Activation of Emergency Department Stroke Protocol by Emergency Medical Services: A Retrospective Cross-Sectional Study
Abstract
1. Introduction
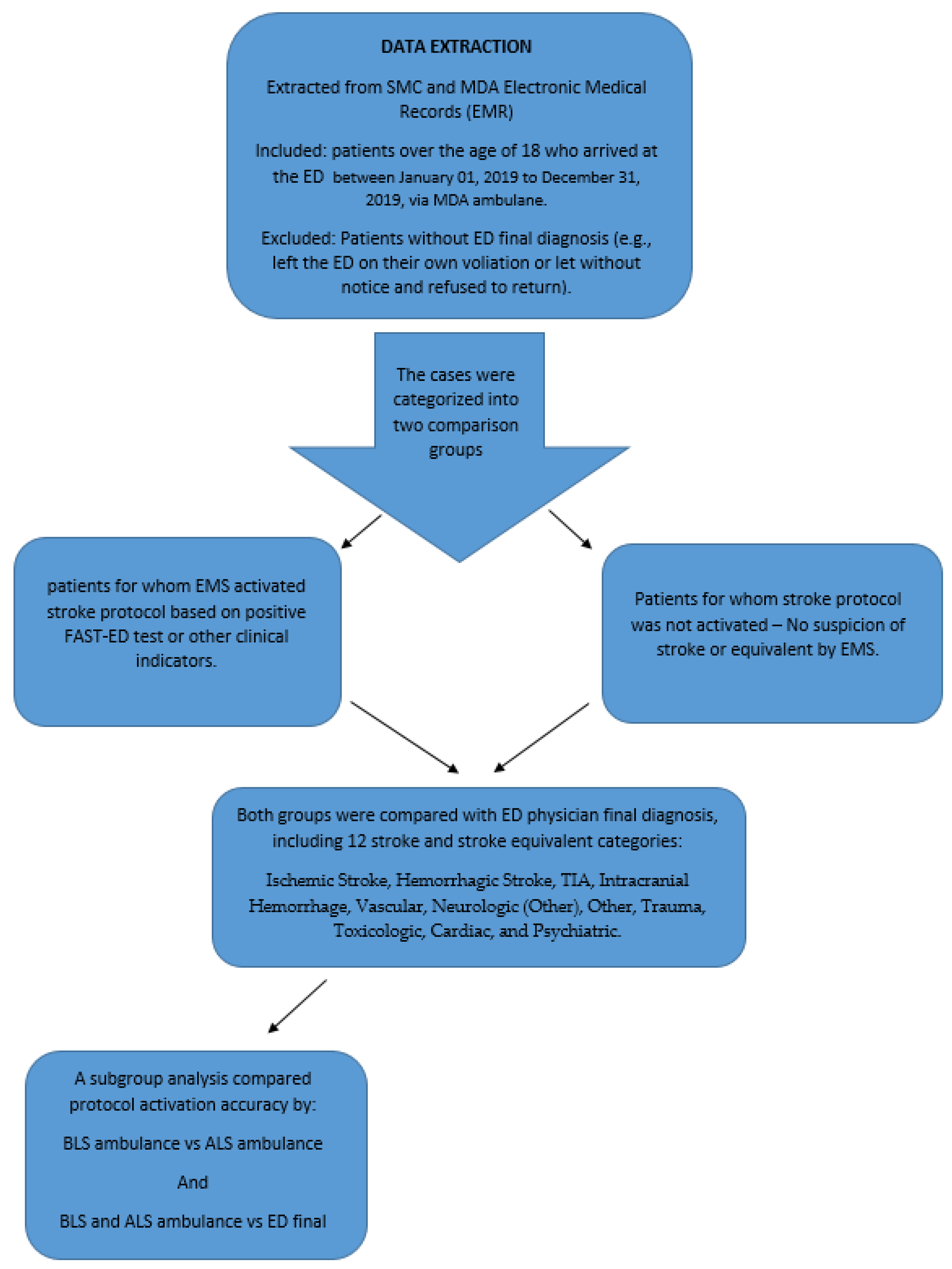
2. Materials and Methods
2.1. Setting
2.2. Study Design and Participants
2.3. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| tPA | Tissue plasminogen activator |
| EMSs | Emergency medical services |
| ED | Emergency department |
| TIA | Transient ischemic attack |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| SBP | Systolic blood pressure |
| DM | Diabetes mellitus |
| FAST-ED | Field assessment stroke triage for emergency destination |
| RACE | Rapid arterial occlusion evaluation |
| LAPSS | Los Angeles prehospital stroke screening |
| PreHAST | PreHospital ambulance stroke test |
| MDA | Magen David Adom |
| SMC | Shamir medical center |
| BLS | Basic life support |
| ALS | Advanced life support |
| EMT | Emergency medical technician |
| RECORD | Reporting of studies conducted using observational routinely collected health data |
| STARD | Standards for reporting diagnostic accuracy |
| IRB | Institutional review board |
| CI | Confidence interval |
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef]
- Lee, J.M.; Grabb, M.C.; Zipfel, G.J.; Choi, D.W. Brain tissue responses to ischemia. J. Clin. Investig. 2000, 106, 723–731. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Saver, J.L. Time is brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef]
- Saver, J.L.; Fonarow, G.C.; Smith, E.E.; Reeves, M.J.; Grau-Sepulveda, M.V.; Pan, W.; Olson, D.M.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013, 309, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Kate, M.P.; Jeerakathil, T.; Buck, B.H.; Khan, K.; Nomani, A.Z.; Butt, A.; Thirunavukkarasu, S.; Nowacki, T.; Kalashyan, H.; Lloret-Villas, M.I.; et al. Pre-hospital triage of suspected acute stroke patients in a mobile stroke unit in the rural Alberta. Sci. Rep. 2021, 11, 4988. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.O.; Silva, G.S.; Furie, K.L.; Frankel, M.R.; Lev, M.H.; Camargo, É.C.; Haussen, D.C.; Singhal, A.B.; Koroshetz, W.J.; Smith, W.S.; et al. Field Assessment Stroke Triage for Emergency Destination: A Simple and Accurate Prehospital Scale to Detect Large Vessel Occlusion Strokes. Stroke 2016, 47, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, H.; Lei, Y.; Gao, D.; Wang, Y.; Wang, Y.; Zhou, Y.; Wang, A.; Wang, W.; Zhao, X. Validation of the Los Angeles pre-hospital stroke screen (LAPSS) in a Chinese urban emergency medical service population. PLoS ONE 2013, 8, e70742. [Google Scholar] [CrossRef]
- Andsberg, G.; Esbjörnsson, M.; Olofsson, A.; Lindgren, A.; Norrving, B.; von Euler, M. PreHospital Ambulance Stroke Test—Pilot study of a novel stroke test. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 37. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Ossa, N.; Carrera, D.; Gorchs, M.; Querol, M.; Millán, M.; Gomis, M.; Dorado, L.; López-Cancio, E.; Hernández-Pérez, M.; Chicharro, V.; et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: The rapid arterial occlusion evaluation scale. Stroke 2014, 45, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Kidwell, C.S.; Starkman, S.; Eckstein, M.; Weems, K.; Saver, J.L. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS). Stroke 2000, 31, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Studnek, J.R.; Asimos, A.; Dodds, J.; Swanson, D. Assessing the validity of the Cincinnati prehospital stroke scale and the medic prehospital assessment for code stroke in an urban emergency medical services agency. Prehospital Emerg. Care 2013, 17, 348–353. [Google Scholar] [CrossRef]
- Asimos, A.W.; Ward, S.; Brice, J.H.; Rosamond, W.D.; Goldstein, L.B.; Studnek, J. Out-of-hospital stroke screen accuracy in a state with an emergency medical services protocol for routing patients to acute stroke centers. Ann. Emerg. Med. 2014, 64, 509–515. [Google Scholar] [CrossRef]
- Heikkilä, I.; Kuusisto, H.; Holmberg, M.; Palomäki, A. Fast Protocol for Treating Acute Ischemic Stroke by Emergency Physicians. Ann. Emerg. Med. 2019, 73, 105–112. [Google Scholar] [CrossRef]
- Sonkin, R.; Goldberg, U.; Jaffe, E. Emergency Medical Services in Israel and Abroad. In Medicine: From Biblical Canaan to Modern Israel; Stanton, S., Collins, K., Eds.; Vallentine Mitchell: London, UK, 2021; pp. 237–257. [Google Scholar]
- Ellis, D.Y.; Sorene, E. Magen David Adom-The EMS in Israel. Resuscitation 2008, 76, 5–10. [Google Scholar] [CrossRef]
- Jaffe, E.; Sonkin, R.; Alpert, E.A.; Zerath, E. Responses of a pre-hospital emergency medical service during military conflict versus COVID-19: A retrospective comparative cohort study. Mil. Med. 2022, 187, e1462–e1468. [Google Scholar] [CrossRef]
- Wacht, O.; Schwartz, D.; Miller, R. The development and history of the paramedic profession in Israel. Int. Paramed. Pract. 2015, 5, 31–34. [Google Scholar] [CrossRef]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M.; RECORD Working Committee. The Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef]
- Kuhn, M. Caret: Classification and Regression Training. Available online: https://CRAN.R-project.org/package=caret (accessed on 22 May 2025).
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 22 May 2025).
- Baldereschi, M.; Piccardi, B.; Di Carlo, A.; Lucente, G.; Guidetti, D.; Consoli, D.; Provinciali, L.; Toni, D.; Sacchetti, M.L.; Polizzi, B.M.; et al. Relevance of prehospital stroke code activation for acute treatment measures in stroke care: A review. Cerebrovasc. Dis. 2012, 34, 182–190. [Google Scholar] [CrossRef]
- Clarke, D.J.; Forster, A. Improving post-stroke recovery: The role of the multidisciplinary health care team. J. Multidiscip. Healthc. 2015, 8, 433–442. [Google Scholar] [CrossRef]
- Newgard, C.D.; Staudenmayer, K.; Hsia, R.Y.; Mann, N.C.; Bulger, E.M.; Holmes, J.F.; Fleischman, R.; Gorman, K.; Haukoos, J.; McConnell, K.J. The cost of overtriage: More than one-third of low-risk injured patients were taken to major trauma centers. Health Aff. 2013, 32, 1591–1599. [Google Scholar] [CrossRef]
- Duvekot, M.H.C.; Venema, E.; Rozeman, A.D.; Moudrous, W.; Vermeij, F.H.; Biekart, M.; Lingsma, H.F.; Maasland, L.; Wijnhoud, A.D.; Mulder, L.J.M.M.; et al. Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): A prospective observational study. Lancet Neurol. 2021, 20, 213–221. [Google Scholar] [CrossRef]

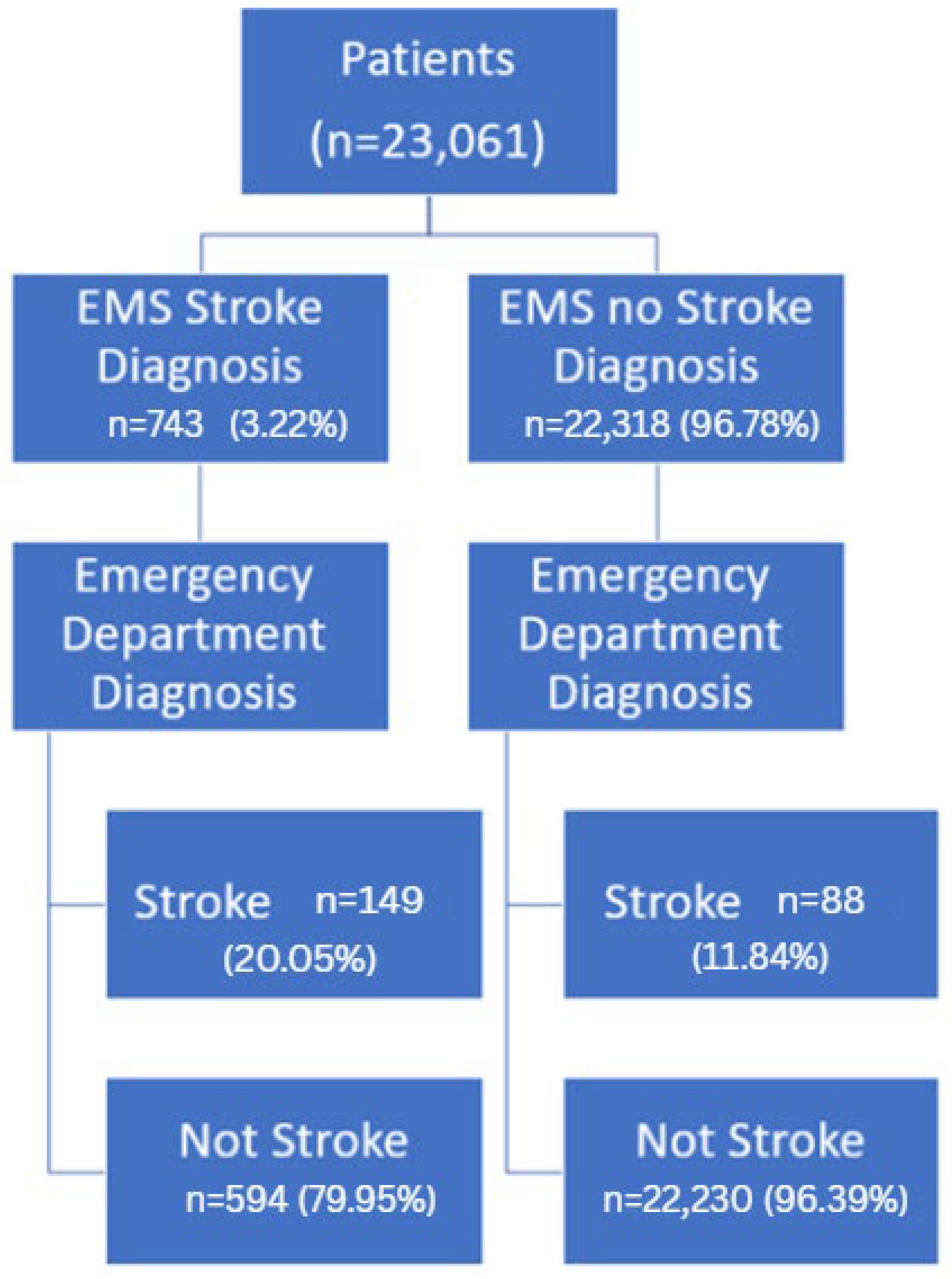
| EMS Response | EMS Diagnosis | Emergency Department Diagnosis | p Value | Sensitivity (CI **) | Specificity (CI) | PPV (CI) | NPV (CI) | |
|---|---|---|---|---|---|---|---|---|
| Stroke * | Not Stroke | |||||||
| BLS Ambulance | Stroke | 111 | 468 | <0.01 | 61% (54–68) | 97% (97–97) | 19% (16–22) | 100% (99–100) |
| Not Stroke | 71 | 14,769 | ||||||
| ALS Ambulance | Stroke | 24 | 81 | <0.01 | 73% (56–89) | 98% (98–99) | 23% (14–31) | 100% (100–100) |
| Not Stroke | 9 | 5254 | ||||||
| Both Responses | Stroke | 14 | 45 | <0.01 | 64% (41–86) | 98% (97–99) | 24% (12–35) | 100% (99–100) |
| Not Stroke | 8 | 2207 | ||||||
| Total | Stroke | 149 | 594 | <0.01 | 63% (57–69) | 97% (97–98) | 20% (17–23) | 100% (100–100) |
| Not Stroke | 88 | 22,230 | ||||||
| ED Diagnosis—Categories | N |
|---|---|
| Hemorrhagic Stroke | 10 |
| Intracranial Hemorrhage | 8 |
| Ischemic Stroke | 35 |
| Other Neurologic | 1 |
| Transient Ischemic Attack | 34 |
| MDA Diagnosis—Categories * | N |
|---|---|
| Cardiac | 6 |
| Other | 21 |
| Other Neuro | 58 |
| Trauma | 12 |
| Toxicologic | 2 |
| Vascular | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arad, N.; Sonkin, R.; Jaffe, E.; Pachys, G.; Strugo, R.; Avisar, S.; Cohen, A.; Levite, R.; Kimiagar, I.; Avnery Kalmanovich, S.; et al. Activation of Emergency Department Stroke Protocol by Emergency Medical Services: A Retrospective Cross-Sectional Study. J. Clin. Med. 2025, 14, 5041. https://doi.org/10.3390/jcm14145041
Arad N, Sonkin R, Jaffe E, Pachys G, Strugo R, Avisar S, Cohen A, Levite R, Kimiagar I, Avnery Kalmanovich S, et al. Activation of Emergency Department Stroke Protocol by Emergency Medical Services: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine. 2025; 14(14):5041. https://doi.org/10.3390/jcm14145041
Chicago/Turabian StyleArad, Noa, Roman Sonkin, Eli Jaffe, Gal Pachys, Refael Strugo, Shiran Avisar, Aya Cohen, Ronen Levite, Itzhak Kimiagar, Shani Avnery Kalmanovich, and et al. 2025. "Activation of Emergency Department Stroke Protocol by Emergency Medical Services: A Retrospective Cross-Sectional Study" Journal of Clinical Medicine 14, no. 14: 5041. https://doi.org/10.3390/jcm14145041
APA StyleArad, N., Sonkin, R., Jaffe, E., Pachys, G., Strugo, R., Avisar, S., Cohen, A., Levite, R., Kimiagar, I., Avnery Kalmanovich, S., Sandler, H., Feig, E., Kagansky, N., & Trotzky, D. (2025). Activation of Emergency Department Stroke Protocol by Emergency Medical Services: A Retrospective Cross-Sectional Study. Journal of Clinical Medicine, 14(14), 5041. https://doi.org/10.3390/jcm14145041





