | PSG | BMI > 32 kg/m2 had 15% increased odds of AF: OR = 1.15, 95% CI 1.05–1.26 | Logistic regression | AF | p < 0.003 | B | 2b |
| Rupprecht et al., 2017 [48] | PS | CABG | 151 | 68 | RP | Respiratory complications: OR 2.40, 95% CI 1.15–4.97 Cardiac complications: OR 1.75, 95% CI 0.93–3.27 Higher risk of sepsis: OR 2.24, 95% CI 0.71–7.02 | Logistic regression | Higher 30-day mortality, nonspecific desaturation events, acute hypoxemia due to pneumonia, sepsis and septic shock, supraventricular arrhythmias, sopor and coma, AKI | Respiratory complications: p = 0.02 Cardiac complications: p = 0.08 Higher risk of sepsis: p = 0.17 | B | 2b |
| Ding et al., 2016 [42] | PS | Valve replacement | 54 | 236 | PSG | Longer ICU stay: OR 2.318, 95% CI 1.241–4.329 Mechanical ventilation: OR 2.050, 95% CI 1.028–4.085 Pacemaker use: OR 2.477, 95% CI 1.196–5.131 | Logistic regression | Longer ICU stay, prolonged mechanical ventilation time (≥20 h), respiratory insufficiency, and pacemaker requirement | Longer ICU stay: p = 0.008 Mechanical ventilation: p = 0.041 Pacemaker use: p = 0.015 | B | 2b |
| Lee et al., 2016 [52] | PS | PCI | 594 | 717 | RP | HR 1.57, 95% CI 1.10–2.24 | Logistic regression | MACCEs | p = 0.013 | B | 2b |
| Kua et al., 2016 [45] | PS | CABG | 75 | 75 | Sleep study | OR 2.89, 95% CI 1.09–7.09 | Logistic regression | AKI | p = 0.03 | B | 2b |
| Uchôa et al., 2015 [40] | PS | CABG | 37 | 30 | PSG | MACCEs: OR 4.10 95% CI 1.94–385.24 New revascularization: OR 2.02 95% CI 1.21–64.22 Typical angina: OR 10.05 95% CI 1.12–62.25 AF: OR 12.56 95% CI 1.44–159.21 | Logistic regression | MACCEs, new revascularization, typical angina and AF | MACCEs: p = 0.004 New revascularization: p = 0.01 Typical angina: p = 0.02 AF: p = 0.006 | B | 2b |
| Szymanski et al., 2015 [55] | PS | catheter ablation of AF | 114 | 137 | RP | OR 2.58 95% CI 1.52–4.38 | Logistic regression | AF recurrence | p < 0.0001 | B | 2b |
| Zhao et al., 2015 [41] | PS | CABG | 69 | 69 | PSG | OR 4.63 95% CI: 1.24–17.31 | Logistic regression | Readmissions due to cardiovascular events | p = 0.023 | B | 2b |
| Wu et al., 2015 [50] | RS | PCI | 390 | RP/PSG | HR 2.13 95% CI 1.19–3.81 | Logistic regression | Repeat revascularization | p = 0.011 | B | 2b | |
| Foldvary-Schaefer et al., 2015 [58] | RS | CABG, single valve repair/replacement, CABG and single valve repair/re-placement or >2 valve repair/replacement, or others, including septal myomectomy, right atrial mass removal, cardiac catheterization, and cardioverter defibrillator placement. | 51 | 56 | PSG | Logistic regression | OR tube time, total tube time, ICU LOS, ICU readmission, insulin infusion in ICU, readmission by 30 days, prolonged intubation, respiratory failure, reintubation, hypoxemia, tracheostomy, myocardial infarction, arrhythmia, encephalopathy, infection, death | p > 0.05 | B | 2b | |
| Utriainen et al., 2014 [49] | PS | elective sub-inguinal revascularisation | 39 | 45 | PSG | HR 4.4 95% CI 1.8–10.6 | Logistic regression | MACCEs | p = 0.001 | B | 2b |
| Roggenbach et al., 2014 [43] | PS | elective coronary artery surgery or heart valve replacement/repair, either with or without coronary bypass grafting | 83 | 9 | RP | OR 6.4 95% CI 2.6–15.4 | Logistic regression | Delirium | p < 0.001 | B | 2b |
| van Oosten et al., 2014 [38] | PS | CABG | 132 | 145 | modified Berlin questionnaire/PSG | AF: OR 2.18 95% CI 1.30–3.65 | Logistic regression | AF, reintubation, postoperative atrial flutter, other postoperative arrhythmias, LOS | AF: p = 0.003 | B | 2b |
| Neilan et al., 2013 [56] | PS | PVI | 142 | 578 | RP | HR 1.61, 95% CI 1.35–1.92 | Logistic regression | AF recurrence | p < 0.001 | B | 2b |
| Fein et al., 2013 [39] | RS | PVI | 62 | 324 | PSG | PVI(+)OSA(+)CPAP(+): HR 0.7 95% CI 0.3–1.59 PVI(+)OSA(+)CPAP(−): HR 2.15 95% CI 1.10–5.44 | Logistic regression | AF recurrence | PVI(+)OSA(+)CPAP(+): p = 0.39 PVI(+)OSA(+)CPAP(−): p = 0.02 | B | 2b |
| Author | Design | Type of Surgery | Sample Size | OSA Diagnosis | Effect Size | Effect Type | Complications | Significance | Oxford Level of Evidence | Grade of Recommendation | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OSA | Non-OSA | ||||||||||
| Passeri LA et al., 2016 [59] | RS | single piece Le Fort I osteotomy, bilateral sagittal split mandibular osteotomies and either a genial tubercle advancement or genioplasty | 28 | 26 | PSG | RR of complications OSA vs. DFD = 3.04 RR for major complications = 10.75 | Descriptive & comparative stats | Dysesthesia, infection, hardware removal, reoperation | p = 0.003 | B | 2b |
| Kandasamy T et al., 2013 [60] | RS | standard Fujita type UPPP (with or without tonsillectomy) is performed with cautery | 345 | PSG | AHI ≥ 22: OR 2.21, 95% CI 1.166–4.188 BMI ≥ 30: OR 2.70, 95% CI 1.48–4.91 both an AHI ≥ 22 and a BMI ≥ 30: OR 3.48, 95% CI 1.56–7.78 | Logistic regression | Oxyhemoglobin desaturation | p < 0.05 | B | 2b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titu, I.-M.; Vulturar, D.M.; Chis, A.F.; Oprea, A.; Manea, A.; Todea, D.A. Impact of Obstructive Sleep Apnea in Surgical Patients: A Systematic Review. J. Clin. Med. 2025, 14, 5095. https://doi.org/10.3390/jcm14145095
Titu I-M, Vulturar DM, Chis AF, Oprea A, Manea A, Todea DA. Impact of Obstructive Sleep Apnea in Surgical Patients: A Systematic Review. Journal of Clinical Medicine. 2025; 14(14):5095. https://doi.org/10.3390/jcm14145095
Chicago/Turabian StyleTitu, Ioana-Medeea, Damiana Maria Vulturar, Ana Florica Chis, Alexandru Oprea, Alexandru Manea, and Doina Adina Todea. 2025. "Impact of Obstructive Sleep Apnea in Surgical Patients: A Systematic Review" Journal of Clinical Medicine 14, no. 14: 5095. https://doi.org/10.3390/jcm14145095
APA StyleTitu, I.-M., Vulturar, D. M., Chis, A. F., Oprea, A., Manea, A., & Todea, D. A. (2025). Impact of Obstructive Sleep Apnea in Surgical Patients: A Systematic Review. Journal of Clinical Medicine, 14(14), 5095. https://doi.org/10.3390/jcm14145095







