Structure of Comorbidities and Causes of Death in Patients with Atrial Fibrillation and Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
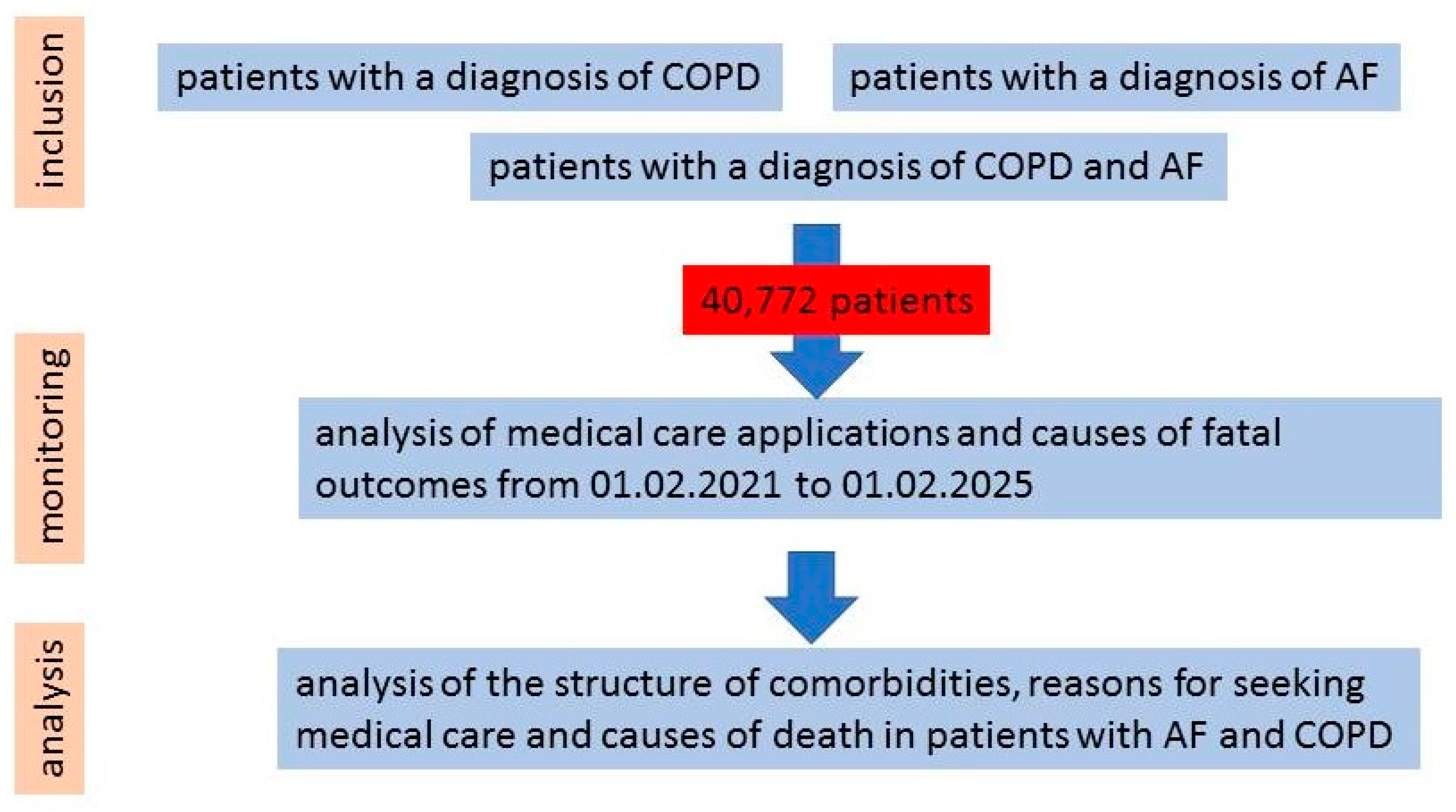
2. Materials and Methods
- -
- Patients older than 18 years of age;
- -
- Confirmed diagnoses of AF and/or COPD, according to current clinical guidelines.
- -
- Absence of a confirmed diagnosis of AF or COPD.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AH | Arterial hypertension |
| ARVI | Acute respiratory viral infection |
| CHD | Chronic ischemic heart disease |
| CHF | Chronic heart failure |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cerebrovascular disease |
| DCMP | Dilated cardiomyopathy |
| DM | Diabetes mellitus |
| ICD | International Classification of Diseases |
| ICH | Intracerebral hemorrhage |
| IS | Ischemic stroke |
| MI | Myocardial infarction |
| PAD | Peripheral artery disease |
| PE | Pulmonary embolism |
References
- May, S.M.; Li, J.T.C. Burden of Chronic Obstructive Pulmonary Disease: Healthcare Costs and Beyond. Allergy Asthma Proc. 2015, 36, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Ahmadian Heris, J.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.-A.; et al. Burden of Chronic Obstructive Pulmonary Disease and Its Attributable Risk Factors in 204 Countries and Territories, 1990-2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378. [Google Scholar] [CrossRef] [PubMed]
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Chronic Obstructive Pulmonary Disease and Atherosclerosis: Common Mechanisms and Novel Therapeutics. Clin. Sci. 2022, 136, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Trinkmann, F.; Saur, J.; Borggrefe, M.; Akin, I. Cardiovascular Comorbidities in Chronic Obstructive Pulmonary Disease (COPD)—Current Considerations for Clinical Practice. J. Clin. Med. 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Ceasovschih, A.; Mantzouranis, E.; Dimitriadis, K.; Sorodoc, V.; Vlachakis, P.K.; Karanikola, A.E.; Theofilis, P.; Koutsopoulos, G.; Drogkaris, S.; Andrikou, I.; et al. Coronary artery thromboembolism as a cause of myocardial infarction with non-obstructive coronary arteries (MINOCA). Hell. J. Cardiol. 2024, 79, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yi, Z.; Cheng, J. Atrial Fibrillation in Aging Population. Aging Med. 2018, 1, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, S.; Ashburner, J.M.; Ellinor, P.T.; McManus, D.D.; Atlas, S.J.; Singer, D.E.; Lubitz, S.A. Prevalence and Incidence of Atrial Fibrillation Among Older Primary Care Patients. JAMA Netw. Open 2023, 6, e2255838. [Google Scholar] [CrossRef] [PubMed]
- Bode, D.; Pronto, J.R.D.; Schiattarella, G.G.; Voigt, N. Metabolic Remodelling in Atrial Fibrillation: Manifestations, Mechanisms and Clinical Implications. Nat. Rev. Cardiol. 2024, 21, 682–700. [Google Scholar] [CrossRef] [PubMed]
- Allessie, M.; Ausma, J.; Schotten, U. Electrical, Contractile and Structural Remodeling during Atrial Fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.R.; Karasik, P.L.; Li, C.; Moubarak, J.; Chavez, M. Electrical Remodeling of the Human Atrium: Similar Effects in Patients with Chronic Atrial Fibrillation and Atrial Flutter. J. Am. Coll. Cardiol. 1997, 30, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, M.; Liu, D.; Zhao, Q. Immune Remodeling and Atrial Fibrillation. Front. Physiol. 2022, 13, 927221. [Google Scholar] [CrossRef] [PubMed]
- Matarese, A.; Sardu, C.; Shu, J.; Santulli, G. Why Is Chronic Obstructive Pulmonary Disease Linked to Atrial Fibrillation? A Systematic Overview of the Underlying Mechanisms. Int. J. Cardiol. 2019, 276, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Solus, J.; Chen, Q.; Rho, Y.H.; Milne, G.; Stein, C.M.; Darbar, D. The Role of Inflammation and Oxidative Stress in Atrial Fibrillation. Heart Rhythm Off. J. Heart Rhythm Soc. 2010, 7, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Chang, J.-P.; Liu, W.-H.; Yang, C.-H.; Chen, Y.-L.; Tsai, T.-H.; Wang, Y.-H.; Pan, K.-L. Increased Inflammatory Cell Infiltration in the Atrial Myocardium of Patients with Atrial Fibrillation. Am. J. Cardiol. 2008, 102, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Bae, B.S.; Kim, J.H.; Jang, H.S.; Lee, B.-R.; Jung, B.-C. The Relationship between Chronic Atrial Fibrillation and Reduced Pulmonary Function in Cases of Preserved Left Ventricular Systolic Function. Korean Circ. J. 2009, 39, 372–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kotlyarov, S.; Lyubavin, A. Early Detection of Atrial Fibrillation in Chronic Obstructive Pulmonary Disease Patients. Medicina 2024, 60, 352. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Laroche, C.; Drozd, M.; Vijgen, J.; Cozma, D.C.; Drozdz, J.; Maggioni, A.P.; Boriani, G.; Lip, G.Y.H. Impact of Chronic Obstructive Pulmonary Disease on Prognosis in Atrial Fibrillation: A Report from the EURObservational Research Programme Pilot Survey on Atrial Fibrillation (EORP-AF) General Registry. Am. Heart J. 2016, 181, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Corica, B.; Pipitone, E.; Vitolo, M.; Raparelli, V.; Basili, S.; Boriani, G.; Harari, S.; Lip, G.Y.H.; Proietti, M.; et al. Prevalence, Management and Impact of Chronic Obstructive Pulmonary Disease in Atrial Fibrillation: A Systematic Review and Meta-Analysis of 4,200,000 Patients. Eur. Heart J. 2021, 42, 3541–3554. [Google Scholar] [CrossRef] [PubMed]
- de-Miguel-Diez, J.; Lopez-de-Andres, A.; Zamorano-Leon, J.J.; Hernández-Barrera, V.; Cuadrado-Corrales, N.; Jimenez-Sierra, A.; Jimenez-Garcia, R.; Carabantes-Alarcon, D. Detrimental Impact of Atrial Fibrillation among Patients Hospitalized for Acute Exacerbation of COPD: Results of a Population-Based Study in Spain from 2016 to 2021. J. Clin. Med. 2024, 13, 2803. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Nguyen, T.D.; Phan, P.T. Prevalence and Associated Factors of Paroxysmal Atrial Fibrillation and Atrial Arrhythmias During Hospitalizations for Exacerbation of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2024, 19, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, A.; Goto, T.; Shimada, Y.J.; Faridi, M.K.; Camargo, C.A.; Hasegawa, K. Acute Exacerbation of Chronic Obstructive Pulmonary Disease and Subsequent Risk of Emergency Department Visits and Hospitalizations for Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006322. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.S.; Eigbire, G.; Ali, M.; Awadalla, M.; Wahab, A.; Ibrahim, H.; Salama, A.; Alweis, R. Relationship of Atrial Fibrillation to Outcomes in Patients Hospitalized for Chronic Obstructive Pulmonary Disease Exacerbation. J. Atr. Fibrillation 2019, 12, 2117. [Google Scholar] [CrossRef] [PubMed]
- Bucci, T.; Romiti, G.F.; Shantsila, A.; Teo, W.; Park, H.; Shimizu, W.; Corica, B.; Proietti, M.; Tse, H.; Chao, T.; et al. Risk of Death and Cardiovascular Events in Asian Patients with Atrial Fibrillation and Chronic Obstructive Pulmonary Disease: A Report from the Prospective APHRS Registry. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2024, 13, e032785. [Google Scholar] [CrossRef] [PubMed]
- Maraey, A.M.; Maqsood, M.H.; Khalil, M.; Hashim, A.; Elzanaty, A.M.; Elsharnoby, H.R.; Elsheikh, E.; Elbatanony, L.; Ong, K.; Chacko, P. Impact of Chronic Obstructive Pulmonary Disease on Atrial Fibrillation Ablation Outcomes According to the National Readmission Database. J. Innov. Card. Rhythm Manag. 2022, 13, 5112–5119. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.F.; Jigoranu, R.A.; Morariu, P.C.; Miftode, R.-S.; Trandabat, B.A.; Iov, D.E.; Cojocaru, E.; Costache, I.I.; Baroi, L.G.; Timofte, D.V.; et al. Atrial Fibrillation and Chronic Coronary Ischemia: A Challenging Vicious Circle. Life 2023, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Almagro, P.; Soler-Cataluña, J.J.; Huerta, A.; González-Segura, D.; Cosío, B.G.; CLAVE Study Investigators. Impact of Comorbidities in COPD Clinical Control Criteria. The CLAVE Study. BMC Pulm. Med. 2024, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Vanfleteren, L.E.G.W.; Spruit, M.A.; Groenen, M.; Gaffron, S.; van Empel, V.P.M.; Bruijnzeel, P.L.B.; Rutten, E.P.A.; Op ’t Roodt, J.; Wouters, E.F.M.; Franssen, F.M.E. Clusters of Comorbidities Based on Validated Objective Measurements and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Durheim, M.T.; Holmes, D.N.; Blanco, R.G.; Allen, L.A.; Chan, P.S.; Freeman, J.V.; Fonarow, G.C.; Go, A.S.; Hylek, E.M.; Mahaffey, K.W.; et al. Characteristics and Outcomes of Adults with Chronic Obstructive Pulmonary Disease and Atrial Fibrillation. Heart Br. Card. Soc. 2018, 104, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañero, M.; López-Pardo, E.; Cordero, A.; Ruano-Ravina, A.; Novo-Platas, J.; Pereira-Vázquez, M.; Martínez-Gómez, Á.; García-Seara, J.; Martínez-Sande, J.-L.; Peña-Gil, C.; et al. A Prospective Study of the Clinical Outcomes and Prognosis Associated with Comorbid COPD in the Atrial Fibrillation Population. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Børvik, T.; Brækkan, S.K.; Enga, K.; Schirmer, H.; Brodin, E.E.; Melbye, H.; Hansen, J.-B. COPD and Risk of Venous Thromboembolism and Mortality in a General Population. Eur. Respir. J. 2016, 47, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.; Chronis, C.; Papapetrou, E.; Tatsioni, A.; Gartzonika, K.; Tsaousi, C.; Gogali, A.; Katsanos, C.; Vaggeli, A.; Tselepi, C.; et al. Prothrombotic State in Patients with Stable COPD: An Observational Study. ERJ Open Res. 2021, 7, 00297-2021. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Seemungal, T.A.; MacCallum, P.K.; Paul, E.A.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Meade, T.W. Acute Exacerbations of Chronic Obstructive Pulmonary Disease Are Accompanied by Elevations of Plasma Fibrinogen and Serum IL-6 Levels. Thromb. Haemost. 2000, 84, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Maclay, J.D.; McAllister, D.A.; Johnston, S.; Raftis, J.; McGuinnes, C.; Deans, A.; Newby, D.E.; Mills, N.L.; MacNee, W. Increased Platelet Activation in Patients with Stable and Acute Exacerbation of COPD. Thorax 2011, 66, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Rahaghi, F.N.; Pistenmaa, C.L. Hypercoagulation in COPD: The Clot Thickens. ERJ Open Res. 2021, 7, 00534–02021. [Google Scholar] [CrossRef] [PubMed]
- Wedzicha, J.A.; Syndercombe-Court, D.; Tan, K.C. Increased Platelet Aggregate Formation in Patients with Chronic Airflow Obstruction and Hypoxaemia. Thorax 1991, 46, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Ma, X.; Zhang, H.; Chen, J.; Liu, M.; Chen, L.; Xu, H. Role of HIF-1α in Hypercoagulable State of COPD in Rats. Arch. Biochem. Biophys. 2024, 753, 109903. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Corica, B.; Mei, D.A.; Frost, F.; Bisson, A.; Boriani, G.; Bucci, T.; Olshansky, B.; Chao, T.-F.; Huisman, M.V.; et al. Impact of Chronic Obstructive Pulmonary Disease in Patients with Atrial Fibrillation: An Analysis from the GLORIA-AF Registry. Europace 2024, 26, euae021. [Google Scholar] [CrossRef] [PubMed]
- Sertcakacilar, G.; Yildiz, G.O. Association between Anemia and New-Onset Atrial Fibrillation in Critically Ill Patients in the Intensive Care Unit: A Retrospective Cohort Analysis. Clin. Pract. 2022, 12, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Inoue, K.; Kobori, A.; Morimoto, T.; Morishima, I.; Yamaji, H.; Nakazawa, Y.; Kusano, K.; Okada, M.; Koyama, Y.; et al. Anemia and Atrial Fibrillation Ablation Recurrence: Insights from a Large-Scale Multicentre Registry. Eur. Heart J. 2023, 44, ehad655.522. [Google Scholar] [CrossRef]
- Sarkar, M.; Rajta, P.N.; Khatana, J. Anemia in Chronic Obstructive Pulmonary Disease: Prevalence, Pathogenesis, and Potential Impact. Lung India Off. Organ Indian Chest Soc. 2015, 32, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Dublin, S.; Glazer, N.L.; Smith, N.L.; Psaty, B.M.; Lumley, T.; Wiggins, K.L.; Page, R.L.; Heckbert, S.R. Diabetes Mellitus, Glycemic Control, and Risk of Atrial Fibrillation. J. Gen. Intern. Med. 2010, 25, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Ochi, A.; Uchimoto, S.; Kakutani, Y.; Yamazaki, Y.; Morioka, T.; Shoji, T.; Inaba, M.; Emoto, M. Factors Associated with Atrial Fibrillation in Japanese Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Diabetol. Int. 2022, 13, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, E.R.; Price, M.V.; Mohler, P.J. Arrhythmogenic Substrates for Atrial Fibrillation in Obesity. Front. Physiol. 2018, 9, 1482. [Google Scholar] [CrossRef] [PubMed]
- Peller, M.; Kapłon-Cieślicka, A.; Rosiak, M.; Tymińska, A.; Ozierański, K.; Eyileten, C.; Kondracka, A.; Mirowska-Guzel, D.; Opolski, G.; Postuła, M.; et al. Are Adipokines Associated with Atrial Fibrillation in Type 2 Diabetes? Endokrynol. Pol. 2020, 71, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Agbaedeng, T.A.; Zacharia, A.L.; Iroga, P.E.; Rathnasekara, V.M.; Munawar, D.A.; Bursill, C.; Noubiap, J.J. Associations between Adipokines and Atrial Fibrillation: A Systematic Review and Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Limpitikul, W.B.; Das, S. Obesity-Related Atrial Fibrillation: Cardiac Manifestation of a Systemic Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Tsukino, M. Clinical Course and Prognosis of Patients with Chronic Obstructive Pulmonary Disease. Curr. Opin. Pulm. Med. 2000, 6, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S. Analysis of Differentially Expressed Genes and Signaling Pathways Involved in Atherosclerosis and Chronic Obstructive Pulmonary Disease. Biomol. Concepts 2022, 13, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Vilkman, S.; Keistinen, T.; Tuuponen, T.; Kivelä, S.L. Survival and Cause of Death among Elderly Chronic Obstructive Pulmonary Disease Patients after First Admission to Hospital. Respir. Int. Rev. Thorac. Dis. 1997, 64, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Gupta, A.; Strollo, P.J.; Fuhrman, C.R.; Leader, J.K.; Bon, J.; Slivka, W.A.; Shoushtari, A.H.; Avolio, J.; Kip, K.E.; et al. Airflow Limitation and Endothelial Dysfunction. Unrelated and Independent Predictors of Atherosclerosis. Am. J. Respir. Crit. Care Med. 2016, 194, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Bukowska, A.; Lillig, C.H.; Lendeckel, U. Oxidative Stress and Microcirculatory Flow Abnormalities in the Ventricles during Atrial Fibrillation. Front. Physiol. 2012, 3, 236. [Google Scholar] [CrossRef] [PubMed]
- Houben-Wilke, S.; Jörres, R.A.; Bals, R.; Franssen, F.M.E.; Gläser, S.; Holle, R.; Karch, A.; Koch, A.; Magnussen, H.; Obst, A.; et al. Peripheral Artery Disease and Its Clinical Relevance in Patients with Chronic Obstructive Pulmonary Disease in the COPD and Systemic Consequences-Comorbidities Network Study. Am. J. Respir. Crit. Care Med. 2017, 195, 189–197. [Google Scholar] [CrossRef] [PubMed]

| Parameter | AF + COPD (n = 1247) | AF (n = 25,474) | COPD (n = 14,051) | Significance Level, p * |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Age, years | 71.82 ± 9.31 | 70.8 ± 10.8 | 59.97 ± 21.48 | p < 0.0001 p 1,2 = 0.0011 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| Male | 773 (61.99%) | 11,243 (44.14%) | 7997 (56.91%) | - |
| Female | 474 (38.01%) | 14,231 (55.86%) | 6054 (43.09%) | - |
| Clinical Condition | AF + COPD (n = 1247) | AF (n = 25,474) | COPD (n = 14,051) | Significance Level, p * | Odds Ratio (OR) and 95% Confidence Interval (CI) * |
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Lung cancer | 58 (4.65%) | 254 (1.0%) | 543 (3.86%) | p < 0.001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.1955 | OR 1,2 4.84 95% CI (3.55–6.51) OR 2,3 0.25 95% CI (0.21–0.29) OR 1,3 1.21 95% CI (0.9–1.6) |
| Anemia | 65 (5.21%) | 927 (3.64%) | 393 (2.8%) | p < 0.0001 p 1,2 = 0.0052 p 2,3=0.0001 p 1,3 < 0.0001 | OR 1,2 1.45 95% CI (1.11–1.89) OR 2,3 1.31 95% CI (1.16–1.48) OR 1,3 1.91 95% CI (1.44–2.51) |
| Hypothyroidism | 103 (8.26%) | 2266 (8.9%) | 788 (5.61%) | p < 0.0001 p 1,2 = 0.4716 p 2,3 < 0.0001 p 1,3 = 0.0002 | OR 1,2 1.11 95% CI (0.82–1.48) OR 2,3 1.69 95% CI (1.49–1.93) OR 1,3 1.88 95% CI (1.38–2.55) |
| Type 1 DM | 53 (4.25%) | 976 (3.83%) | 323 (2.3%) | p < 0.001 p 1,2 = 0.4996 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.11 95% CI (0.82–1.48) OR 2,3 1.69 95% CI (1.49–1.93) OR 1,3 1.88 95% CI (1.38–2.55) |
| Type 2 DM | 350 (28.07%) | 5782 (22.7%) | 2012 (14.32%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.32 95% CI (1.17–1.51) OR 2,3 1.75 95% CI (1.66–1.86) OR 1,3 2.33 95% CI (2.04–2.67) |
| Obesity | 310 (24.86%) | 5654 (22.2%) | 2490 (17.72%) | p < 0.0001 p 1,2 = 0.0299 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.15 95% CI (1.01–1.32) OR 2,3 1.32 95% CI (1.26–1.4) OR 1,3 1.53 95% CI (1.34–1.76) |
| AH | 1002 (80.35%) | 19419 (76.23%) | 7980 (56.79%) | p < 0.0001 p 1,2 = 0.0009 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.27 95% CI (1.1–1.48) OR 2,3 2.43 95% CI (2.33–2.55) OR 1,3 3.11 95% CI (2.69–3.61) |
| Angina pectoris | 215 (17.24%) | 3085 (12.11%) | 911 (6.48%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.51 95% CI (1.29–1.76) OR 2,3 1.98 95% CI (1.84–2.15) OR 1,3 3.00 95% CI (2.54–3.54) |
| Primary MI | 19 (1.52%) | 651 (2.56%) | 185 (1.32%) | p < 0.0001 p 1,2 = 0.029 p 2,3 < 0.0001 p 1,3 = 0.5413 | OR 1,2 0.58 95% CI (0.35–0.93) OR 2,3 1.96 95% CI (1.66–2.33) OR 1,3 1.15 95% CI (0.68–1.87) |
| Recurrent MI | 8 (0.64%) | 68 (0.27%) | 23 (0.16%) | p 1,2 = 0.0313 p 2,3 = 0.0523 p 1,3 = 0.0011 | OR 1,2 2.41 95% CI (1.0–5.04) OR 2,3 1.63 95% CI (1.0–2.75) OR 1,3 3.93 95% CI (1.52–9.14) |
| CHD | 1113 (89.25%) | 20045 (78.69%) | 6929 (49.31%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 2.24 95% CI (1.87–2.72) OR 2,3 3.79 95% CI (3.63–3.97) OR 1,3 8.53 95% CI (7.11–10.32) |
| PE | 25 (2.0%) | 133 (0.52%) | 65 (0.46%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 = 0.4669 p 1,3 < 0.0001 | OR 1,2 3.89 95% CI (2.43–6.04) OR 2,3 1.12 95% CI (0.83–1.54) OR 1,3 4.40 95% CI (2.65–7.11) |
| Aortic stenosis | 13 (1.04%) | 257 (1.01%) | 50 (0.36%) | p < 0.0001 p 1,2 = 0.90 p 2,3 < 0.0001 p 1,3 = 0.0007 | OR 1,2 1.03 95% CI (0.54–1.81) OR 2,3 2.85 95% CI (2.1–3.95) OR 1,3 2.94 95% CI (1.47–5.53) |
| DCMP | 49 (3.93%) | 633 (2.48%) | 142 (1.01%) | p < 0.0001 p 1,2 = 0.0022 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.60 95% CI (1.17–2.16) OR 2,3 2.49 95% CI (2.07–3.02) OR 1,3 4.00 95% CI (2.82–5.61) |
| CHF | 209 (16.76%) | 2412 (9.47%) | 453 (3.22%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.92 95% CI (1.64–2.25) OR 2,3 3.13 95% CI (2.83–3.49) OR 1,3 6.04 95% CI (5.04–7.22) |
| ICH | 4 (0.32%) | 149 (0.58%) | 38 (0.27%) | p < 0.0001 p 1,2 = 0.3102 p 2,3 < 0.0001 p 1,3 = 0.7447 | OR 1,2 0.54 95% CI (0.15–1.43) OR 2,3 2.16 95% CI (1.51–3.19) OR 1,3 1.18 95% CI (0.31–3.3) |
| IS | 112 (8.98%) | 2355 (9.24%) | 438 (3.12%) | p < 0.0001 p 1,2 = 0.7539 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 0.96 95% CI (0.79–1.18) OR 2,3 3.16 95% CI (2.85–3.52) OR 1,3 3.06 95% CI (2.45–3.82) |
| CVD | 601 (48.2%) | 10311 (40.48%) | 4461 (31.75%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.36 95% CI (1.22–1.54) OR 2,3 1.46 95% CI (1.4–1.53) OR 1,3 1.99 95% CI (1.78–2.25) |
| PAD | 13 (1.04%) | 102 (0.4%) | 58 (0.41%) | p = 0.0029 p 1,2 = 0.0016 p 2,3 = 0.9182 p 1,3 = 0.0035 | OR 1,2 2.62 95% CI (1.35–4.7) OR 2,3 0.96 95% CI (0.7–1.36) OR 1,3 2.54 95% CI (1.27–4.7) |
| ARVI | 588 (47.15%) | 10079 (39.57%) | 7227 (51.43%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.0041 | OR 1,2 1.36 95% CI (1.21–1.53) OR 2,3 0.61 95% CI (0.59–0.64) OR 1,3 0.84 95% CI (0.75–0.95) |
| Viral pneumonia | 180 (14.43%) | 2030 (7.97%) | 1504 (10.7%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 | OR 1,2 1.94 95% CI (1.64–2.3) OR 2,3 0.72 95% CI (0.67–0.78) OR 1,3 1.40 95% CI (1.18–1.67) |
| Bacterial pneumonia | 188 (15.08%) | 1787 (7.01%) | 1615 (11.49%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.0002 | OR 1,2 2.35 95% CI (1.99–2.77) OR 2,3 0.58 95% CI (0.54–0.62) OR 1,3 1.36 95% CI (1.15–1.61) |
| Clinical Condition | AF + COPD (n = 1247) | AF (n = 25,474) | COPD (n = 14,051) | Significance Level, p * |
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Lung cancer | 0.54 ± 3.35 | 0.13 ± 1.83 | 0.55 ± 3.87 | p < 0.001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.9311 |
| Anemia | 0.1 ± 0.59 | 0.07 ± 0.53 | 0.06 ± 0.49 | p < 0.001 p 1,2 = 0.1014 p 2,3 = 0.0014 p 1,3 = 0.0035 |
| Hypothyroidism | 0.19 ± 0.85 | 0.26 ± 1.14 | 0.15 ± 0.87 | p < 0.001 p 1,2 = 0.0405 p 2,3 < 0.0001 p 1,3 = 0.1623 |
| Type 1 DM | 0.2 ± 1.72 | 0.19 ± 1.83 | 0.14 ± 1.56 | p < 0.001 p 1,2 = 0.8544 p 2,3 = 0.0031 p 1,3 = 0.1693 |
| Type 2 DM | 2.8 ± 6.93 | 2.24 ± 6.5 | 1.41 ± 5.18 | p < 0.001 p 1,2 = 0.0032 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| Obesity | 0.37 ± 0.75 | 0.34 ± 0.76 | 0.29 ± 0.77 | p < 0.001 p 1,2 = 0.1603 p 2,3 < 0.0001 p 1,3 = 0.0004 |
| AH | 5.76 ± 6.42 | 4.98 ± 5.64 | 3.68 ± 5.37 | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| Angina pectoris | 0.61 ± 2.18 | 0.41 ± 1.89 | 0.25 ± 1.57 | p < 0.0001 p 1,2 = 0.0003 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| Primary MI | 0.03 ± 0.34 | 0.05 ± 0.38 | 0.03 ± 0.28 | p < 0.001 p 1,2 = 0.1737 p 2,3 < 0.0001 p 1,3 = 0.3039 |
| Recurrent MI | 0.01 ± 0.11 | 0.0 ± 0.12 | 0.0 ± 0.05 | p < 0.001 p 1,2 = 0.338 p 2,3 = 0.0058 p 1,3 = 0.0006 |
| CHD | 7.4 ± 7.29 | 5.52 ± 6.45 | 3.07 ± 5.32 | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| PE | 0.04 ± 0.43 | 0.01 ± 0.17 | 0.01 ± 0.2 | p < 0.0001 p 1,2 < 0.0001 p 2,3 = 0.6958 p 1,3 < 0.0001 |
| Aortic stenosis | 0.04 ± 0.69 | 0.05 ± 0.77 | 0.02 ± 0.42 | p < 0.001 p 1,2 = 0.8218 p 2,3 < 0.0001 p 1,3 = 0.0345 |
| DCMP | 0.25 ± 1.78 | 0.12 ± 1.28 | 0.05 ± 0.77 | p < 0.0001 p 1,2 = 0.0007 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| CHF | 0.43 ± 1.3 | 0.23 ± 0.96 | 0.07 ± 0.55 | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| ICH | 0.0 ± 0.09 | 0.01 ± 0.16 | 0.0 ± 0.1 | p < 0.001 p 1,2 = 0.2998 p 2,3 = 0.0005 p 1,3 = 0.8751 |
| IS | 0.21 ± 0.92 | 0.21 ± 0.91 | 0.06 ± 0.48 | p < 0.0001 p 1,2 = 0.8041 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| CVD | 1.67 ± 2.86 | 1.37 ± 2.83 | 1.07 ± 2.56 | p < 0.0001 p 1,2 = 0.0002 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| PAD | 0.06 ± 0.72 | 0.01 ± 0.3 | 0.02 ± 0.35 | p < 0.0001 p 1,2 < 0.0001 p 2,3 = 0.4396 p 1,3 = 0.0005 |
| ARVI | 0.11 ± 0.39 | 0.1 ± 0.4 | 0.15 ± 0.53 | p < 0.0001 p 1,2 = 0.5533 p 2,3 < 0.0001 p 1,3 = 0.0085 |
| Viral pneumonia | 0.26 ± 0.79 | 0.12 ± 0.48 | 0.17 ± 0.61 | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 < 0.0001 |
| Bacterial pneumonia | 0.25 ± 0.8 | 0.1 ± 0.42 | 0.19 ± 0.65 | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.0043 |
| Parameter | AF + COPD (n = 1247) | AF (n = 25,474) | COPD (n = 14,051) | Significance Level, p * | Hazard Ratio (HR) and 95% Confidence Interval (CI) * |
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Male | 206 (73.57%) | 1967 (49.42%) | 1655 (75.06%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.6407 | HR 1,2 1.58 95% CI (1.37–1.82) HR 2,3 1.20 95% CI (1.13–1.28) |
| Female | 74 (26.43%) | 2013 (50.58%) | 550 (24.94%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.6407 | HR2,3 0.62 95% CI (0.57–0.68) |
| Age | 73.46 ± 9.94 | 75.69 ± 10.54 | 72.89 ± 11.37 | p < 0.0001 p 1,2 = 0.0006 p 2,3 < 0.0001 p 1,3 = 0.4265 | - |
| Parameter | AF + COPD (n = 1247) | AF (n = 25,474) | COPD (n = 14,051) | Significance Level, p * | Hazard Ratio (HR) and 95% Confidence Interval (CI) * |
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
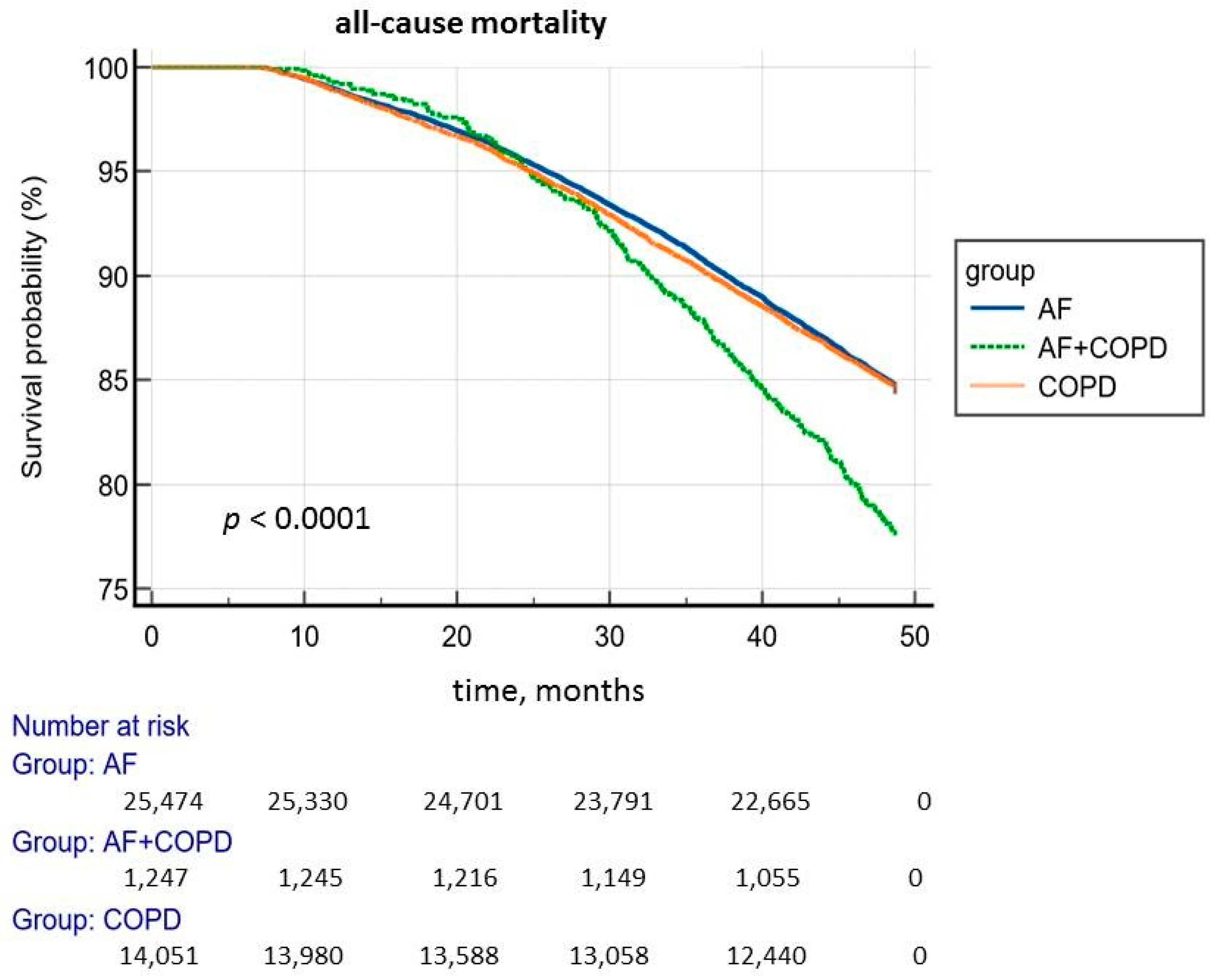
| Total number of deceased | 280 (22.45%) | 3980 (15.62%) | 2205 (15.69%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 = 0.8678 p 1,3 < 0.0001 | HR 1,2 1.47 95% CI (1.31–1.67) HR 1,3 1.46 95% CI (1.29–1.65) |
| Over 70 years old | 176 (24.89%) | 2800 (20.64%) | 1279 (27.19%) | p < 0.0001 p 1,2 = 0.0076 p 2,3 < 0.0001 p 1,3 = 0.2157 | HR 1,2 1.26 95% CI (1.09–1.47) HR 2,3 1.38 95% CI (1.29–1.47) |
| Diseases Group (ICD-10) | AF + COPD (n = 1247) | AF (n = 25,474) | COPD (n = 14,051) | Significance Level, p * | Hazard Ratio (HR) and 95% Confidence Interval (CI) * |
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Neoplasms | 13 (1.04%) | 167 (0.66%) | 132 (0.94%) | p = 0.0043 p 1,2 = 0.146 p 2,3 = 0.0022 p 1,3 = 0.7189 | HR 2,3 1.26 95% CI (1.05–1.52) |
| Diseases of the nervous system | 28 (2.25%) | 737 (2.89%) | 215 (1.53%) | p < 0.0001 p 1,2 = 0.2105 p 2,3 < 0.0001 p 1,3 = 0.0691 | HR 2,3 0.59 95% CI (0.52–0.68) |
| Diseases of the circulatory system | 171 (13.71%) | 2127 (8.35%) | 1070 (7.62%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 = 0.0109 p 1,3 < 0.0001 | HR 1,2 1.64 95% CI (1.41–1.92) HR 2,3 0.92 95% CI (0.85–0.99) HR 1,3 1.78 95% CI (1.52–2.09) |
| Pulmonary embolism | 15 (1.20%) | 109 (0.43%) | 58 (0.41%) | p = 0.0258 p 1,2 = 0.3125 p 2,3 = 0.0716 p 1,3 = 0.0388 | HR 1,3 1.70 95% CI (1.02–2.83) |
| I50.0 congestive heart failure | 53 (4.25%) | 941 (3.69%) | 249 (1.77%) | p < 0.0001 p 1,2 = 0.3489 p 2,3 < 0.0001 p 1,3 < 0.0001 | HR 2,3 0.54 95% CI (0.47–0.61) HR 1,3 2.12 95% CI (1.59–2.83) |
| I50.1 left ventricular failure | 36 (2.89%) | 694 (2.72%) | 267 (1.9%) | p < 0.0001 p 1,2 = 0.7988 p 2,3 < 0.0001 p 1,3 = 0.022 | HR 2,3 0.74 95% CI (0.65–0.84) HR 1,3 1.39 95% CI (1.00–1.95) |
| Diseases of the respiratory system | 30 (2.41%) | 198 (0.78%) | 375 (2.67%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.6437 | HR 1,2 2.27 95% CI (1.57–3.26) HR 2,3 2.53 95% CI (2.19–2.93) |
| J96.0 acute respiratory failure | 12 (0.96%) | 127 (0.5%) | 172 (1.22%) | p < 0.0001 p 1,2 = 0.0433 p 2,3 < 0.0001 p 1,3 = 0.4982 | HR 2,3 1.77 95% CI (1.48–2.12) |
| J96.1 chronic respiratory failure | 14 (1.12%) | 28 (0.11%) | 145 (1.03%) | p < 0.0001 p 1,2 < 0.0001 p 2,3 < 0.0001 p 1,3 = 0.8751 | HR 1,2 2.52 95% CI (1.50–4.23) HR 2,3 2.68 95% CI (2.16–3.32) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlyarov, S.; Lyubavin, A. Structure of Comorbidities and Causes of Death in Patients with Atrial Fibrillation and Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2025, 14, 5045. https://doi.org/10.3390/jcm14145045
Kotlyarov S, Lyubavin A. Structure of Comorbidities and Causes of Death in Patients with Atrial Fibrillation and Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2025; 14(14):5045. https://doi.org/10.3390/jcm14145045
Chicago/Turabian StyleKotlyarov, Stanislav, and Alexander Lyubavin. 2025. "Structure of Comorbidities and Causes of Death in Patients with Atrial Fibrillation and Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 14, no. 14: 5045. https://doi.org/10.3390/jcm14145045
APA StyleKotlyarov, S., & Lyubavin, A. (2025). Structure of Comorbidities and Causes of Death in Patients with Atrial Fibrillation and Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine, 14(14), 5045. https://doi.org/10.3390/jcm14145045







